Abstract
Pretreatment with scutellaria baicalensis stem-leaf total flavonoid has protective effects against ischemia and attenuates myocardial ischemia-reperfusion injury. In this study, rats were given scutellaria baicalensis stem-leaf total flavonoid intragastrically at 50, 100, and 200 mg/kg per day for 7 days before focal cerebral ischemia-reperfusion injury models were established using the suture method. We then determined the protective effects of scutellaria baicalensis stem-leaf total flavonoid pretreatment on focal cerebral ischemia-reperfusion injury. Results showed that neurological deficit scores increased, infarct volumes enlarged, apoptosis increased and Bcl-2 and Bax protein expression were upregulated at 24 hours after reperfusion. Pretreatment with scutellaria baicalensis stem-leaf total flavonoid at any dose lowered the neurological deficit scores, reduced the infarct volume, prevented apoptosis in hippocampal cells, attenuated neuronal and blood-brain barrier damage and upregulated Bcl-2 protein expression but inhibited Bax protein expression. Doses of 100 and 200 mg/kg were the most efficacious. Our findings indicate that pretreatment with scutellaria baicalensis stem-leaf total flavonoid at 100 and 200 mg/kg can improve the neurological functions and have preventive and protective roles after focal cerebral ischemia-reperfusion injury.
Keywords: neural regeneration, brain injury, scutellaria baicalensis stem-leaf total flavonoid, ischemia-reperfusion injury, pretreatment, hippocampus, apoptosis, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
Scutellaria baicalensis stem-leaf total flavonoids reduced the infarct volume and apoptosis in hippocampal neurons, restored neurological deficits, and improved the integrity of the blood-brain barrier in rats with cerebral ischemia-reperfusion injury.
-
(2)
In rats with cerebral ischemia-reperfusion injury, scutellaria baicalensis stem-leaf total flavonoids increased the expression of the anti-apoptotic protein Bcl-2 and decreased the expression of the pro-apoptotic protein Bax, but could not prevent apoptosis.
-
(3)
Scutellaria baicalensis stem-leaf total flavonoids may act on multiple areas and pathways to protect rat hippocampal tissue from ischemia-reperfusion injury, and thus may be useful for the clinical prevention and treatment of ischemic brain injury.
INTRODUCTION
Focal cerebral ischemia-reperfusion may lead to neuronal loss, but strongly promotes the activation and proliferation of glial cells in the hippocampus[1]. Loss of hippocampal neurons is regarded as one of the basic pathological mechanisms underlying cognitive impairment[2]. The loss of neurons by apoptosis plays an important role in cerebral infarction[3]. Therefore, inhibiting apoptosis is a necessary measure in the treatment of cerebral ischemia-reperfusion injury.
Growing evidence has suggested that Chinese herbs, such as Radix Astragali, Radix Puerariae, ginkgo leaf and their extracts, can inhibit hippocampal apoptosis caused by ischemia-reperfusion[4]. Scutellaria baicalensis stem-leaf total flavonoid (SSTF), a flavonoid extracted from Radix Scutellariae, has anti-inflammatory[5], antioxidant[6] and anti-apoptotic[7] effects. It also protects microvessels and improves ischemia-related memory disorders[8]. One-week of SSTF pretreatment prior to the ischemia can interfere with the expression of cardiac apoptosis-related genes, block the apoptotic process, restore cell function and decrease the infarct volume, thus resulting in a preconditioning-like protective effect and a reduction in subsequent ischemia-reperfusion injury[9]. However, whether SSTF can prevent cerebral ischemia-reperfusion injury remains unclear. In this study, focal cerebral ischemia-reperfusion injury rats were pretreated with different doses of SSTF to explore the protective effects of SSTF on cerebral ischemia-reperfusion injury through the observations of infarct volume, hippocampal neuronal apoptosis, anti-apoptotic Bcl-2 and pro-apoptotic Bax expression, ultra-microstructure of neurons and the blood-brain barrier (BBB), as well as neurological deficits.
RESULTS
Quantitative analysis of experimental animals
One hundred and eight rats were randomly divided into six groups: control group (intragastric saline), sham group (sham operation + intragastric saline), model group (ischemia-reperfusion model + intragastric saline), and SSTF low-, medium- and high-dose groups (ischemia-reperfusion model + intragastric SSTF at 50, 100, and 200 mg/kg, respectively). After cerebral ischemia-reperfusion was completed, six rats were graded as a 0 or 4 by the first neurological deficit scale assessment, and eight rats exhibited excessive intraoperative bleeding and subarachnoid hemorrhage. These rats were excluded from the study and supplemented randomly. Finally, 108 rats were involved in the analysis of results.
SSTF improved neurological functions of cerebral ischemia-reperfusion injury rats
Rats in the control and sham groups showed normal activities and no neurological deficit symptoms, with a 0 neurological deficit score at the time when the other groups had received cerebral ischemia-reperfusion 24 hours previously. The neurological deficit scores of the model group, however, were significantly increased (P < 0.01). Compared with the model group, neurological deficit scores were significantly decreased in the SSTF groups (P < 0.01). Among the SSTF groups, the neurological deficit scores in the SSTF medium- and high-dose groups were significantly lower than that in the SSTF low-dose group (P < 0.01). There was no significant difference between the SSTF medium- and high-dose groups (P > 0.05; Figure 1).
Figure 1.
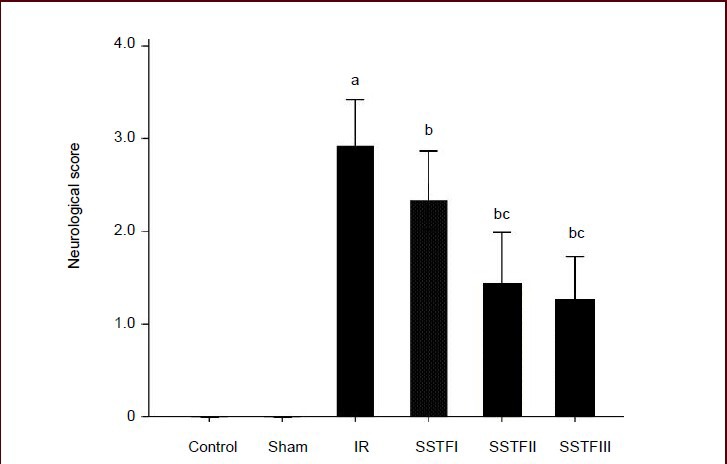
Effect of scutellaria baicalensis stem-leaf total flavonoid (SSTF) on neurological function in rats with cerebral ischemia-reperfusion injury.
After rats were pretreated with SSTF 50, 100, or 200 mg/kg per day (SFTFI, II, III) for 7 days, and underwent 2-hour ischemia and 24-hour reperfusion, the neurological functions were evaluated with Longa's method[10] using a 5-point (0–4) scoring scale: higher scores indicate more severe neurological deficits.
Data are expressed as mean ± SD; there were eighteen rats in each group. aP < 0.01, vs. control group and sham group; bP < 0.01, vs. model group; cP < 0.01, vs. SSTF low-dose group (one-way analysis of variance and least significant difference test).
SSTF reduced infarct volumes in cerebral ischemia-reperfusion injured rats
2,3,5-Triphenyltetrazolium chloride (TTC) staining revealed that rat brains from the control and sham groups were dyed red, no infarction was observed. In the model group and SSTF groups, coronal sections of brain tissue showed irregular unstained (white) areas with clear boundaries between them and the surrounding, and contralateral, non-ischemic brain tissue (red; Figure 2A-F). In the model group, white infarct lesions were clearly visible in the fronto-parietal cortex, which is supplied by the right middle cerebral artery, and some subcortical areas such as the striatum and the hippocampus. Sections through the optic chiasm showed the most obvious lesions. The infarct volumes were significantly reduced in the SSTF groups compared with those in the model group (P < 0.01). The infarct volumes in the medium- and high-dose groups were significantly lower than that in the low-dose group (P < 0.05; Figure 2G).
Figure 2.
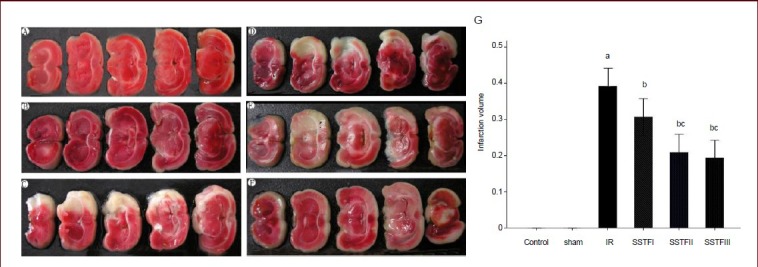
Effect of scutellaria baicalensis stem-leaf total flavonoid (SSTF) on infarct volume in rats with cerebral ischemia-reperfusion injury.
After rats were pretreated with SSTF at 50, 100, or 200 mg/kg per day for 7 days and underwent 2-hour ischemia and 24-hour reperfusion, brain tissues were analyzed with 2,3,5-triphenyltetrazolium chloride (TTC) staining.
(A) Control group; (B) sham group; (C) model (IR) group; (D–F) SSTF low-, medium-, high-dose groups (SSTFI, II, III). Red is normal tissue and white is ischemic tissue.
(G) Brain infarction volume in each group. Data are expressed as mean ± SD; there were six rats in each group. aP < 0.01, vs. control and sham groups; bP < 0.01, vs. model group; cP < 0.05, vs. SSTF low-dose group (one-way analysis of variance and least significant difference test). Infarction percentage (%) = white tissue weight/total weight × 100%[11].
SSTF inhibited apoptosis in the hippocampus of cerebral ischemia-reperfusion injury rats
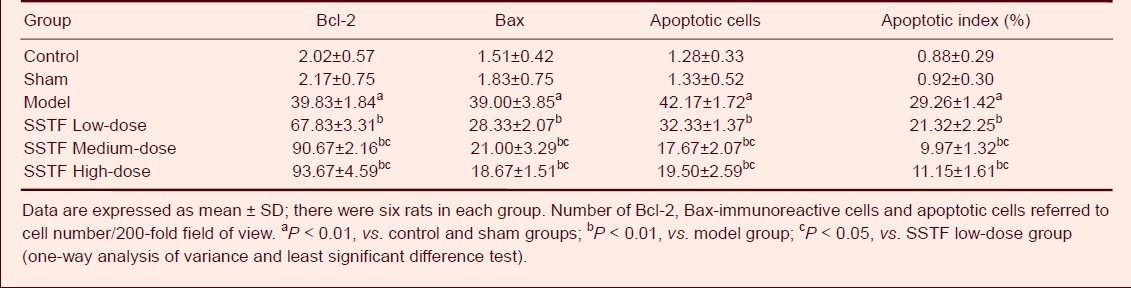
Terminal deoxynucleotidyl transferase (TUNEL) staining showed that only a few apoptotic cells were observed in the control and sham groups; after cerebral ischemia-reperfusion, many apoptotic cells were visible in the hippocampus (Figure 3). The number of apoptotic cells in the hippocampus and the apoptotic index were significantly higher in the model group than those in the control and sham groups (P < 0.01). Compared with those in the model group, the number of apoptotic cells in the hippocampus and the apoptotic index were significantly decreased in the SSTF low-, medium- and high-dose groups (P < 0.01). The number of apoptotic cells in the medium- and high-dose groups was significantly lower than that in the low-dose group (P < 0.01) (Table 1, Figure 3).
Figure 3.
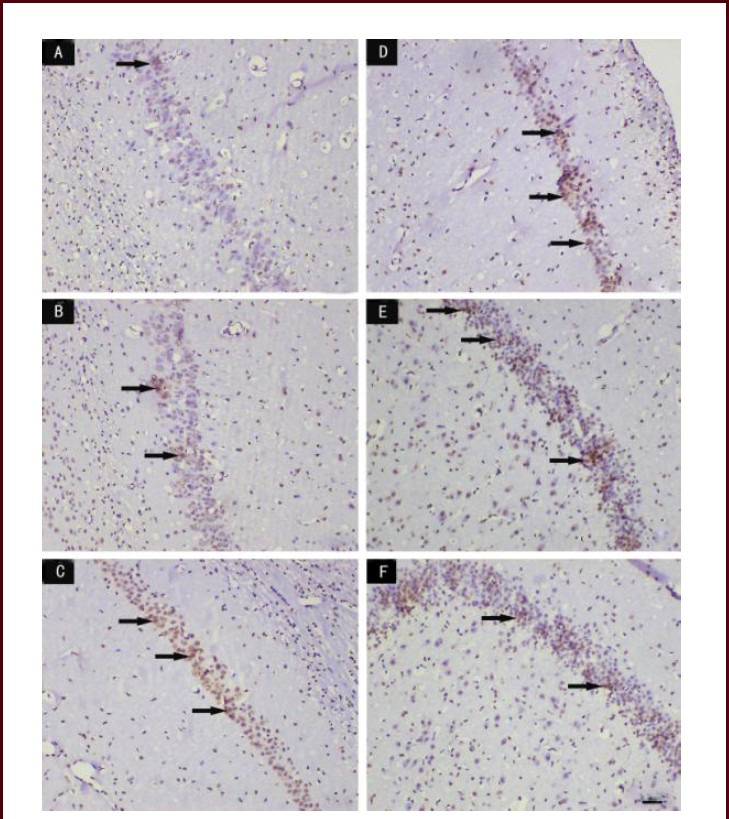
Effect of scutellaria baicalensis stem-leaf total flavonoid (SSTF) on apoptosis of the hippocampal CA1 region in rats with cerebral ischemia-reperfusion injury (TUNEL staining, light microscopy, scale bar: 50 μm).
(A) In the control group, apoptotic cells were rarely seen. (B) In the sham group, few apoptotic cells were observed. (C) In the model group, the number of apoptotic cells was increased. (D–F) In the SSTF low-, medium-, and high-dose groups, the number of apoptotic cells was significantly reduced compared with the model group, and only a few apoptotic cells were visible in the SSTF high-dose group. Arrows indicate apoptotic cells.
Table 1.
Effect of scutellaria baicalensis stem-leaf total flavonoid (SSTF) on hippocampal apoptosis and on Bcl-2- and Bax-immunoreactive cells in cerebral ischemia-reperfusion injured rats
Effect of SSTF on Bcl-2 and Bax immunoreactivity in the hippocampus of cerebral ischemia-reperfusion injury rats
Immunohistochemical staining showed that there were very weak immunoreactivity of Bcl-2 and Bax in the control and sham groups. After cerebral ischemia-reperfusion was induced, the number of Bcl-2- and Bax-immunoreactive cells began to increase (Figures 4, 5). Compared with those in the model group, the number of Bcl-2-immunoreactive cells in the hippocampus was significantly increased. By contrast, Bax-immunoreactive cells were significantly reduced in SSTF low-, medium- and high-dose groups (P < 0.01) compared with those in the model group. The medium- and high-dose groups showed more evident changes compared with those in the low-dose group (Table 1, Figures 4, 5).
Figure 4.
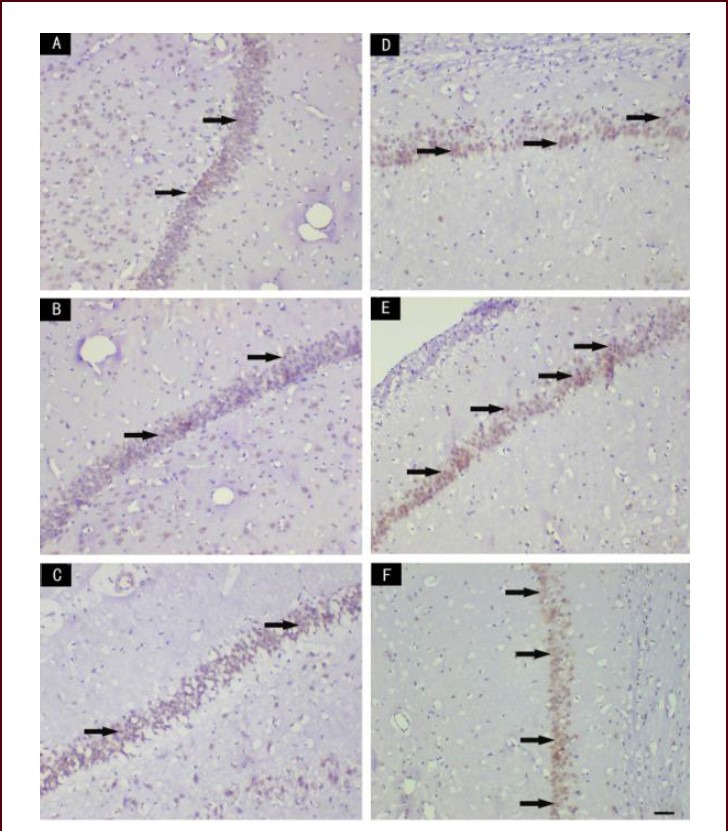
Effect of scutellaria baicalensis stem-leaf total flavonoid (SSTF) on Bcl-2 immunoreactivity in the hippocampal CA1 region of cerebral ischemia-reperfusion injured rats (immunohistochemical staining, light microscopy, scale bar: 50 μm)
In the normal control group (A) and the sham group (B), only a few Bcl-2-immunoreactive cells were seen in the hippocampal CA1 region; in the model group (C), some Bcl-2-immunoreactive cells were observed; in the SSTF low-, medium- and high-dose groups (D, E), the number of Bcl-2-immunoreactive cells increased significantly. Arrows indicate Bcl-2-immunoreactive cells.
Figure 5.
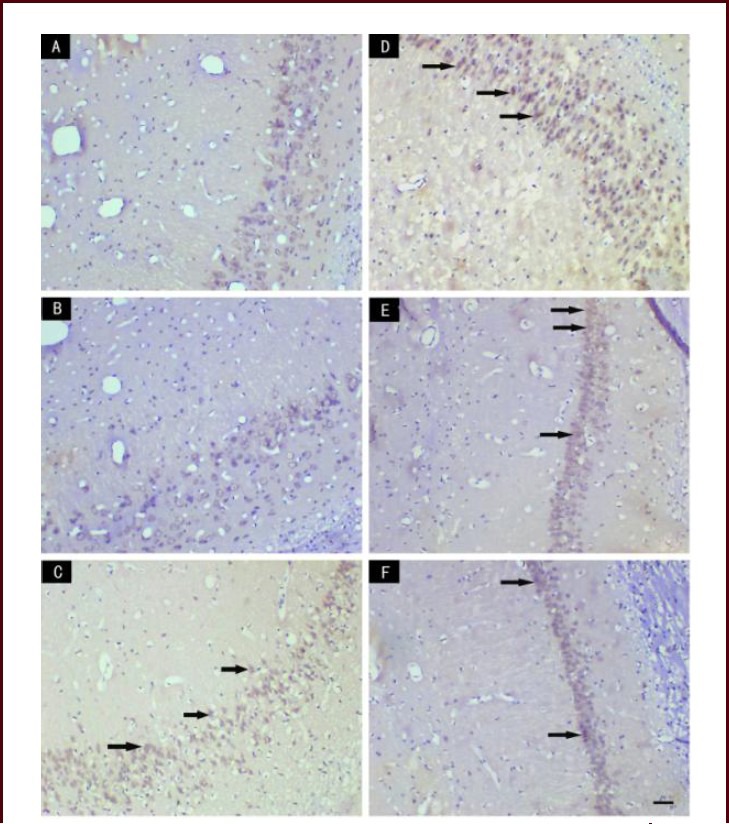
Effect of scutellaria baicalensis stem-leaf total flavonoid (SSTF) on Bax immunoreactivity in the hippocampal CA1 region of cerebral ischemia-reperfusion injured rats (immunohistochemical staining, light microscopy, scale bar: 50 μm).
In the normal control group (A) and the sham group (B), no Bax-immunoreactive cells were seen in the hippocampal CA1 region; in the model group (C), many Bcl-2-immunoreactive cells were observed; in the SSTF low-dose group (D), the number of Bax-immunoreactive cells was lower than that in the model group; in the SSTF medium- and high-dose groups (E, F), the number of Bax-immunoreactive cells decreased significantly. Arrows indicate Bax-immunoreactive cells.
Effect of SSTF on the ultrastructure of hippocampal neurons and the BBB of cerebral ischemia-reperfusion injury rats
Transmission electron microscopy on samples from the control group showed that hippocampal neurons had clear membranes, prominent nucleoli and evenly distributed chromatin. The mitochondria and endoplasmic reticulum (ER) were intact with abundant ribosomes (Figure 6A). Capillary endothelial cells, basement membrane and glial cells, which are components of the BBB, presented continuous and complete pedal plates (Figure 7A).
Figure 6.
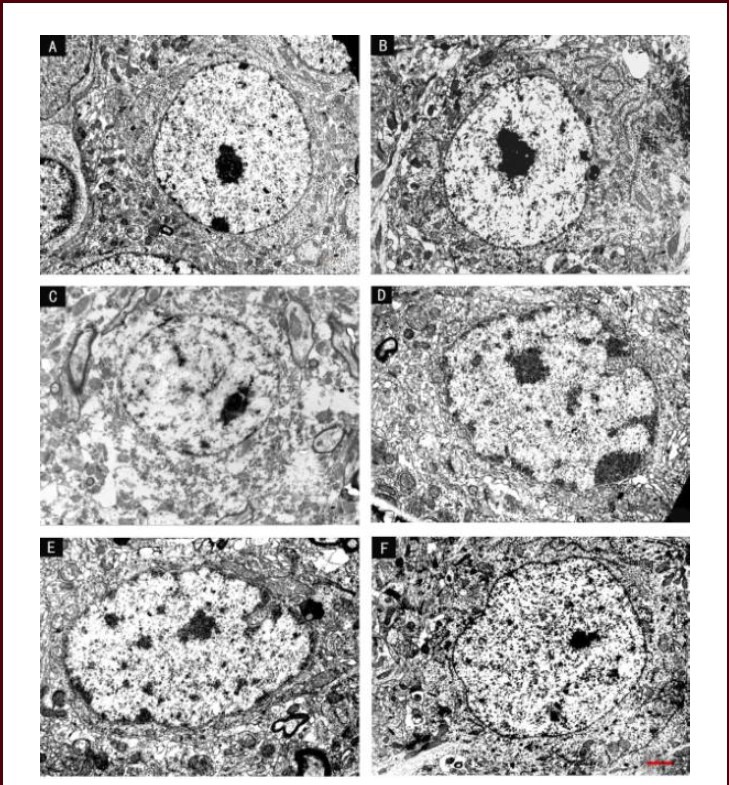
Effect of scutellaria baicalensis stem-leaf total flavonoid (SSTF) on the ultrastructure of hippocampal neurons in cerebral ischemia-reperfusion injured rats (transmission electron microscopy, scale bar: 2 μm).
(A) In the control group, hippocampal neurons had clear boundaries and a large, central nucleolus, the plasma membrane was continuous and clear, and many organelles were visible. (B) In the sham group, the neuronal nucleolus was large, chromatin was uniformly distributed, the rough endoplasmic reticulum and ribosomes were abundant, and the Golgi and mitochondria were visible.
(C) In the model group, the majority of neurons were disintegrating, the cell membrane and nuclear membrane have ruptured, and nucleoli appeared significantly distorted. The chromatin condensed or dissolved, and the mitochondria had disintegrated. (D) In the SSTF low-dose group, the nuclear membrane and nucleolus were irregular, the cytoplasm appeared dissolved, and mitochondrial swelling or vacuolization was observed. The membranes of the endoplasmic reticulum and the Golgi were balloon-dilated.
(E) In the SSTF medium-dose group, hippocampal neurons were mildly swollen, the plasma and nuclear membrane were continuous, most of the mitochondria were intact, rough endoplasmic reticulum and ribosomes were abundant, and the Golgi was complete and clear. (F) In the SSTF high-dose group, hippocampal neuronal structures were normal, nuclear membrane was intact and the nucleolus was close to the center. Chromatin was uniform, mitochondria were intact, and the rough endoplasmic reticulum and ribosomes were abundant.
Figure 7.
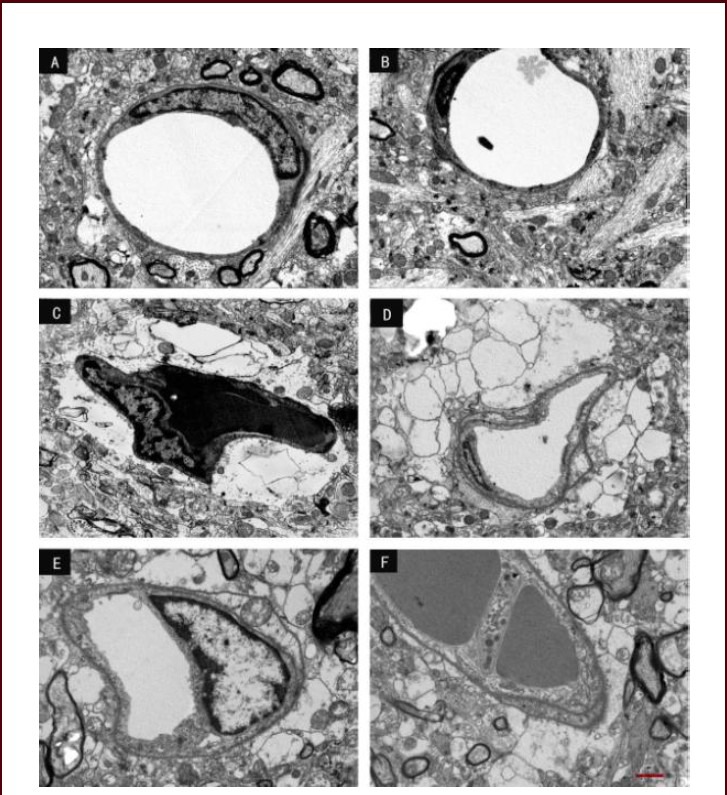
Effect of scutellaria baicalensis stem-leaf total flavonoid (SSTF) on the blood-brain barrier ultrastructure in cerebral ischemia-reperfusion injured rats (transmission electron microscopy, scale bar: 2 μm).
(A) In the control group, capillary endothelial cells, the basement membrane and the glial cell pedal plate were continuous and intact. (B) In the sham group, the ultrastructure was similar to that in the control group. The structure was intact.
(C) In the model group, capillaries were closed, endothelial cytoplasm was condensed, the basement membrane and glial membrane were dissolved, and the pedal plate was reticulated. (D) In the SSTF low-dose group, the capillary endothelium was continuous, the basement membrane was not intact, and the glial pedal plate had begun to degenerate.
(E) In the SSTF medium-dose group, the capillary endothelium and the basement membrane were continuous and intact, and the glial pedal plate partially degenerated, and the blank areas were significantly reduced. (F) In the SSTF high-dose group, the capillary endothelium was close to normal, the basement membrane was uniform and smooth, and a small amount of the glial pedal plate was lost. However, vesicles, filaments and mitochondria were still visible.
In the sham group, hippocampal neurons were morphologically normal with large nucleoli, uniform chromatin, abundant rough ER and ribosomes. The mitochondria, Golgi and secondary lysosomes were clearly visible (Figure 6B). The BBB was similar to that in the control group, showing a complete and continuous structure (Figure 7B).
In the model group, plasma and nuclear membranes were disrupted with unclear boundaries, nucleoli were dislocated, and chromatin was condensed or was dissolved and unevenly distributed. There was mitochondrial swelling and the crest appeared in a homogeneous state, the rough ER was broken and ribosomes were not observable (Figure 6C). The BBB capillary lumen had shrunk, the endothelial cytoplasm had aggregated, there was lysis of the basement membrane and glial membrane, and the pedal plate was reticular (Figure 7C).
In the SSTF low-dose group, the neuronal nuclear membrane was irregular, showing nuclear pyknosis and nucleolus deviation. Compared with the model group, there was less heterochromatin scattered in the nuclear membrane, euchromatin was unevenly distributed and the cytoplasm had shrunk. However, mitochondrial swelling, crista rupture or vacuolar degeneration were visible, the rough ER and Golgi were expanded and the numbers of ribosomes reduced (Figure 6D). The BBB injury was reduced compared with the model group and the capillary endothelium was continuous. Despite the basement membrane being incomplete, the glial pedal plate was less damaged than that in the model group (Figure 7D).
In the SSTF medium-dose group, neuronal swelling was reduced, the cell membrane was continuous, the nuclear membrane was complete, and the bilayer structure was visible. The mitochondria were largely intact but some mitochondrial cristae had ruptured. The rough ER, ribosomes, Golgi and lysosomes were clearly visible in the SSTF medium-dose group (Figure 6E).
The BBB damage was significantly reduced compared with the ischemia-reperfusion group and the SSTF low-dose group, the capillary endothelium and basement membrane were continuous and complete, the glial cell pedal plates were only partially dissolved. Blank areas around the capillaries were also significantly reduced compared with the ischemia-reperfusion and the SSTF low-dose groups (Figure 7E).
In the SSTF high-dose group, neuronal damage was significantly reduced compared with the ischemia-reperfusion and the SSTF high-dose groups and the nuclear membrane was complete and close to the center. There was little heterochromatin and the euchromatin was distributed evenly. Most of the mitochondria were intact, with only a few ruptured cristae, and the rough ER and ribosomes were abundant (Figure 6F). The BBB capillary endothelial structure was close to normal. The basement membrane was uniform and smooth, with only a small amount of glial cell pedal plates dissolved into granules. However, vesicles, filaments, and mitochondria were still observed (Figure 7F).
DISCUSSION
SSTF pretreatment at different doses ameliorates these neurological deficits in rats with cerebral ischemia-reperfusion injury, confirming that SSTF can attenuate cerebral ischemia-reperfusion injury. TTC histological results showed that in rats with cerebral ischemia-reperfusion injury, the brain tissue served by the right middle cerebral artery was obviously swollen and poorly stained, showing a clear boundary between the infarct tissue and surrounding normal tissues. The affected lesions involved the right frontoparietal cortex, striatum and hippocampus. Our findings are consistent with previous studies[12]. The different doses of SSTF pretreatment significantly reduced brain tissue swelling and infarct volume compared with that in rats that did not receive SSTF pretreatment. The medium- and high-dose groups showed greater efficacy than that shown by the low-dose group. These results suggested that SSTF pretreatment can significantly reduce neurological deficits and decrease the infarct volume following cerebral ischemia-reperfusion in a dose-dependent manner.
Shang and Cao[13] found that SSTF significantly improved traumatic brain edema and reduced the cerebral ischemic area; however, it was ineffective in reducing brain damage that had already formed. This evidence implies that SSTF should be used as early as possible for ischemic cerebrovascular injury treatment. Our results showed that focal cerebral ischemia-reperfusion injury can lead to apoptosis in rat hippocampus, while SSTF pretreatment reduces the number of apoptotic cells. Furthermore, our findings indicate that SSTF pretreatment dose-dependently inhibits the apoptosis caused by cerebral ischemia-reperfusion injury.
The neuroprotective effects of scutellaria extracts are mediated by inhibiting protein kinases, relaxing vascular smooth muscle, increasing the tissue blood supply, decreasing the ability of antagonizing capillary motor protein activity and reducing pyramidal cell delayed death[14]. We found that SSTF pretreatment significantly increased Bcl-2 immunoreactivity and decreased Bax immunoreactivity in the rats with cerebral ischemia-reperfusion injury, which was supportive of a previous study[15]. Our findings suggest that SSTF pretreatment may upregulate the expression of Bcl-2 and downregulate the expression of Bax, thus protecting cells against cerebral ischemia-reperfusion injury.
Drug treatment before ischemia-reperfusion can prevent neuronal necrosis and apoptosis, reducing ischemia-reperfusion injury[16] and changes in ultrastructure, which are regarded as an indicator of cell damage and repair[17]. Mitochondria maintain cellular oxidation and energy production as well as remove oxygen free radicals[14]. Mitochondria are very sensitive to hypoxia and can directly reflect neuronal injury[18]. Intracellular Ca2+ overload can induce neuronal apoptosis and the endonuclease activation pathway, which results in nuclear chromatin condensation, margination, and apoptotic bodies[19]. The intact plasma membrane can prevent water, Ca2+, Mg2+ and Cl- influx into neurons, reducing Ca2+ overload and inhibiting Ca2+ - and Mg2+ -dependent endonuclease activity[20]. We observed that SSTF pretreatment reduced damage to neuronal membrane structures and various organelles, especially the mitochondria. This morphological evidence suggests that SSTF pretreatment may protect neuronal structures and inhibit ischemia-reperfusion-induced apoptosis by reducing intracellular Ca2+ overload, and may be anti-ischemic and anti-hypoxic by promoting free radicals scavenging[20,21].
The BBB is a complex cellular system that exists between the brain and its blood supply. This physiological barrier functions to prevent water-soluble substances, such as small-molecule electrolytes, from entering the brain. The BBB also selectively removes harmful or excess materials from the brain and maintains brain homeostasis[22]. After ischemic brain tissue restores blood perfusion, free radical production is increased, intracellular Ca2+ is overloaded[23], excitatory amino acids are released excessively[22,23,24], proteases are activated[22], endothelial cell adhesion molecules are expressed[22,23], prostaglandin levels are increased[25,26], and production of cytokines is raised[24]. These changes lead to endothelial cell injury and alter the capillary structure. Subsequent to cerebral ischemia-reperfusion injury, glial cell protrusions are more sensitive than microvessels to the ischemia, and swell then degenerate[27]. When both neuronal apoptosis and necrosis occur, glial cells experience similar changes, and the structure and functions of the BBB are destroyed[27,28]. Our observations of the BBB ultrastructural changes following ischemia-reperfusion are consistent with previous experimental results[29,30]. Once the BBB is breached, the stability of the microenvironment of the nerve cells is inevitably lost, leading to apoptosis or necrosis and neurological deficits[22,29,30]. SSTF pretreatment reduces BBB damage following cerebral ischemia-reperfusion, so effective control of this pathophysiological process is important for the prevention and treatment of cerebral ischemia-reperfusion injury.
In summary, SSTF pretreatment can inhibit apoptosis in the hippocampus, reduce neuronal structural damage and maintain BBB integrity, thus protecting neurological functions following cerebral ischemia-reperfusion. SSTF pretreatment may regulate certain aspects of the pathogenesis after cerebral ischemia-reperfusion, such as lack of energy, release of excitatory amino acids, Ca2+ overload, free radical release and mitochondrial damage. Finally, SSTF affects the expression of apoptosis-related proteins and helps prevent apoptosis.
MATERIALS AND METHODS
Design
A randomized controlled animal experiment.
Time and setting
The experiment was performed from September 2008 to April 2011 in the Electron Microscope Room and the Institute of Basic Medical Sciences, Chengde Medical College, China.
Materials
Animals
One hundred and eight healthy Sprague-Dawley rats (54 males and 54 females), aged 4 months, weighing 180–220 g were provided by the Experimental Animal Center of Hebei Medical University, China (license No. SCXK (Ji) 2008-1-003). Experimental procedures were approved by the Animal Ethics Committee of Chengde Medical College, China.
Drugs
SSTF (powder, batch No. 010608) was provided by the Laboratory of Chinese Medicine Research and Development (Hebei Province Key Laboratory, Chengde Medical College, Hebei Province, China). The powder was dissolved in 0.1 mol/L PBS (pH 7.4) before administration. SSTF purity was 61.8%; it is mainly composed of scutellarin, containing the crude drug at 5.5 g/100 mL.
Methods
SSTF pretreatment
Rats were allowed free access to water and food for 7 days to adapt to the environment. One week before modeling, rats in the SSTF low-, medium- and high-dose groups were given SSTF intragastrically at 50, 100 or 200 mg/kg for 7 successive days[31], while the control group, sham group and model group were given a similar volume of physiological saline.
Establishing focal cerebral ischemia-reperfusion injury model
Twenty-four hours after the last administration, focal cerebral ischemia-reperfusion injury models were established using Zea Longa's method[12] with some modifications in the model group and SSTF groups. In brief, after the rats were anesthetized, the right common carotid artery, internal carotid artery and external carotid artery were isolated, the external carotid artery branches were coagulated and the external carotid arteries were ligated. A thread embolus (diameter 0.22 mm) was inserted into the right common carotid artery incision, to a depth of 18.5 ± 0.5 mm. When resistance was felt, it confirmed that the embolus head had crossed the middle cerebral artery. The common carotid artery was then ligated at the proximal end. The wounds were sutured and the thread was retained by 1 cm to occlude the middle cerebral artery for 2 hours. Then the thread was pulled out to recover the middle cerebral artery recanalization, thus achieving reperfusion over a 24-hour period. After rats recovered from the anesthesia, neurological functions were evaluated with the methods of Longa et al[10] : 0 point, no neurological deficit symptoms, normal activities; 1 point, rats cannot fully extend the left forelimb; 2 points, circling to the left side when walking; 3 points, inclining or falling to the left side during independent movement; 4 points, rats cannot spontaneously walk, level of consciousness decreases. Only rats that scored 2 or 3 points were included in the experiments. After 24-hour reperfusion, rats were tested again.
Infarct volume determination
Six rats in each group were sacrificed. The brain was immediately harvested and preserved at –20°C for 20 minutes, and then the brains were cut into coronal slices at a thickness of 2 mm. The slices were incubated in 2% TTC solution (Sigma, St. Louis, MO, USA) at 37°C, in the dark for 30 minutes, the slices were turned over every 7–8 minutes. Normal brain tissue stained red, while infarct tissue remained white. The whole brain slices and infarct area were weighed to calculate the percentage of infarct area. Infarct percentage (%) = white area mass/total mass × 100%[15].
Specimens
Six rats in each group were anesthetized using 10% chloral hydrate and underwent thoracotomy, perfused for 5 minutes through the ascending aorta, and fixed in 4% paraformaldehyde. The right brain tissue was paraffin embedded and sliced into serial coronal slices at 5 μm thickness, then mounted on to pretreated slides. Adjacent sections were used for detection of apoptosis or Bcl-2 and Bax immunohistochemical staining.
TUNEL assay of apoptosis
Paraffin sections were dewaxed, treated with 3% H2O2 at room temperature, and incubated in 20 μg/mL proteinase K solution for 15 minutes; rinsed with 0.01 mol/L PBS and incubated with 50 μL TUNEL reaction mixture (Boehringer, Mannheim, Germany) at 37°C for 60 minutes; rinsed with PBS and incubated with peroxidase at 37°C for 30 minutes; rinsed with PBS and developed with 3,3’-diaminobenzidine (Fuzhou Maixin Biotechnology Development Co., Ltd., Fuzhou, Fujian Province, China) for 10 minutes; and counterstained with hematoxylin, dehydrated, cleared and mounted. Slices were observed under a light microscope (Olympus, Tokyo, Japan) and the brown cells were counted as apoptotic cells. Five fields in each of three slices from each rat were randomly selected to count the number of apoptotic cells and total cells. The apoptotic index = number of positive cells/total cell number × 100%[32].
Immunohistochemical staining for Bcl-2 and Bax protein expression
Slices were dewaxed, incubated with 3% H2O2 deionized water for 10 minutes, and rinsed with distilled water; heat repaired with 0.01 mol/L citrate buffer in a microwave for 17 minutes; incubated with normal goat serum for 10 minutes and a rabbit anti-Bcl-2 or anti-Bax polyclonal antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. The following day, they were rinsed with PBS five times for 3 minutes each, incubated with biotinylated goat anti-rabbit IgG (1:50; Santa Cruz Biotechnology) for 12 minutes and rinsed with PBS five times for 3 minutes each. They were then incubated with horseradish peroxidase-conjugated streptavidin (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) for 12 minutes and rinsed with PBS five times for 3 minutes each; developed with 3,3’-diaminobenzidine, counterstained with hematoxylin, and rinsed with tap water. The samples were then dehydrated, cleared, mounted, and observed under a light microscope. Cells with a brownish-yellow cytoplasm were counted as positive cells. Five fields in each of three slices from each rat were randomly selected for image processing using MiVnt (Nikon, Tokyo, Japan). The numbers of Bcl-2- and Bax-immunoreactive cells in the hippocampus on the ischemic side were calculated and averaged.
Transmission electron microscopy observation of hippocampal ultrastructure
Six rats in each group were anesthetized using 10% chloral hydrate and underwent thoracotomy, perfused with saline through the ascending aorta, and fixed in 4% paraformaldehyde and 2% glutaraldehyde. The hippocampal tissue at the ischemic area and ischemic border zone was cut into 1 mm3 blocks, and fixed with 3.1% glutaraldehyde for 2 hours. Hippocampal tissues were conventionally embedded and sliced into ultrathin sections of 50 nm thickness, then stained with uranium and lead, and photographed under an H-7650 transmission electron microscope (Hitachi, Tokyo, Japan). The ultrastructure of neurons and the BBB was observed.
Statistical analysis
Data were analyzed using SPSS 17.0 statistical package (SPSS, Chicago, IL, USA) and were expressed as mean ± SD. The difference between groups was compared by one-way analysis of variance followed by least significant difference test. P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was financially supported by the Science and Technology Department of Hebei Province, No. 07276101D-46, and the Education Ministry of Hebei Province, No. 2005227.
Conflicts of interest: None declared.
(Reviewed by Dawes EA, Frenchman B, Lian XY, Liu P)
(Edited by Wang LM, Yang Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Butler TL, Kassed CA, Sanberg PR, et al. Neurodegeneration in the rat hippocampus and striatum after middle cerebral artery occlusion. Brain Res. 2002;929(2):252–260. doi: 10.1016/s0006-8993(01)03371-6. [DOI] [PubMed] [Google Scholar]
- [2].Leverenz JB, Agustin CM, Tsuang D, et al. Clinical and neuropathological characteristics of hippocampal sclerosis: a community-based study. Arch Neurol. 2002;59(7):1099–1106. doi: 10.1001/archneur.59.7.1099. [DOI] [PubMed] [Google Scholar]
- [3].Hill IE, MacManus JP, Rasquinha I, et al. DNA fragmentation indicative of apoptosis following unilateral cerebral hypoxia-ischemia in the neonatal rat. Brain Res. 1995;676(2):398–403. doi: 10.1016/0006-8993(95)00145-g. [DOI] [PubMed] [Google Scholar]
- [4].Wang Y, Li WY, Zhang HX, et al. The effect of astragaloside IV on neural cell apoptosis and the expression of apoptosis-related genes in hippocampus after cerebral ischemia-reperfusion in rats. Zhongguo Linchuang Baojian Zazhi. 2011;14(4):401–403. [Google Scholar]
- [5].Wang YM, Liu YP, Cao K, et al. Effects of flavonoids from Scutellaria stems and leaves on memory impairment and nerve inflammation in chronic cerebral ischemia rats. Zhongguo Yaoli Xue yu Duli Xue Zazhi. 2011;25(2):135–140. [Google Scholar]
- [6].Wang RT, Zhang JX, Dong YJ. Protective effect of scutellaria ba icalensis stem-leaf total flavonoid against hippocampus neuron damage induced by injection A25-35. Zhongguo Laonian Xue Zazhi. 2010;30(7):926–928. [Google Scholar]
- [7].Zhou XH, Qi JM, Mei LX, et al. Effects of SSTF on the protein expression of apoptosis-associated bcl-2 and bax genes in cultured neonatal rat cardiomyocytes with hypoxia/reoxygenation. Sichuan Zhongyi. 2007;25(9):18–20. [Google Scholar]
- [8].Wang YM, Cao K, Liu YP, et al. Effects of flavonoids from scutellaria stem and leaf on memory impairment and the expression of choline acetyl transferase and nitric oxide synthase in chronic cerebral ischemia rats. Shoudu Yike Daxue Xuebao. 2011;32(4):494–500. [Google Scholar]
- [9].Zhao SM, Han L, Ma LH, et al. Pre-treatment with radix axtragali for myocardial cell apoptosis and its relative genes in rats with ischemia reperfusion. Zhongguo Linchuang Kangfu. 2005;9(21):3908–3910. [Google Scholar]
- [10].Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- [11].Peng FH, Li L, Huang HW, et al. The study on cerebral infarct volumes and EEG after theombolytic therapy in different therapeutic time windows in rats. Zuzhong yu Shenjing Jibing Zazhi. 1999;6(3):129–131. [Google Scholar]
- [12].Chen M, Zhao SM, Li H, et al. Effect of pretreatment with scutellaria baicalensis stem-leaf total flavonoid on cerebral infarction volume and lipid peroxidation induced by focal cerebral ischemia reperfusion rats. Jiepou Xue Zazhi. 2010;33(4):495–497. [Google Scholar]
- [13].Shang YZ, Cao K. Protective effects of total flavonoids from stems and leaves of scutellaria baicalensis George on cerebral hypoxia in mice. Zhongguo Linchuang Kangfu. 2004;8(19):3908–3909. [Google Scholar]
- [14].Wang XS, Miao L, Yao XM, et al. The ultrastructure changes of blood brain barrier after cerebral ischemic reperfusion in rats. Tianjin Yiyao. 2006;34(3):188–189. [Google Scholar]
- [15].Liu KY, Huang XZ. Effects of Magnolol on apoptosis and the expression of bcl-2 and Bax in rats following cerebral ischemia and reperfusion. Hubei Minzu Xueyuan Xuebao: Yixue Ban. 2005;22(3):15–17. [Google Scholar]
- [16].Zhao SM, Chen M, Li H, et al. Expression of neuron in hippocampal apoptosis Bcl-2, Bax gene of focal brain ischemia-reperfusion and the interventional effect of scutellaria baicalensis stem-leaf total flavonoid. Jiepou Xue Zazhi. 2009;32(Suppl):72. [Google Scholar]
- [17].Zheng XY, Kong XY, Zhao SM. Protective effect of scutellaria baicalensis stem-leaf total flavonoid on expression of heat shock protein 70 and ultrastructure induced by cerebral ischemia reperfusion cortex. Jiepou Xue Zazhi. 2011;34(1):52–54. [Google Scholar]
- [18].Kong XY, Zhao SM. Study on the ultrastructure changes of hippocampus neurons in ischemia reperfusion rats. Chengde Yixueyuan Xuebao. 2005;22(4):281–283. [Google Scholar]
- [19].Kong XY, Zhao SM, Guo S. Protective effects of preconditioning of total fiavonoid from the stem and leaf of scutellaria baicalensis on cardiocyte ultrastructure of rats with ischemic reperfusion. Zhongguo Linchuang Kangfu. 2006;10(31):49–52. [Google Scholar]
- [20].Zheng XY, Kong W, Kong XY, et al. Protective effect of scutellaria baicalensis stem-leaf total flavonoid on injury of microvascular architecture and lipid peroxidation induced by cerebral ischemia reperfusion cortex in rats. Jiepou Xue Zazhi. 2012;35(2):198–200. [Google Scholar]
- [21].Lou XL, Zhou XX, Su PQ, et al. Effects of Scutellaria baicalensis stem-leaf total flavonoid on proliferation of vassel smooth muscle cells stimulated by high triglyceride blood serum. Zhongguo Zhongyao Zazhi. 2009;34(21):1–5. [PubMed] [Google Scholar]
- [22].Zou W, Sun XW, Yu XP, et al. Research progress on blood-brain barrier and cerebral ischemia-reperfusion injury. Zhonghua Zhongyiyao Xuekan. 2009;27(3):466–469. [Google Scholar]
- [23].Zhou FX. Continuing medical education of correspondence: Perfusion of tissue blood and pathological physiology of micro-circulation(7) —Ischemia-reperfusion injury. Waike Lilun yu Shijian. 2008;13(3):38–42. [Google Scholar]
- [24].Xian XB. Research progress of ischemic cerebrovascular disease. Hainan Yixue. 2008;19(1):131–134. [Google Scholar]
- [25].Liu ZQ, Hou YL. Research progress of Chinese medicine intervention in the micro circulation obstacles. Weixunhuan Xue Zazhi. 2010;20(1):56–59. [Google Scholar]
- [26].Wu Z, Xia ZY, Huang HB, et al. Effects of salvia miltiorrhazae compound on serum lipid peroxidation and PGI2 during oppen heart surgery. Linchuang Mazui Xue Zazhi. 2002;18(5):242–244. [Google Scholar]
- [27].Wang YT, Zeng LL, Lv HY, et al. The research on etiology and pathogenesis of ischemic stroke in ten years. Zhongguo Xiandai Shenjing Jibing Zazhi. 2010;10(2):2–26. [Google Scholar]
- [28].Li Q, Li BZ, Liu H. Pharmacological effect and clinical application of Ligustrazine injection. Yixue Zongshu. 2009;15(9):1402–1405. [Google Scholar]
- [29].Chen M, Kong Wei, Zhao SM, et al. Effect of pretreatment with scutellaria baicalensis stem-leaf total flavonoid on morphologic changes of hippocampus neurons and blood brain barrier followed by focal cerebral ischemia reperfusion rats. Jiepou Xue Zazhi. 2012;35(3):341–344. [Google Scholar]
- [30].Xu K, Huang HD, Shen TZ, et al. Blood-brain barrier changes after acute cerebral ischemia reperfusion in rats. Zhongguo Yixue Yingxiang Jishu. 2005;21(4):519–522. [Google Scholar]
- [31].Zhou XX, Su PQ, Liu Z. Experimental study of total flavonoids from stems and leaves of scatellaria baicalensis in preventing and curing diabetes mellitus. Zhongyao Xinyao yu Linchuang Yaoli. 2006;17(6):418–420. [Google Scholar]
- [32].Wang YS, Zhang LF, Li JQ, et al. Propofol protects against global cerebral ischemia-reperfusion injury in rats. Zhonghua Mazui Xue Zazhi. 2004;24(8):596–600. [Google Scholar]


