Abstract
Proteoglycans in the central nervous system play integral roles as “traffic signals” for the direction of neurite outgrowth. This attribute of proteoglycans is a major factor in regeneration of the injured central nervous system. In this review, the structures of proteoglycans and the evidence suggesting their involvement in the response following spinal cord injury are presented. The review further describes the methods routinely used to determine the effect proteoglycans have on neurite outgrowth. The effects of proteoglycans on neurite outgrowth are not completely understood as there is disagreement on what component of the molecule is interacting with growing neurites and this ambiguity is chronicled in an historical context. Finally, the most recent findings suggesting possible receptors, interactions, and sulfation patterns that may be important in eliciting the effect of proteoglycans on neurite outgrowth are discussed. A greater understanding of the proteoglycan-neurite interaction is necessary for successfully promoting regeneration in the injured central nervous system.
Keywords: chondroitin sulfate proteoglycans, heparan sulfate proteoglycans, glycosaminoglycans, protein core, extracellular matrix, neuronal growth cones, axon outgrowth and regeneration, spinal cord injury, glial scar, tissue culture
Introduction
Understanding the complex causes of disease and the inability of some tissues to repair following injury is a major priority in biomedical research. Historically, biomedical research has followed a “cell-centric” model to explain and treat the progression and pathophysiology of disease. Considering the role of the extracellular matrix (ECM) in many diseases is a relatively recent and departing approach. Though many diseases are manifested by cellular insufficiencies, the environments in which the cells reside likely contribute and initiate many pathophysiologic mechanisms. In this review, we discuss one example of this interaction in a very unlikely place, the central nervous system (CNS).
Until recently, the study of the CNS was predominated by a “neuro-centric” model. The neuron is the “processor” cell of the CNS. The neuron receives chemical signals, translates them into electrical signals, and passes this message over to the next neuron in a re-translated chemical signal. This process, known as synaptic signaling, is the work of the neuron. However, the other three major cells of the CNS also play important roles in proper functioning. Astrocytes, oligodendrocytes and microglia regulate the strength, response, rate, and structure of neuronal signaling (Doretto et al., 2011; Jourdain et al., 2007; Perea et al., 2009; Wake et al., 2009). In addition, the astrocyte regulates the amount of chemical signal reaching the neuron, the amount of neurotransmitter stored for release, energy demands, and ionic homeostasis (Belanger et al., 2011; Beller et al., 2011; Haydon and Carmignoto, 2006; Mack and Wolburg, 2013). Most importantly, these three glial types contribute to the production of integral ECM components, specifically proteoglycans (Jander and Stoll, 1996; Jones et al., 2002; Lander et al., 1998; Shioi et al., 1995).
Our understanding of the ECM's function in the CNS is miniscule compared to our functional knowledge of the various CNS cell types themselves. Advances, which have gradually appeared over the past few decades, implicate the ECM as a dynamic and important contributor to the development of the CNS, as well as to the normal and aberrant function of the CNS. A major impetus to investigate the role of the ECM in the CNS was the observed interaction of proteoglycans with elongating neurons.
Proteoglycans are a complex component of the extracellular matrix
Proteoglycans (PGs) are complex molecules comprised of a core protein that is glycosylated and contains various types of carbohydrate chains. PGs were originally isolated from cartilage tissue in 1891. At this time, PGs’ sole biological function was believed to be as a molecule to fill space and supply structural support within the extracellular matrix (ECM) (for review: Yanagishita, 1993). Contrast this assessment with the current view of the ECM's role in the progression of disease, which has more recently gained great attention (Cox and Erler, 2011; Curinga et al., 2008; Lu et al., 2011; Snow, 2011). In all, the ECM plays an important role in development, regulation of synapses, the progression of neurodegenerative disease, and repair following injury (Fawcett and Asher, 1999; Franco and Muller, 2011; Lukes et al., 1999; Pyka et al., 2011). Of the many components of the ECM, proteoglycans appear to be the major influencer in the ECM's role in CNS physiology (Fitch and Silver, 2008; Galtrey and Fawcett, 2007; Hartmann and Maurer, 2001).
Proteoglycans
There are three major classes of PG including heparan sulfate proteoglycans (HSPGs), chondroitin sulfate proteoglycans (CSPGs), and keratan sulfate proteoglycans (KSPGs). Each class of proteoglycan is classified by the type of carbohydrate chain attached to the core protein. Of particular interest to CNS physiology are the CSPGs and HSPGs, which play pivotal roles in repair and reorganization following CNS injury (Carulli et al., 2005; Galtrey and Fawcett, 2007; Jones et al., 2003).
CSPGs are currently the most widely discussed proteoglycan in the field of neuronal regeneration. In the late 1980s, when initial discoveries were being made about CSPGs and/or their carbohydrate side chains, and affects on axonal outgrowth (Carbonetto et al., 1983; Verna et al, 1989; Snow et al., 1990 a and b), conference presentations about CPSGs, e.g., the Society for Neuroscience annual meeting, consisted of only a few abstracts on the topic, while in 2013, there were > 50 abstracts devoted to CSPGs alone, and a magnitude more devoted to the involvement of ECM components in outgrowth and regeneration, as determined by titles alone (SfN Proceedings; website; 2013). Clearly, there has been an explosion of interest and pronounced scientific advancement made on this topic.
CSPGs
CSPGs are linked to carbohydrate chains containing N-acetylgalactosamine (GalNAc) and glucuronic acid (GlcA) (Hardingham and Fosang, 1992; Poole, 1986), and exist in both cell membrane bound, excreted and ECM embedded forms. The family of CSPGs is highly variable having multiple core proteins, differing number and length of associated sugar chains, as well as a variety of differing sulfation patterns, which imbue diversity of function. The GlcA can be sulfated in the 2-carbon position, referred to as CS-2. The GalNAc sugar amine can be sulfated at either the 4- or 6- position, or at both the 4- and 6- position (DeLuca et al., 1973). These are referred to as CS-2, CS-4, CS-6, or CS-4,6 respectively (Figure 1A, B).
Figure 1.
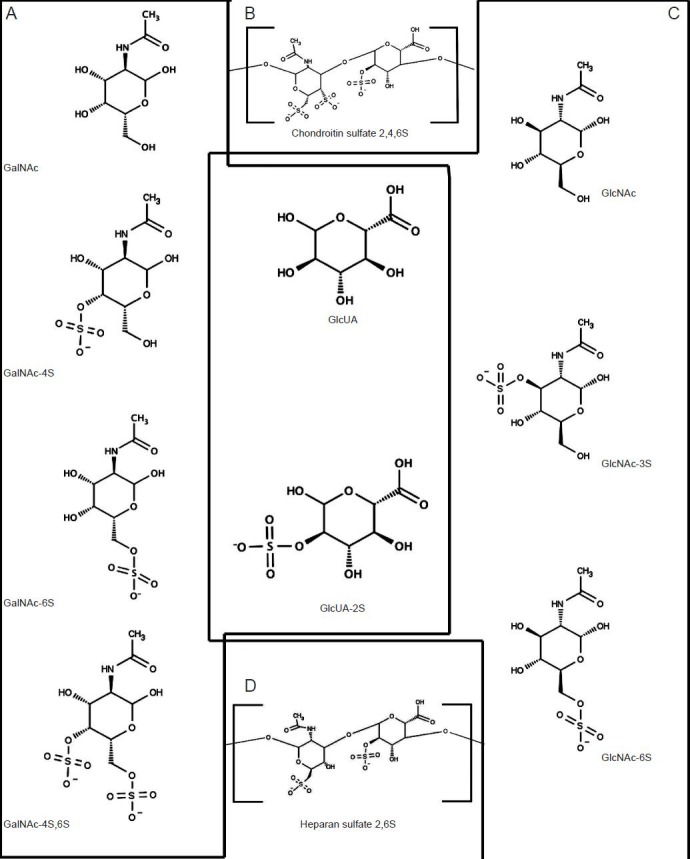
Structural components of chondroitin sulfate and heparan sulfate glycosaminoglycans.
Structural components of chondroitin sulfate and heparan sulfate glycosaminoglycans. (A) Chondroitin sulfate chains are comprised of repeat-ing disaccharide units of N-acetylgalactosamine (GalNAc) and glucuronic acid. GalNAc is either not sulfated, or sulfated at the 4, 6 or both 4 and 6 carbon positions. (B) These chains are arranged by the formation of a 1,4-β-glycosidic linkage. (C) Heparan sulfated chains are comprised of repeating disaccharide units of N-acetylglucosamine (GlcNAc) and glucuronic acid (GlcA). GlcNAc is either not sulfated, or sulfated at the 3 or 6 carbon position. GlcA may be sulfated at the 2 carbon position. (D) Like chondroitin sulfate these disaccharides are linked through a 1,4-β-glyco-sidic linkage. The chemical structures in were produced using Marvin Sketch Version 6.1.0, which was used under the ChemAxon Ltd. free educational license.
HSPGs
The HSPG family of PGs contain both cell membrane bound and excreted forms. Major transmembrane bound HSPGs are the syndecans and glypicans. Major components of the ECM include perlecan, agrin, and collagen XVII. Heparan sulfate chains are variably sulfated at either carbon 3 or 6 of GlcNAc or oxygen 2 position of GlcA (Figure 1C, D).
Figure 2 is a schematic representation of the wide variety of chondroitin and heparan sulfate proteoglycans. As also depicted in Figure 2, PGs have different sizes, levels of glycosylation, and cellular localization. These different characteristics are plausible factors that determine the type of interaction PGs have with elongating neurites, which is a major agenda of this review.
Figure 2.
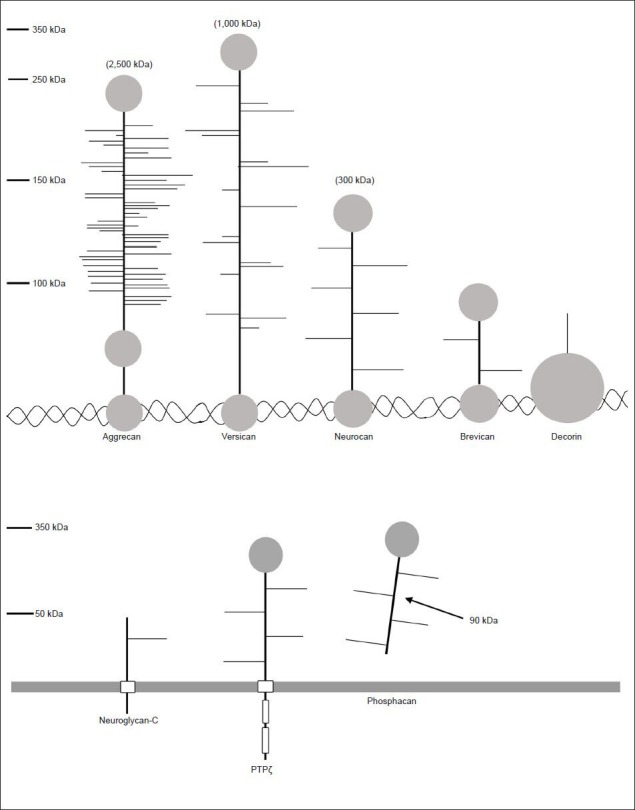
Proteoglycans are proteins that vary in size, localization, and degree of post-translational modifications.
There are many different proteoglycans that vary in size and degree of glycosylation. In addition, the localization of these molecules varies depend-ing on the type and tissue, as some are bound to the extracellular matrix through hyaluronan or collagen (top panel) or found in the membrane or excreted into the extracellular space (bottom panel).
Proteoglycans role in the inability for the CNS to repair following injury
The inability for the spinal cord to regenerate is a major reason for the long-term disabilities and high societal costs spinal cord injuries bare. As most tissues have an innate ability to regenerate, the innate CNS regeneration response does not enable full anatomical and functional recovery. As regeneration of neurons often occurs locally, in a process called “sprouting”, the new neuronal connections made are often insufficient in providing functional recovery, but can sometimes be further burdensome in the form of pathological pain (Harris, 1999; Shortland et al., 1997; Zhang et al., 2004; Zimmermann, 2001). This makes CNS injury a particularly challenging injury to overcome. Greater understanding of the physiological response following SCI reveals PGs to be culpable in preventing the injured spinal cord from regenerating. As with many damaged tissues, following injury to the spinal cord, a scar forms. This scar, commonly referred to as the glial scar, is caused by a proliferation of reactive astrocytes and acts as a barrier that surrounds the damaged tissue. The glial scar reduces the damaging secondary insults that can follow injury to the CNS, such as cytotoxic, excitotoxic, and oxidative insults (Beller et al., 2011; Rolls et al., 2009; Sofroniew, 2005). However, the formation of this scar is also responsible for inhibiting the regeneration of the injured spinal cord (Bovolenta et al., 1992; Hu et al., 2010; Rudge and Silver, 1990). Intriguingly, the formation of the glial scar is closely associated with increased expression of CSPGs.
A variety of CSPGs are elevated both acutely and chronically following SCI and localized within the glial scar and surrounding areas. Following SCI in the rat, neurocan, brevican, and versican expression was increased in the area surrounding the injury up to 2 months post-injury. In addition, neurocan, tenascin-C, and NG2 were significantly increased and reached peak levels 8 days post-injury, while expression of other CSPGs, phosphacan and brevican, are increased and reach peak levels 1 month post-injury in the rat. These increases in PG protein were localized to regions that contain fibronectin scar tissue (Jones et al., 2003; Tang et al., 2003). Furthermore, the expression of some CSPGs following spinal cord injury is closely associated with the areas adjacent to the forward process of transected axons, appearing as a stop sign to a regenerating neuron (Jones et al., 2002).
The increase of PG expression within the glial scar and the inability for the injured spinal cord to grow beyond the glial scar implicates a direct role of CSPGs in the inability for the injured spinal cord to regenerate. In vitro experimentation has demonstrated CSPGs ability to inhibit neurite outgrowth and regeneration. For instance, PGs extracted from the epicenter of a rodent SCI inhibited neurite outgrowth from various neuronal cell-types (McKeon et al., 1995). While, versican and brevican (which are elevated following injury) were demonstrated to contribute to the growth inhibitory activity of myelin, as treatment with β-xyloside (a PG synthesis inhibitor) greatly increased the growth permissiveness of oligodendrocytes (producers of myelin) (Niederost et al., 1999). In addition, CSPGs produced in reactive astrocytes, like those that makeup the glial scar inhibit neurite outgrowth in vitro (Bovolenta et al., 1997; Canning et al., 1996). The historical precedent for CSPGs ability to inhibit neurite outgrowth is discussed, in detail, later in the review.
Further evidence for the role of CSPGs following SCI comes from animal models of SCI. Treatments that have directly targeted the CSPGs of the glial scar have been modestly effective at promoting growth across the injury site and functional recovery in rodents. For instance, treatment of the injured spinal cord with the bacterial enzyme chondroitinase ABC (cABC), which removes the chondroitin sulfate chains of CSPGs, can improve neuronal growth across the glial scar and improve functional outcome (Bradbury and Carter, 2011; Bradbury et al., 2002; Garcia-Alias et al., 2009; Huang et al., 2006; Massey et al., 2006; Tom et al., 2009). In addition, degradation of a specific CSPG core protein, aggrecan, enhances functional recovery following SCI (Tauchi et al., 2012; Snow, et al., unpublished data).
The connection between SCI, CSPGs, and failed regeneration is based on experimental observations. The increase in PG expression in the glial scar, the ability for these PGs to inhibit neurite outgrowth, and the inability for neurons to grow across the glial scar suggests that there is a direct interaction between PGs and an outgrowing/regenerating neurite. Though this evidence implicates PGs as having a profound effect on neurite outgrowth the exact mechanisms responsible for these interactions are unknown. Therefore, it is the goal of our review to explain where and how we got to the current understanding of this interaction and where this field of research is heading. As PGs’ role following SCI has been, and continues to be, a major impetus for examining the exact mechanisms by which PGs interact with outgrowing neurites, specifically, with the sensorimotor leading end of the neurite, the neuronal growth cone (Beller et al., 2013; Cajal, 1892; Letourneau et al., 1994). A deeper understanding of this interaction will yield new modes of therapy aimed at improving neuroregeneration following SCI.
Traditional and novel methods to analyze PG interactions with neuronal growth cones, axons, and cell bodies
There are three major in vitro methods for analyzing the interaction of PGs with outgrowing neurons, neurites andgrowth cones.
Soluble PG outgrowth models
The simplest and first method used to analyze the influence of PGs on neurite outgrowth was the introduction of PGs in solution, i.e., delivering PGs in the media to attached, outgrowing neuronal cells. The advantage to this method, still used, is that multiple concentrations of PGs can be easily and quickly tested for their effect on neurite outgrowth (Snow et al., 1996). The disadvantage to this approach is the physiological relevance. For instance, in the extracellular matrix, PGs are commonly physically attached to hyaluronan and other ECM molecules and not simply floating in the extracellular milieu (Frantz et al., 2010). This may alter the observed effect of the PG on the outgrowing neurite, and it may allow for interactions with the PG, that when coupled to the ECM, may not be possible. For instance, when laminin, a growth permissive substrate for neurons, and aggrecan, a growth inhibitory CSPG, are both substratum adsorbed in vitro, the order of application of these compounds effects the behavior of the neurons (Condic et al., 1999). In particular, when aggrecan was adsorbed to the substrata in the presence of laminin in solution, the aggrecan was more inhibitory than when the aggrecan was bound either before or after adsorption of laminin to the substratum. This suggests that an interaction between laminin and aggrecan is essential for the inhibitory nature of aggrecan. In addition, electrochemical and steric interactions between the PG molecules themselves may be altered when not physically coupled to the ECM. These were early “lessons learned” and have shaped the specifics of more refined methods now in use. For example, to account for these interactions, methods in which PGs are substratum bound and presented directly to outgrowing neurites were, and are, also still widely used.
The “stripe assay” (also “patterned assay” or “choice assay”)
The “stripe assay” is a widely used patterned outgrowth assay developed to analyze the response of elongating neurites to CSPGs in much the same way a developing or regenerating neurite might encounter cells expressing CSPGs in vivo, e.g., the glial scar (Snow et al., 1990). The “stripe assay” uses an alternating pattern of substratum-bound growth-permissive molecules, often laminin but also fibronectin, collagen, NCAM, or others, and the experimental PG (Snow et al., 1990; 2001). Commonly, neuronal explants or dissociated cells, e.g., sensory dorsal root ganglion (DRG), are placed alongside a substrate-bound PG stripe and the effect on neurite outgrowth is determined in a variety of ways (i.e., inhibition of forward elongation by examining the number of neurites turning versus crossing the stripe; growth cone morphology; growth cone retraction, etc.). For instance, when the CS/KSPG, aggrecan, the V2 splice variant of versican, and tenascin, were tested using the “stripe assay”, there was a significant reduction in the number of neurites growing on the stripes containing the PG (Krull et al., 1994; Schmalfeldt et al., 2000; Snow et al., 1990; Treloar et al., 2009). In addition, this method is also extremely useful in examining other effects than just total outgrowth. For example, the “stripe assay” was also used to measure the effect PGs have on calcium signaling, rate of neurite elongation, and filipodia behavior (Snow et al., 1994; 1996). Further, the method was also used to develop semi-automated approaches to quantitatively determine the relative level of inhibition of various PGs by calculating an “inhibitory index” (Hynds and Snow, 2002). More recently, the method was adapted to measure subtle changes in growth cone morphology, filipodia behavior, and growth cone velocity in response to structural variants of the CSPG aggrecan (Beller et al., 2013). This highly useful and commonly employed method provides a means to examine the effect a steep change from a growth permissive to a growth inhibitory substratum has on neuronal outgrowth. This represents the pattern of CSPG an outgrowing neurite may encounter when approaching the ECM presented by the glial scar. Next we describe another type of patterned assay that examines a gradient of PG expression, i.e., a less steep presentation of PGs. This model represents a different way the PGs expressed by glial scars or other key depositions of ECM may present themselves to outgrowing neurites.
Gradient assays
Gradient assays facilitate the examination of the effect a more gradual change in concentration of substrate-bound PG and growth-permissive substrata have on neurite outgrowth. There are two major methods to examine PG gradients, namely the “step-gradient” (Snow and Letourneau, 1992) and the “spot gradient” (Tom et al., 2004). The “step-gradient” is a modified stripe assay with increasing concentrations of PGs on each subsequent step of the gradient. The “spot-gradient” is a gradient formed by adhering a spot of PG to the substratum (often laminin) and diffusion of the PG around the initial spot forms a gradient. These assays have been used to study the effects of an approaching growth cone interacting with increasing concentrations of PG, the formation and behavior of dystrophic growth cones, identifying PG receptors, and to model the effect PGs have on neurite outgrowth following macrophage induced axonal dieback (Busch et al., 2010; Shen et al., 2009; Snow and Letourneau, 1992; Tom et al., 2004). Together these substratum-bound methods used to analyze the PG-neurite interaction successfully model various aspects of neuronal interactions with the glial scar formed following injury, and represent the predominate methods currently used to study this interaction.
Tracing the history of proteoglycan interactions with neuronal growth cones
The complex structure of PGs suggests a number of ways a neuronal growth cone may interact with PG molecules (Figure 3). Though the field has grown since its beginnings (Carbonetto et al., 1983; Snow et al., 1990; Verna et al., 1989), there is no consensus as to what part of the PG molecule is the key player. There are at least two possible ways in which a PG can physically interact with a neuronal growth cone in a receptor-mediated fashion: interactions through the PG core protein or through the associated GAG chains.
Figure 3.
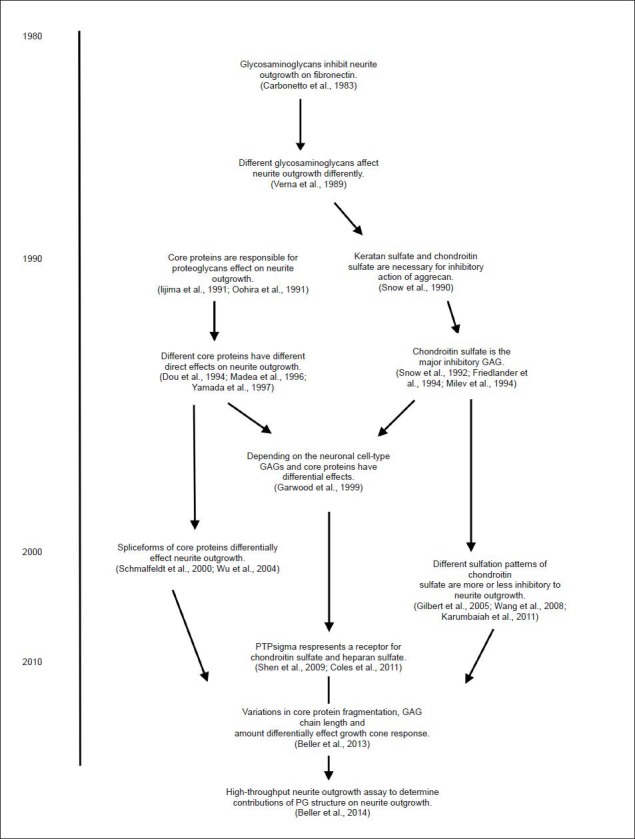
Timeline of advancements in the understanding of the role proteoglycans play in neuronal outgrowth (Selective list; not intended to be exhaustive).
Interactions via the proteoglycan core proteins.
The hypothesis that the core protein is involved in neurite inhibition arose from two studies conducted in 1991, in which, CSPGs were extracted from rat brain and were enzymatically degraded to remove their attached GAG chains. Intriguingly, these two studies revealed different effects of the extracted PG purifications. Specifically, CSPGs extracted from rat brain were adsorbed to the bottom of cell culture plates as either intact CSPGs, the isolated CSPG core proteins (no GAG chains), or the isolated GAG chains. There was an increase in neurite outgrowth of neocortical neurons in response to the intact CSPGs and the intact CSPG core proteins, but not with the purified GAG chains (Iijima et al., 1991). This suggested that CSPG core proteins promote neurite outgrowth, while GAG chains have no effect. In contrast, CSPGs extracted from 10-day-old rat brain and directly added to the media of PC12D cell cultures caused an opposite effect. Intact CSPGs and CSPGs with the GAG chains enzymatically removed inhibited neurite outgrowth. In addition, the purified GAG from this extraction did not inhibit neurite outgrowth, further suggesting that the core proteins themselves were responsible for inhibition (Oohira et al., 1991). Although the measured effects on neurite outgrowth of these two early experiments were different, neurite outgrowth promotion in the first and inhibition in the latter, they both suggested that PGs effect on neurite outgrowth was dependent on the core protein.
Further experimentation analyzed specific PG core proteins and their effect on neurite outgrowth. In 1994, experimentation with purified NG2 further supported the role of CSPG core proteins involvement in neurite inhibition. Embryonic rat DRG neurons outgrowth was inhibited by intact NG2 and was also inhibited after enzymatic removal of CS chains by chondroitinase ABC, thus suggesting a direct role of the NG2 core protein in neurite outgrowth inhibition (Dou and Levine, 1994).
A repulsive and differentiation-promoting influence of the phosphacan core protein was demonstrated by Maeda and Noda (Maeda and Noda, 1996). In brief, cortical neurons exposed to purified phosphacan (intact and following chABC/keratanase digestion) displayed less neurite outgrowth and enhanced morphological differentiation. Similarly, brevican bound to astrocyte cell surfaces through the C-terminal portion of the core protein, inhibited neurite outgrowth in vitro in cerebellar granule cells independent of GAG chains (Yamada et al., 1997). In 1999, evidence suggesting that different neuronal types may respond differently to the same PG was reported. The mouse homolog of phosphacan (DSD-1), caused opposing effects on neurite outgrowth dependent on the neuronal cell type, as the core protein inhibited neurite outgrowth from DRG explants and did not affect outgrowth of hippocampal neurons (Garwood et al., 1999). This important study not only strengthened the belief that core proteins affect outgrowth, but also suggested the important idea that different cell-types react differently to PGs. Further studies of phosphacan and neurocan revealed that core proteins inhibited neurite outgrowth of retinal ganglion cells independent of chondroitinase digestion (Inatani et al., 2001). In 2003, the outgrowth of peripheral and CNS-derived neurons in response to versican V2 was measured. Purified versican V2 core protein was inhibitory to neurite outgrowth, as an undigested PG, following digestion with chondroitinase ABC and removal of N- and O-linked oligosaccharides. This versican V2 inhibited neurite outgrowth from both peripheral and CNS-derived neurons. Only digestion of the core protein itself abolished versican V2 mediated neurite outgrowth inhibition (Schmalfeldt et al., 2000). More recently, PI3-K activation was measured in response to neuroglycan-C and a recombinant neuroglycan-C that lacked associated GAG chains. Both the intact and recombinant neuroglycan-C activated the PI3-K pathway and promoted neurite outgrowth, suggesting the core protein was involved in both (Nakanishi et al., 2006). Based on this long list of results from a variety of laboratories, it is clear that PG core proteins regulate neurite outgrowth, although the specific mechanisms and differences between results are not well understood.
Interactions via the glycosaminoglycan chains
The alternative, and to many, the leading hypothesis in the field of PGs influence on neurite outgrowth is that there are direct interactions between the elongating/regenerating growth cone and the GAG chains of PGs. This hypothesis is supported by many results that conflict with those discussed above. For example, in 1982, the first evidence suggesting the role of GAG chains in the control of neurite outgrowth was reported, while experimenting with heparan sulfate. Rat sympathetic neurons were placed onto matrix produced by cultured bovine endothelial cells. The neurons extended neurites in the absence of nerve growth factor. Only treatment with heparanase abolished this neurite extension in the absence of NGF. This pivotal study led to the hypothesis that PG GAG chains have a direct effect on neurite outgrowth (Lander et al., 1982). In 1990, a pivotal study examining the proteoglycan aggrecan (at the time known as KS/CS-PG) was published. In this report, a novel, and now classic, method for testing neurite outgrowth was presented (see Ways to Measure Outgrowth). Using enzymatic digestion, it was determined that KS and CS were essential for complete inhibition (Snow et al., 1990). Following this study, in 1991, a keratan sulfate proteoglycan extracted from developing chick CNS inhibited neurite outgrowth in vitro and the inhibition of this PG was abolished with keratanase treatment (Cole and McCabe, 1991). These three studies presented an alternative hypothesis to that presented above, in particular, that the GAG chains of PGs were responsible for imparting either the growth promoting or inhibiting activity of PGs.
In 1994, the effect of neurocan and phosphacan on the adhesion molecules N-CAM and Ng-CAM were described. N-CAM and Ng-CAM are members of the cadherin family and are integral mediators of neurite outgrowth, as cadherins act as substrates for neuronal adhesion. Neurocan and phosphacan bind to N-CAM and Ng-CAM and inhibit neurite outgrowth and cell attachment. In addition, the binding of neurocan and phosphacan to N-CAM and Ng-CAM was significantly reduced following chondroitinase digestion, implicating the role of neurocan and phosphacan GAG chains in the inhibition of neurite outgrowth and cell adhesion (Friedlander et al., 1994; Milev et al., 1994). Further supporting the role of GAG chains in outgrowth determination, in 1995, chemical inhibition of GAG formation by treating astrocytes with beta-D-xylosides or sodium chlorate caused a significant increase in neurite outgrowth in treated-astrocyte-conditioned media and on the treated-astrocytes themselves (Smith-Thomas et al., 1995). Additionally, in 1999, NG2 produced by astrocytes with CS chains attached was inhibitory to DRG neurons, while removal of NG2 CS chains was growth permissive (Fidler et al., 1999). This suggests that GAG formation is necessary for the inhibitory activity of astrocytic PGs, like those found in the injured spinal cord.
Thus, both components of PGs have measureable effects on neurite outgrowth. However, the mechanisms of each of these regulatory effects are not well delineated and underlie the basis of many lines of current research in the field (Beller et al., 2013; Brown et al., 2012; Dickendesher et al., 2012; Matsuo and Kimura-Yoshida, 2013; Yu et al., 2013).
New ways of thinking about the interaction between PGs and neurite outgrowth
HSPGs versus CSPGs: Go versus Stop
As reviewed above, there is a large amount of uncertainty as to how PGs can elicit such a diverse spectrum of effects on neurite outgrowth. There is no model that can predict the effect a particular PG will have on an outgrowing neurite solely based on the type of core protein. However, when comparing different GAG types a clearer delineation can be drawn. In particular, the differences in the response to HSPGs versus CSPGs.
As presented earlier, Lander et al.'s study conducted in 1982, showed that removal of heparan sulfate (HS) through the action of heparanase abolishes the NGF-independent outgrowth of sympathetic neurons, thus promoting neurite outgrowth (Lander et al., 1982). In agreement, outgrowth of spinal cord neurons, chick retinal neurons, motor neurons and PC12 cells were also promoted by HSPGs (Campagna et al., 1995; Hantaz-Ambroise et al., 1987; Kim et al., 2003). It is widely suggested that the growth promoting effect of HSPGs is due to their ability to bind and interact with growth factors. For instance, the transmembrane HSPG syndecan-3 is a receptor for the glial cell-derived neurotrophic factor (GDNF) and syndecan-3 binding to GDNF mediates cell spreading and neurite outgrowth. In addition, knockout of syndecan-3 in mice caused a reduction in cortical GABAergic neurons, suggesting an important role in cortical development (Bespalov et al., 2011). Similarly, the cell-surface-attached HSPGs (i.e., glypican, perlecan, and agrin) are important modulators of fibroblast growth factor signaling (Matsuo and Kimura-Yoshida, 2013). HSPGs also interact with other neurite outgrowth regulating proteins. For instance, the growth repulsive cue semaphorin-1a (for more discussion of semaphorins; see Shen et al. in this issue) was ineffective in motor neurons lacking the HSPG perlecan (Cho et al., 2012). HSPGs also interact with Netrin-DCC (growth permissive), and Robo-Slit (growth inhibitory) signaling to coordinate the lateral positioning of dopaminergic axons leading into the spinal cord by modulating the repulsive cues produced by the Robo-Slit interaction (Kastenhuber et al., 2009). Indeed, HSPGs appear to transduce a positive cue to outgrowing neurites by promoting continued growth.
Unlike the HSPGs, CSPGs provide inhibitory cues to outgrowing neurites. For instance, brevican, aggrecan, NG2, neurocan and phosphacan are inhibitory to DRG neurons, cortical neurons, cerebellar granule neurons, and retinal ganglion cells, respectively (Beller et al., 2013; Dou and Levine, 1997; Inatani et al., 2001; Snow et al., 1990; Yamada et al., 1997). However, there is conflicting data, which suggests that not all CSPGs interact with neurons to produce the same effect, and that this may be CSPG dependent or neuronal cell-type dependent. For instance, DSD-1, the mouse homolog of phosphacan, inhibited neurite outgrowth from neonatal DRG neurons yet, it had no effect on embryonic hippocampal neurons (Garwood et al., 1999). Further, different forms of the same CSPG may elicit different effects in a class of neuron. For instance, the short isoform of phosphacan, a non-proteoglycan variant known as receptor protein tyrosine phosphatase-β (RPTPβ), promoted outgrowth of neonatal cortical neurons (Garwood et al., 2003). In addition, differences in splicing of a particular PG can affect its effect on neurite outgrowth. For instance, the V1 isoform of versican promoted neurite outgrowth of hippocampal neurons, whilst the V2 isoform inhibited neurite outgrowth of hippocampal neurons, retinal ganglion and DRG neurons (Schmalfeldt et al., 2000; Wu et al., 2004). Other than neuronal cell-type, the form of the PG, or the splice variants, the differential effect of particular CSPGs on neurite outgrowth may be explained by the differences in the varied forms of sulfation of the GAGs. This idea is one of the more recent developments in the study of PGs, specifically, the effect of different sulfation patterns on neurite outgrowth.
CSPG sulfation may affect signal
Investigating the effects that sulfation patterns and epitopes have on neurite outgrowth is taking the forefront as the most intricate understanding of the interaction of proteoglycans and neurite outgrowth (Habuchi et al., 2004; Holt and Dickson, 2005). As explained earlier, there are four major forms of CS, CS-2, CS-4, CS-6, and CS-4,6. Initial experimentation examining the activity of these different sulfation forms of GAGs studied purified preparations of the CS chains alone. Though unlikely to perfectly mimic the effect of CS attached to the core protein, these experiments did begin to unravel the role sulfation may play in the differential response to CSPGs. These experiments have demonstrated that depending on the neuronal cell-type and predominant sulfation form of the PG, differential responses in neurite outgrowth were reported. When CS-4 and CS-6 were added into the cell media, adhesion of thalamic and hippocampal neurons to laminin and poly-l-lysine were reduced. However, only thalamic neuron outgrowth was affected as CS-6 increased the amount of outgrowth from thalamic neurons (Fernaud-Espinosa et al., 1994). Similarly, in a different study, CS-4 was found to be strongly inhibitory to cerebellar granule cells, while CS-6 was not (Wang et al., 2008). These results seem to suggest that one can predict a particular neuronal cell-type response based on the interaction with CS specific sulfation pattern. However, the association is not necessarily clear. For instance, substrate bound CS-4,6 increased neurite outgrowth of embryonic hippocampal neurons, while it strongly inhibited DRG neurite outgrowth (Gilbert et al., 2005). In partial agreement with the previous study, genetic suppression of CS-4,6 in astrocytes through siRNA targeting of the sulfotransferases that produce the 4,6 sulfation pattern, produced astrocytic proteoglycans with reduced inhibition of neurite outgrowth in embryonic cortical neurons (Karumbaiah et al., 2011). Though developments are expanding the role different sulfation patterns play in the regulation of neurite outgrowth, the mechanism(s) and the target(s) involved in this interaction are not known.
How do CSPGs regulate neurite outgrowth?
The regulation of neurite outgrowth is often envisioned as the summation of negative and positive signals received by a neuron through receptor-mediated mechanisms. Though CSPGs may act as a physical wall or barrier to extending neurites, it is likely that CSPGs interact with an outgrowing neurite either directly or indirectly with receptors located on the neuron.
Do PGs interact with the neuronal growth cone, axon, or cell body?
Anatomically, the ECM surrounds all parts of cells including the growth cone, axon and cell body. Less clear is what part or parts of a neuron are interacting with the PGs in the ECM. Immunocytochemical and immuno-electron microscopic analysis of the distribution of phosphacan in the olfactory nerve displayed a widespread distribution of the PG on both the surfaces of neuronal cell bodies and neurites (Nishizuka et al., 1996). In the CNS, neuronal cell bodies are commonly surrounded by structures known as perineuronal nets (PNN), which are enriched in proteoglycans (Celio and Blumcke, 1994). In addition, these PNNs regulate synaptic plasticity and synapse formation, by both interacting with the cell body and neurites growing towards the surrounded cell body (Pizzorusso et al., 2002; Wang and Fawcett, 2012). Work conducted by our group previously demonstrated that contact with CSPGs through either the cell body or growth cone caused a significant increase in intracellular [Ca2+] (Snow et al., 1994). Neuronal cell bodies in contact with CSPGs located in Schwann cell membranes extend neurites that are inhibited by contact with CSPG, however when the Schwann cell membrane CSPGs are digested with chondroitinase, neuronal cell bodies in contact with these Schwann cells extend neurites that are no longer inhibited by contact with CSPGs (Castro and Kuffler, 2006). Likewise, postnatal cerebellar granule cell bodies and neurites are both repulsed through contact with CSPGs (Kaneko et al., 2007). It is not clear at what locale the CSPGs are eliciting their effect.
Work in our group has focused on the rapid changes occurring following growth cone contact with CSPGs. Following growth cone contact with CSPG, there are rapid changes in filipodia number, length, growth cone width, growth cone length, and growth cone velocity (Beller et al., 2013). In addition, the majority of studies are focused on the interaction of the neuronal growth cone's contact with CSPGs, though CSPG contact with the neuronal cell body also affects the interaction of the neuronal growth cone with CSPG. For instance, neurons have the ability to “adapt” to the presence of CSPGs contacting the cell bodies through the upregulation of integrins and are no longer inhibited by CSPGs (Condic et al., 1999; Lemons et al., 2005). It is clear that both the neuronal cell body and growth cone interact with CSPGs. However, the similarities and differences in the neuronal response and their dependence on where the CSPG is contacted is unclear.
PGs as receptor “shuttles”
PGs may interact with an outgrowing neurite by acting as a ligand “shuttle” and present a bound ligand to a recipient receptor. For instance, the syndecans are a class of HSPG, which play a key role in growth factor signaling sensitivity. Through the interaction with the 2-O-sulfated iduronic acid units of the HS on syndecan, many growth factors are able to bind. Once bound to the HS, the growth factor is presented and bound to its respective receptor. Signaling pathways dependent upon syndecan binding include the GDNF, FGF, Wnt, VEGF, and HGF pathways (Chen et al., 2013; Chernousov and Carey, 1993; Tkachenko et al., 2005). In particular, removal of N-syndecan HS chains reduced neurite outgrowth in vitro and ex vivo in response to growth factor stimulation, thus suggesting a growth-permissive role of N-syndecan binding (Kinnunen et al., 1996). N-syndecan is implicated to signal through a Src kinase signaling pathway, contribute to the development of axon tracts, and the process of hippocampal long-term potentiation, all requiring neurite outgrowth (Kinnunen et al., 1998; Lauri et al., 1999). Syndecans represent a way a PG may affect neurite outgrowth as a receptor or “shuttle” for growth factors. However, there is also evidence of PGs as a ligand, binding to cell-surface receptors.
PGs as ligands
Protein tyrosine phosphatase sigma (PTPσ) was recently discovered to be a receptor for CSPGs. This represents an important step in understanding the manner in which, CSPGs inhibit neurite outgrowth. Through a series of elaborate experimentation, PTPσ bound specifically to the CS chains of CSPGs, and inhibited neurite outgrowth (Shen et al., 2009). Further, HSPGs also bound to the PTPσ receptor and acted as a switch between growth-promotion and inhibition. As binding to CSPG reduced neurite outgrowth, while binding to HSPG increased neurite outgrowth (Coles et al., 2011). PTPσ is not the only CSPG receptor as two members of the Nogo receptor family are also CSPG receptors. NgR1 and NgR3 knockout mice have reduced CS binding and an enhanced ability for axonal regeneration following optic nerve crush, and reduced outgrowth inhibition in neurons cultured on CSPG (Dickendesher et al., 2012). Initial steps at elucidating the pathways these receptors signal through is underway. Global analysis of the neuronal phosphoproteome in response to CSPGs identified a number of proteins whose phosphorylation state is regulated by CSPGs. The majority of these proteins are found in important cellular pathways that regulate cell morphology, nervous system development, and RNA post-transcriptional modifications (Yu et al., 2013).
Knowledge of PGs role in the CNS has evolved overtime from the discovery that PGs produced by cultured endothelial cells improve neurite outgrowth (Lander et al., 1982), to the conclusion that both the PG core protein and GAG chain affect neurite outgrowth in a neuron cell-type specific manner (Beller et al., 2013; Nakanishi et al., 2006; Snow et al., 1990). The added knowledge on how PGs interact with outgrowing neurites allows for the continuing acceleration of knowledge, as the role of PGs as receptor and ligand opens up a new field of mechanistic and pharmacological research (Beller et al., in press; Dickendesher et al., 2012; Shen et al., 2009; Tkachenko et al., 2005).
Future directions
Though the 30+ years of research into the role of PGs in the regulation of neurite outgrowth has not led to a consensus on the way they interact with an outgrowing neurite, many tools and ideas integral to cracking the PG code have been revealed. For instance, the recent discovery that PTPσ, NgR1 and NgR3 are CSPG receptors opens the path to a new field of pharmacology. Now that CSPG targets are identified, pharmacological analysis of the varying post-translational modifications can be conducted. The binding affinities of these substrates to the receptors can be compared to their effect on neurite outgrowth and the relationship between structure and function can be understood. In addition, development of a high throughput method to analyze a large number of proteoglycans on different neuronal cell types will enhance our ability to determine neuronal specificity and their response to PGs. For instance, through a series of elaborate experimentation, a synthetic GAG containing only CS-4,6 was found to bind with high affinity to the PTPσ receptor and inhibited neurite outgrowth of dissociated and intact DRG neurons. In addition, masking of this epitope through a specific monoclonal antibody against CS-4,6 promoted neuroregeneration through the CSPG-rich glial scar (Brown et al., 2012).
Our laboratory is currently producing CSPGs that have defined sulfation patterns. Through reverse transcriptase PCR we determined that HEK293T cells expressed all the carbohydrate sulfotransferases that produce each of the CSPG sulfation patterns, specifically CS-2, CS-4, CS-6, and CS-4,6. By producing stable transfectants of these HEK293T cells with shRNA directed towards specific CHSTs, we are producing CSPGs that lack one of the specific sulfation patterns. This allows us to determine the contribution of each of these sulfation patterns on neurite outgrowth. This will require a large number of outgrowth assays. Therefore, our laboratory has developed a high-throughput assay (Beller et al., in press). This assay will allow the analysis of a large number of proteoglycans and neuronal cell-types, in order to determine the contribution of each sulfation pattern, proteoglycan core protein, and post-translational modification. Results from these studies may lead to the development of novel pharmacological approaches to enhance regeneration of the injured spinal cord.
Conclusion
The complexity of both PG structure and the process of neuronal outgrowth makes deciphering the interaction between PG and neurite an arduous task. Due to the high variability in PG composition, the limitations in the models available for assessing PG effect, the multiple ways PGs and neurites are capable of interacting; a full understanding of the mechanism(s) at play are likely far off. However, it is clear that PGs play an important role in the process of neurite outgrowth and contribute to the inability of the injured spinal cord to regenerate. With continued persistence, a clearer picture of the PG neurite interaction may lead to the ability to not only regenerate, but also rewire, the injured spinal cord and brain.
Acknowledgments:
We wish to acknowledge the contributions of Thomas M. Hering, Ph.D. to ongoing work on CSPGs and neurite outgrowth, some of which is cited in this review. We would also like to acknowledge Chris Calulot, and Adrian Centers, M.S., for any cited data to which they contributed.
Footnotes
Funding: The study was supported by the NIH (NS53470), the Kentucky Spinal Cord and Head Injury Research Trust (#10-11A), and the Department of Defense, CDMRP (SC090248/W81XWH-10-1-0778).
Conflicts of interest: None declared.
Copyedited by Li CH, Song LP, Zhao M
References
- [1].Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- [2].Beller JA, Gurkoff GG, Berman RF, Lyeth BG. Pharmacological enhancement of glutamate transport reduces excitotoxicity in vitro. Restor Neurol Neurosci. 2011;29:331–346. doi: 10.3233/RNN-2011-603. [DOI] [PubMed] [Google Scholar]
- [3].Beller JA, Kulengowski B, Kobraei EM, Curinga G, Calulot CM, Bahrami A, Hering TM, Snow DM. Comparison of sensory neuron growth cone and filopodial responses to structurally diverse aggrecan variants, in vitro. Exp Neurol. 2013;247:143–157. doi: 10.1016/j.expneurol.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beller JA, Hering TM, Calulot CM, Snow DM. High-throughput quantitative assay for analyzing neurite outgrowth on a uniform substratum: the cell-substratum assay. In: Leach JB, Powell EM, editors. Neuromethods: Extracellular Matrix. New York: Humana Press; 2014. in press. [Google Scholar]
- [5].Bespalov MM, Sidorova YA, Tumova S, Ahonen-Bishopp A, Magalhaes AC, Kulesskiy E, Paveliev M, Rivera C, Rauvala H, Saarma M. Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. J Cell Biol. 2011;192:153–169. doi: 10.1083/jcb.201009136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bovolenta P, Wandosell F, Nieto-Sampedro M. CNS glial scar tissue: a source of molecules which inhibit central neurite outgrowth. Prog Brain Res. 1992;94:367–379. doi: 10.1016/s0079-6123(08)61765-3. [DOI] [PubMed] [Google Scholar]
- [7].Bovolenta P, Fernaud-Espinosa I, Mendez-Otero R, Nieto-Sampedro M. Neurite outgrowth inhibitor of gliotic brain tissue. Mode of action and cellular localization, studied with specific monoclonal antibodies. Eur J Neurosci. 1997;9:977–989. doi: 10.1111/j.1460-9568.1997.tb01448.x. [DOI] [PubMed] [Google Scholar]
- [8].Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- [9].Bradbury EJ, Carter LM. Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res Bull. 2011;84:306–316. doi: 10.1016/j.brainresbull.2010.06.015. [DOI] [PubMed] [Google Scholar]
- [10].Brown JM, Xia J, Zhuang B, Cho KS, Rogers CJ, Gama CI, Rawat M, Tully SE, Uetani N, Mason DE, Tremblay ML, Peters EC, Habuchi O, Chen DF, Hsieh-Wilson LC. A sulfated carbohydrate epitope inhibits axon regeneration after injury. Proc Natl Acad Sci U S A. 2012;109:4768–4773. doi: 10.1073/pnas.1121318109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Busch SA, Horn KP, Cuascut FX, Hawthorne AL, Bai L, Miller RH, Silver J. Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury. J Neurosci. 2010;30:255–265. doi: 10.1523/JNEUROSCI.3705-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cajal SR. La retine des vertebres. La Cellule. 1892;9:121–133. [Google Scholar]
- [13].Campagna JA, Ruegg MA, Bixby JL. Agrin is a differentiation-inducing “stop signal” for motoneurons in vitro. Neuron. 1995;15:1365–1374. doi: 10.1016/0896-6273(95)90014-4. [DOI] [PubMed] [Google Scholar]
- [14].Canning DR, Hoke A, Malemud CJ, Silver J. A potent inhibitor of neurite outgrowth that predominates in the extracellular matrix of reactive astrocytes. Int J Dev Neurosci. 1996;14:153–175. doi: 10.1016/0736-5748(96)00004-4. [DOI] [PubMed] [Google Scholar]
- [15].Carbonetto S, Gruver MM, Turner DC. Nerve fiber growth in culture on fibronectin, collagen, and glycosaminoglycan substrates. J Neurosci. 1983;3:2324–2335. doi: 10.1523/JNEUROSCI.03-11-02324.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Carulli D, Laabs T, Geller HM, Fawcett JW. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol. 2005;15:116–120. doi: 10.1016/j.conb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- [17].Castro C, Kuffler DP. Membrane-bound CSPG mediates growth cone outgrowth and substrate specificity by Schwann cell contact with the DRG neuron cell body and not via growth cone contact. Exp Neurol. 2006;200:19–25. doi: 10.1016/j.expneurol.2006.02.001. [DOI] [PubMed] [Google Scholar]
- [18].Celio MR, Blumcke I. Perineuronal nets--a specialized form of extracellular matrix in the adult nervous system. Brain Res Brain Res Rev. 1994;19:128–145. doi: 10.1016/0165-0173(94)90006-x. [DOI] [PubMed] [Google Scholar]
- [19].Chen J, Repunte-Canonigo V, Kawamura T, Lefebvre C, Shin W, Howell LL, Hemby SE, Harvey BK, Califano A, Morales M, Koob GF, Sanna PP. Hypothalamic proteoglycan syndecan-3 is a novel cocaine addiction resilience factor. Nat Commun. 2013;4:1955. doi: 10.1038/ncomms2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chernousov MA, Carey DJ. N-syndecan (syndecan 3) from neonatal rat brain binds basic fibroblast growth factor. J Biol Chem. 1993;268:16810–16814. [PubMed] [Google Scholar]
- [21].Cho JY, Chak K, Andreone BJ, Wooley JR, Kolodkin AL. The extracellular matrix proteoglycan perlecan facilitates transmembrane semaphorin-mediated repulsive guidance. Genes Dev. 2012;26:2222–2235. doi: 10.1101/gad.193136.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cole GJ, McCabe CF. Identification of a developmentally regulated keratan sulfate proteoglycan that inhibits cell adhesion and neurite outgrowth. Neuron. 1991;7:1007–1018. doi: 10.1016/0896-6273(91)90345-z. [DOI] [PubMed] [Google Scholar]
- [23].Coles CH, Shen Y, Tenney AP, Siebold C, Sutton GC, Lu W, Gallagher JT, Jones EY, Flanagan JG, Aricescu AR. Proteoglycan-specific molecular switch for RPTPsigma clustering and neuronal extension. Science. 2011;332:484–488. doi: 10.1126/science.1200840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Condic ML, Snow DM, Letourneau PC. Embryonic neurons adapt to the inhibitory proteoglycan aggrecan by increasing integrin expression. J Neurosci. 1999;19:10036–10043. doi: 10.1523/JNEUROSCI.19-22-10036.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Curinga G, Snow DM, Smith GM. Mechanisms regulating interpretation of guidance cues during development, maturation and following injury. Rev Neurosci. 2008;19:213–226. doi: 10.1515/revneuro.2008.19.4-5.213. [DOI] [PubMed] [Google Scholar]
- [27].DeLuca S, Richmond ME, Silbert JE. Biosynthesis of chondroitin sulfate. Sulfation of the polysaccharide chain. Biochemistry. 1973;12:3911–3915. doi: 10.1021/bi00744a019. [DOI] [PubMed] [Google Scholar]
- [28].Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, Katagiri Y, Benowitz LI, Geller HM, Giger RJ. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15:703–712. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Doretto S, Malerba M, Ramos M, Ikrar T, Kinoshita C, De Mei C, Tirotta E, Xu X, Borrelli E. Oligodendrocytes as regulators of neuronal networks during early postnatal development. PLoS One. 2011;6:e19849. doi: 10.1371/journal.pone.0019849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dou CL, Levine JM. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci. 1994;14:7616–7628. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dou CL, Levine JM. Identification of a neuronal cell surface receptor for a growth inhibitory chondroitin sulfate proteoglycan (NG2) J Neurochem. 1997;68:1021–1030. doi: 10.1046/j.1471-4159.1997.68031021.x. [DOI] [PubMed] [Google Scholar]
- [32].Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- [33].Fernaud-Espinosa I, Nieto-Sampedro M, Bovolenta P. Differential effects of glycosaminoglycans on neurite outgrowth from hippocampal and thalamic neurones. J Cell Sci. 1994;107:1437–1448. doi: 10.1242/jcs.107.6.1437. [DOI] [PubMed] [Google Scholar]
- [34].Fidler PS, Schuette K, Asher RA, Dobbertin A, Thornton SR, Calle-Patino Y, Muir E, Levine JM, Geller HM, Rogers JH, Faissner A, Fawcett JW. Comparing astrocytic cell lines that are inhibitory or permissive for axon growth: the major axon-inhibitory proteoglycan is NG2. J Neurosci. 1999;19:8778–8788. doi: 10.1523/JNEUROSCI.19-20-08778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Franco SJ, Muller U. Extracellular matrix functions during neuronal migration and lamination in the mammalian central nervous system. Dev Neurobiol. 2011;71:889–900. doi: 10.1002/dneu.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- [40].Garcia-Alias G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- [41].Garwood J, Schnadelbach O, Clement A, Schutte K, Bach A, Faissner A. DSD-1-proteoglycan is the mouse homolog of phosphacan and displays opposing effects on neurite outgrowth dependent on neuronal lineage. J Neurosci. 1999;19:3888–3899. doi: 10.1523/JNEUROSCI.19-10-03888.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Garwood J, Heck N, Reichardt F, Faissner A. Phosphacan short isoform, a novel non-proteoglycan variant of phosphacan/receptor protein tyrosine phosphatase-beta, interacts with neuronal receptors and promotes neurite outgrowth. J Biol Chem. 2003;278:24164–24173. doi: 10.1074/jbc.M211721200. [DOI] [PubMed] [Google Scholar]
- [43].Gilbert RJ, McKeon RJ, Darr A, Calabro A, Hascall VC, Bellamkonda RV. CS-4 6 is differentially upregulated in glial scar and is a potent inhibitor of neurite extension. Mol Cell Neurosci. 2005;29:545–558. doi: 10.1016/j.mcn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- [44].Habuchi H, Habuchi O, Kimata K. Sulfation pattern in glycosaminoglycan: does it have a code. Glycoconj J. 2004;21:47–52. doi: 10.1023/B:GLYC.0000043747.87325.5e. [DOI] [PubMed] [Google Scholar]
- [45].Hantaz-Ambroise D, Vigny M, Koenig J. Heparan sulfate proteoglycan and laminin mediate two different types of neurite outgrowth. J Neurosci. 1987;7:2293–2304. [PMC free article] [PubMed] [Google Scholar]
- [46].Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. FASEB J. 1992;6:861–870. [PubMed] [Google Scholar]
- [47].Harris AJ. Cortical origin of pathological pain. Lancet. 1999;354:1464–1466. doi: 10.1016/S0140-6736(99)05003-5. [DOI] [PubMed] [Google Scholar]
- [48].Hartmann U, Maurer P. Proteoglycans in the nervous system--the quest for functional roles in vivo. Matrix Biol. 2001;20:23–35. doi: 10.1016/s0945-053x(00)00137-2. [DOI] [PubMed] [Google Scholar]
- [49].Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- [50].Holt CE, Dickson BJ. Sugar codes for axons. Neuron. 2005;46:169–172. doi: 10.1016/j.neuron.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hu R, Zhou J, Luo C, Lin J, Wang X, Li X, Bian X, Li Y, Wan Q, Yu Y, Feng H. Glial scar and neuroregeneration: histological, functional, and magnetic resonance imaging analysis in chronic spinal cord injury. J Neurosurg Spine. 2010;13:169–180. doi: 10.3171/2010.3.SPINE09190. [DOI] [PubMed] [Google Scholar]
- [52].Huang WC, Kuo WC, Cherng JH, Hsu SH, Chen PR, Huang SH, Huang MC, Liu JC, Cheng H. Chondroitinase ABC promotes axonal re-growth and behavior recovery in spinal cord injury. Biochem Biophys Res Commun. 2006;349:963–968. doi: 10.1016/j.bbrc.2006.08.136. [DOI] [PubMed] [Google Scholar]
- [53].Hynds DL, Snow DM. A semi-automated image analysis method to quantify neurite preference/axon guidance on a patterned substratum. J Neurosci Methods. 2002;121:53–64. doi: 10.1016/s0165-0270(02)00231-5. [DOI] [PubMed] [Google Scholar]
- [54].Iijima N, Oohira A, Mori T, Kitabatake K, Kohsaka S. Core protein of chondroitin sulfate proteoglycan promotes neurite outgrowth from cultured neocortical neurons. J Neurochem. 1991;56:706–708. doi: 10.1111/j.1471-4159.1991.tb08207.x. [DOI] [PubMed] [Google Scholar]
- [55].Inatani M, Honjo M, Otori Y, Oohira A, Kido N, Tano Y, Honda Y, Tanihara H. Inhibitory effects of neurocan and phosphacan on neurite outgrowth from retinal ganglion cells in culture. Invest Ophthalmol Vis Sci. 2001;42:1930–1938. [PubMed] [Google Scholar]
- [56].Jander S, Stoll G. Strain-specific expression of microglial keratan sulfate proteoglycans in the normal rat central nervous system: inverse correlation with constitutive expression of major histocompatibility complex class II antigens. Glia. 1996;18:255–260. doi: 10.1002/(SICI)1098-1136(199611)18:3<255::AID-GLIA9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- [57].Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182:399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- [59].Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci. 2003;23:9276–9288. doi: 10.1523/JNEUROSCI.23-28-09276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- [61].Kaneko M, Kubo T, Hata K, Yamaguchi A, Yamashita T. Repulsion of cerebellar granule neurons by chondroitin sulfate proteoglycans is mediated by MAPK pathway. Neurosci Lett. 2007;423:62–67. doi: 10.1016/j.neulet.2007.06.038. [DOI] [PubMed] [Google Scholar]
- [62].Karumbaiah L, Anand S, Thazhath R, Zhong Y, McKeon RJ, Bellamkonda RV. Targeted downregulation of N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase significantly mitigates chondroitin sulfate proteoglycan-mediated inhibition. Glia. 2011;59:981–996. doi: 10.1002/glia.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kastenhuber E, Kern U, Bonkowsky JL, Chien CB, Driever W, Schweitzer J. Netrin-DCC Robo-Slit, and heparan sulfate proteoglycans coordinate lateral positioning of longitudinal dopaminergic diencephalospinal axons. J Neurosci. 2009;29:8914–8926. doi: 10.1523/JNEUROSCI.0568-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kim MJ, Cotman SL, Halfter W, Cole GJ. The heparan sulfate proteoglycan agrin modulates neurite outgrowth mediated by FGF-2. J Neurobiol. 2003;55:261–277. doi: 10.1002/neu.10213. [DOI] [PubMed] [Google Scholar]
- [65].Kinnunen A, Kinnunen T, Kaksonen M, Nolo R, Panula P, Rauvala H. N-syndecan and HB-GAM (heparin-binding growth-associated molecule) associate with early axonal tracts in the rat brain. Eur J Neurosci. 1998;10:635–648. doi: 10.1046/j.1460-9568.1998.00082.x. [DOI] [PubMed] [Google Scholar]
- [66].Kinnunen T, Raulo E, Nolo R, Maccarana M, Lindahl U, Rauvala H. Neurite outgrowth in brain neurons induced by heparin-binding growth-associated molecule (HB-GAM) depends on the specific interaction of HB-GAM with heparan sulfate at the cell surface. J Biol Chem. 1996;271:2243–2248. doi: 10.1074/jbc.271.4.2243. [DOI] [PubMed] [Google Scholar]
- [67].Kinnunen T, Kaksonen M, Saarinen J, Kalkkinen N, Peng HB, Rauvala H. Cortactin-Src kinase signaling pathway is involved in N-syndecan-dependent neurite outgrowth. J Biol Chem. 1998;273:10702–10708. doi: 10.1074/jbc.273.17.10702. [DOI] [PubMed] [Google Scholar]
- [68].Krull CE, Oland LA, Faissner A, Schachner M, Tolbert LP. In vitro analyses of neurite outgrowth indicate a potential role for tenascin-like molecules in the development of insect olfactory glomeruli. J Neurobiol. 1994;25:989–1004. doi: 10.1002/neu.480250808. [DOI] [PubMed] [Google Scholar]
- [69].Lander AD, Fujii DK, Gospodarowicz D, Reichardt LF. Characterization of a factor that promotes neurite outgrowth: evidence linking activity to a heparan sulfate proteoglycan. J Cell Biol. 1982;94:574–585. doi: 10.1083/jcb.94.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lander C, Zhang H, Hockfield S. Neurons produce a neuronal cell surface-associated chondroitin sulfate proteoglycan. J Neurosci. 1998;18:174–183. doi: 10.1523/JNEUROSCI.18-01-00174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lauri SE, Kaukinen S, Kinnunen T, Ylinen A, Imai S, Kaila K, Taira T, Rauvala H. Regulatory role and molecular interactions of a cell-surface heparan sulfate proteoglycan (N-syndecan) in hippocampal long-term potentiation. J Neurosci. 1999;19:1226–1235. doi: 10.1523/JNEUROSCI.19-04-01226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lemons ML, Barua S, Abanto ML, Halfter W, Condic ML. Adaptation of sensory neurons to hyalectin and decorin proteoglycans. J Neurosci. 2005;25:4964–4973. doi: 10.1523/JNEUROSCI.0773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Letourneau PC, Condic ML, Snow DM. Interactions of developing neurons with the extracellular matrix. J Neurosci. 1994;14:915–928. doi: 10.1523/JNEUROSCI.14-03-00915.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lukes A, Mun-Bryce S, Lukes M, Rosenberg GA. Extracellular matrix degradation by metalloproteinases and central nervous system diseases. Mol Neurobiol. 1999;19:267–284. doi: 10.1007/BF02821717. [DOI] [PubMed] [Google Scholar]
- [76].Mack AF, Wolburg H. A novel look at astrocytes: aquaporins ionic homeostasis, and the role of the microenvironment for regeneration in the CNS. Neuroscientist. 2013;19:195–207. doi: 10.1177/1073858412447981. [DOI] [PubMed] [Google Scholar]
- [77].Maeda N, Noda M. 6B4 proteoglycan/phosphacan is a repulsive substratum but promotes morphological differentiation of cortical neurons. Development. 1996;122:647–658. doi: 10.1242/dev.122.2.647. [DOI] [PubMed] [Google Scholar]
- [78].Massey JM, Hubscher CH, Wagoner MR, Decker JA, Amps J, Silver J, Onifer SM. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neurosci. 2006;26:4406–4414. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Matsuo I, Kimura-Yoshida C. Extracellular modulation of Fibroblast Growth Factor signaling through heparan sulfate proteoglycans in mammalian development. Curr Opin Genet Dev. 2013;23:399–407. doi: 10.1016/j.gde.2013.02.004. [DOI] [PubMed] [Google Scholar]
- [80].McKeon RJ, Hoke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol. 1995;136:32–43. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- [81].Milev P, Friedlander DR, Sakurai T, Karthikeyan L, Flad M, Margolis RK, Grumet M, Margolis RU. Interactions of the chondroitin sulfate proteoglycan phosphacan, the extracellular domain of a receptor-type protein tyrosine phosphatase, with neurons, glia, and neural cell adhesion molecules. J Cell Biol. 1994;127:1703–1715. doi: 10.1083/jcb.127.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Nakanishi K, Aono S, Hirano K, Kuroda Y, Ida M, Tokita Y, Matsui F, Oohira A. Identification of neurite outgrowth-promoting domains of neuroglycan C, a brain-specific chondroitin sulfate proteoglycan, and involvement of phosphatidylinositol 3-kinase and protein kinase C signaling pathways in neuritogenesis. J Biol Chem. 2006;281:24970–24978. doi: 10.1074/jbc.M601498200. [DOI] [PubMed] [Google Scholar]
- [83].Niederost BP, Zimmermann DR, Schwab ME, Bandtlow CE. Bovine CNS myelin contains neurite growth-inhibitory activity associated with chondroitin sulfate proteoglycans. J Neurosci. 1999;19:8979–8989. doi: 10.1523/JNEUROSCI.19-20-08979.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Nishizuka M, Ikeda S, Arai Y, Maeda N, Noda M. Cell surface-associated extracellular distribution of a neural proteoglycan, 6B4 proteoglycan/phosphacan in the olfactory epithelium, olfactory nerve and cells migrating along the olfactory nerve in chick embryos. Neurosci Res. 1996;24:345–355. doi: 10.1016/0168-0102(95)01010-6. [DOI] [PubMed] [Google Scholar]
- [85].Oohira A, Matsui F, Katoh-Semba R. Inhibitory effects of brain chondroitin sulfate proteoglycans on neurite outgrowth from PC12D cells. J Neurosci. 1991;11:822–827. doi: 10.1523/JNEUROSCI.11-03-00822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- [87].Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- [88].Poole AR. Proteoglycans in health and disease: structures and functions. Biochem J. 1986;236:1–14. doi: 10.1042/bj2360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Pyka M, Wetzel C, Aguado A, Geissler M, Hatt H, Faissner A. Chondroitin sulfate proteoglycans regulate astrocyte-dependent synaptogenesis and modulate synaptic activity in primary embryonic hippocampal neurons. Eur J Neurosci. 2011;33:2187–2202. doi: 10.1111/j.1460-9568.2011.07690.x. [DOI] [PubMed] [Google Scholar]
- [90].Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009;10:235–241. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- [91].Rudge JS, Silver J. Inhibition of neurite outgrowth on astroglial scars in vitro. J Neurosci. 1990;10:3594–3603. doi: 10.1523/JNEUROSCI.10-11-03594.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Schmalfeldt M, Bandtlow CE, Dours-Zimmermann MT, Winterhalter KH, Zimmermann DR. Brain derived versican V2 is a potent inhibitor of axonal growth. J Cell Sci. 2000;113:807–816. doi: 10.1242/jcs.113.5.807. [DOI] [PubMed] [Google Scholar]
- [93].Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Shioi J, Pangalos MN, Ripellino JA, Vassilacopoulou D, Mytilineou C, Margolis RU, Robakis NK. The Alzheimer amyloid precursor proteoglycan (appican) is present in brain and is produced by astrocytes but not by neurons in primary neural cultures. J Biol Chem. 1995;270:11839–11844. doi: 10.1074/jbc.270.20.11839. [DOI] [PubMed] [Google Scholar]
- [95].Shortland P, Kinman E, Molander C. Sprouting of A-fibre primary afferents into lamina II in two rat models of neuropathic pain. Eur J Pain. 1997;1:215–227. doi: 10.1016/s1090-3801(97)90107-5. [DOI] [PubMed] [Google Scholar]
- [96].Smith-Thomas LC, Stevens J, Fok-Seang J, Faissner A, Rogers JH, Fawcett JW. Increased axon regeneration in astrocytes grown in the presence of proteoglycan synthesis inhibitors. J Cell Sci. 1995;108:1307–1315. doi: 10.1242/jcs.108.3.1307. [DOI] [PubMed] [Google Scholar]
- [97].Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- [98].Snow DM, Letourneau PC. Neurite outgrowth on a step gradient of chondroitin sulfate proteoglycan (CS-PG) J Neurobiol. 1992;23:322–336. doi: 10.1002/neu.480230311. [DOI] [PubMed] [Google Scholar]
- [99].Snow DM, Atkinson PB, Hassinger TD, Letourneau PC, Kater SB. Chondroitin sulfate proteoglycan elevates cytoplasmic calcium in DRG neurons. Dev Biol. 1994;166:87–100. doi: 10.1006/dbio.1994.1298. [DOI] [PubMed] [Google Scholar]
- [100].Snow DM, Brown EM, Letourneau PC. Growth cone behavior in the presence of soluble chondroitin sulfate proteoglycan (CSPG), compared to behavior on CSPG bound to laminin or fibronectin. Int J Dev Neurosci. 1996;14:331–349. doi: 10.1016/0736-5748(96)00017-2. [DOI] [PubMed] [Google Scholar]
- [101].Snow DM, Mullins N, Hynds DL. Nervous system-derived chondroitin sulfate proteoglycans regulate growth cone morphology and inhibit neurite outgrowth: a light, epifluorescence, and electron microscopy study. Microsc Res Tech. 2001;54:273–286. doi: 10.1002/jemt.1140. [DOI] [PubMed] [Google Scholar]
- [102].Snow DM. Pioneering studies on the mechanisms of axonal regeneration. Dev Neurobiol. 2011;71:785–789. doi: 10.1002/dneu.20904. [DOI] [PubMed] [Google Scholar]
- [103].Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- [104].Tang X, Davies JE, Davies SJ. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J Neurosci Res. 2003;71:427–444. doi: 10.1002/jnr.10523. [DOI] [PubMed] [Google Scholar]
- [105].Tauchi R, Imagama S, Natori T, Ohgomori T, Muramoto A, Shinjo R, Matsuyama Y, Ishiguro N, Kadomatsu K. The endogenous proteoglycan-degrading enzyme ADAMTS-4 promotes functional recovery after spinal cord injury. J Neuroinflammation. 2012;9:53. doi: 10.1186/1742-2094-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Tkachenko E, Rhodes JM, Simons M. Syndecans: new kids on the signaling block. Circ Res. 2005;96:488–500. doi: 10.1161/01.RES.0000159708.71142.c8. [DOI] [PubMed] [Google Scholar]
- [107].Tom VJ, Steinmetz MP, Miller JH, Doller CM, Silver J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J Neurosci. 2004;24:6531–6539. doi: 10.1523/JNEUROSCI.0994-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Tom VJ, Kadakia R, Santi L, Houle JD. Administration of chondroitinase ABC rostral or caudal to a spinal cord injury site promotes anatomical but not functional plasticity. J Neurotrauma. 2009;26:2323–2333. doi: 10.1089/neu.2009.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Treloar HB, Ray A, Dinglasan LA, Schachner M, Greer CA. Tenascin-C is an inhibitory boundary molecule in the developing olfactory bulb. J Neurosci. 2009;29:9405–9416. doi: 10.1523/JNEUROSCI.2356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Verna JM, Fichard A, Saxod R. Influence of glycosaminoglycans on neurite morphology and outgrowth patterns in vitro. Int J Dev Neurosci. 1989;7:389–399. doi: 10.1016/0736-5748(89)90060-9. [DOI] [PubMed] [Google Scholar]
- [111].Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Wang D, Fawcett J. The perineuronal net and the control of CNS plasticity. Cell Tissue Res. 2012;349:147–160. doi: 10.1007/s00441-012-1375-y. [DOI] [PubMed] [Google Scholar]
- [113].Wang H, Katagiri Y, McCann TE, Unsworth E, Goldsmith P, Yu ZX, Tan F, Santiago L, Mills EM, Wang Y, Symes AJ, Geller HM. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci. 2008;121:3083–3091. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Wu Y, Sheng W, Chen L, Dong H, Lee V, Lu F, Wong CS, Lu WY, Yang BB. Versican V1 isoform induces neuronal differentiation and promotes neurite outgrowth. Mol Biol Cell. 2004;15:2093–2104. doi: 10.1091/mbc.E03-09-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Yamada H, Fredette B, Shitara K, Hagihara K, Miura R, Ranscht B, Stallcup WB, Yamaguchi Y. The brain chondroitin sulfate proteoglycan brevican associates with astrocytes ensheathing cerebellar glomeruli and inhibits neurite outgrowth from granule neurons. J Neurosci. 1997;17:7784–7795. doi: 10.1523/JNEUROSCI.17-20-07784.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Yanagishita M. A brief history of proteoglycans. Experientia. 1993;49:366–368. doi: 10.1007/BF01923581. [DOI] [PubMed] [Google Scholar]
- [117].Yu P, Pisitkun T, Wang G, Wang R, Katagiri Y, Gucek M, Knepper MA, Geller HM. Global analysis of neuronal phosphoproteome regulation by chondroitin sulfate proteoglycans. PLoS One. 2013;8:e59285. doi: 10.1371/journal.pone.0059285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Zhang JM, Li H, Munir MA. Decreasing sympathetic sprouting in pathologic sensory ganglia: a new mechanism for treating neuropathic pain using lidocaine. Pain. 2004;109:143–149. doi: 10.1016/j.pain.2004.01.033. [DOI] [PubMed] [Google Scholar]
- [119].Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]


