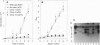Abstract
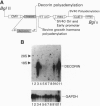
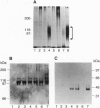
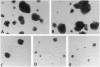
The rapid progress in the cloning of proteoglycan genes has enabled investigators to examine in depth the functional roles these polyhedric molecules play in the control of cell proliferation. Decorin, a leucine-rich proteoglycan expressed by most connective tissues, is a prototype molecule that regulates cellular growth via two mechanisms: modulation of growth factor activity and matrix assembly. We now provide direct evidence that human colon cancer cells stably transfected with decorin cDNA exhibit a marked suppression of the transformed phenotype: the cells have a reduced growth rate in vitro, form small colonies in soft agar, and do not generate tumors in scid/scid mice. Several independent clones are arrested in the G1 phase of the cell cycle, and their growth suppression can be restored by treatment with decorin antisense oligodeoxynucleotides. These effects are independent of growth factors and are not due to either clonal selection or integration site of the decorin gene. These findings correlate well with the observation that decorin gene expression is markedly up-regulated during quiescence. Decorin thus appears to be one component of a negative loop that controls cell growth.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adany R., Heimer R., Caterson B., Sorrell J. M., Iozzo R. V. Altered expression of chondroitin sulfate proteoglycan in the stroma of human colon carcinoma. Hypomethylation of PG-40 gene correlates with increased PG-40 content and mRNA levels. J Biol Chem. 1990 Jul 5;265(19):11389–11396. [PubMed] [Google Scholar]
- Adany R., Iozzo R. V. Hypomethylation of the decorin proteoglycan gene in human colon cancer. Biochem J. 1991 Jun 1;276(Pt 2):301–306. doi: 10.1042/bj2760301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asundi V. K., Dreher K. L. Molecular characterization of vascular smooth muscle decorin: deduced core protein structure and regulation of gene expression. Eur J Cell Biol. 1992 Dec;59(2):314–321. [PubMed] [Google Scholar]
- Bianco P., Fisher L. W., Young M. F., Termine J. D., Robey P. G. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem. 1990 Nov;38(11):1549–1563. doi: 10.1177/38.11.2212616. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Buttke T. M., McCubrey J. A., Owen T. C. Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphokine-dependent cell lines. J Immunol Methods. 1993 Jan 4;157(1-2):233–240. doi: 10.1016/0022-1759(93)90092-l. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coppock D. L., Kopman C., Scandalis S., Gilleran S. Preferential gene expression in quiescent human lung fibroblasts. Cell Growth Differ. 1993 Jun;4(6):483–493. [PubMed] [Google Scholar]
- Danielson K. G., Fazzio A., Cohen I., Cannizzaro L. A., Eichstetter I., Iozzo R. V. The human decorin gene: intron-exon organization, discovery of two alternatively spliced exons in the 5' untranslated region, and mapping of the gene to chromosome 12q23. Genomics. 1993 Jan;15(1):146–160. doi: 10.1006/geno.1993.1022. [DOI] [PubMed] [Google Scholar]
- Dodge G. R., Kovalszky I., Hassell J. R., Iozzo R. V. Transforming growth factor beta alters the expression of heparan sulfate proteoglycan in human colon carcinoma cells. J Biol Chem. 1990 Oct 15;265(29):18023–18029. [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- Iozzo R. V. Biosynthesis of heparan sulfate proteoglycan by human colon carcinoma cells and its localization at the cell surface. J Cell Biol. 1984 Aug;99(2):403–417. doi: 10.1083/jcb.99.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo R. V., Cohen I. R., Grässel S., Murdoch A. D. The biology of perlecan: the multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem J. 1994 Sep 15;302(Pt 3):625–639. doi: 10.1042/bj3020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo R. V., Cohen I. Altered proteoglycan gene expression and the tumor stroma. Experientia. 1993 May 15;49(5):447–455. doi: 10.1007/BF01923588. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V. Neoplastic modulation of extracellular matrix. Colon carcinoma cells release polypeptides that alter proteoglycan metabolism in colon fibroblasts. J Biol Chem. 1985 Jun 25;260(12):7464–7473. [PubMed] [Google Scholar]
- Iozzo R. V., Sampson P. M., Schmitt G. K. Neoplastic modulation of extracellular matrix: stimulation of chondroitin sulfate proteoglycan and hyaluronic acid synthesis in co-cultures of human colon carcinoma and smooth muscle cells. J Cell Biochem. 1989 Apr;39(4):355–378. doi: 10.1002/jcb.240390403. [DOI] [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava H., Häkkinen L., Rahemtulla F. A biochemical analysis of human periodontal tissue proteoglycans. Biochem J. 1992 May 15;284(Pt 1):267–274. doi: 10.1042/bj2840267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppä S., Mali M., Miettinen H. M., Jalkanen M. Syndecan expression regulates cell morphology and growth of mouse mammary epithelial tumor cells. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):932–936. doi: 10.1073/pnas.89.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebersbach B. F., Sanderson R. D. Expression of syndecan-1 inhibits cell invasion into type I collagen. J Biol Chem. 1994 Aug 5;269(31):20013–20019. [PubMed] [Google Scholar]
- Mali M., Andtfolk H., Miettinen H. M., Jalkanen M. Suppression of tumor cell growth by syndecan-1 ectodomain. J Biol Chem. 1994 Nov 11;269(45):27795–27798. [PubMed] [Google Scholar]
- Mauviel A., Santra M., Chen Y. Q., Uitto J., Iozzo R. V. Transcriptional regulation of decorin gene expression. Induction by quiescence and repression by tumor necrosis factor-alpha. J Biol Chem. 1995 May 12;270(19):11692–11700. doi: 10.1074/jbc.270.19.11692. [DOI] [PubMed] [Google Scholar]
- Murdoch A. D., Dodge G. R., Cohen I., Tuan R. S., Iozzo R. V. Primary structure of the human heparan sulfate proteoglycan from basement membrane (HSPG2/perlecan). A chimeric molecule with multiple domains homologous to the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor. J Biol Chem. 1992 Apr 25;267(12):8544–8557. [PubMed] [Google Scholar]
- Murdoch A. D., Liu B., Schwarting R., Tuan R. S., Iozzo R. V. Widespread expression of perlecan proteoglycan in basement membranes and extracellular matrices of human tissues as detected by a novel monoclonal antibody against domain III and by in situ hybridization. J Histochem Cytochem. 1994 Feb;42(2):239–249. doi: 10.1177/42.2.7507142. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991 Mar 8;64(5):867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Santra M., Danielson K. G., Iozzo R. V. Structural and functional characterization of the human decorin gene promoter. A homopurine-homopyrimidine S1 nuclease-sensitive region is involved in transcriptional control. J Biol Chem. 1994 Jan 7;269(1):579–587. [PubMed] [Google Scholar]
- Schaeffer W. I., Friend K. Efficient detection of soft agar grown colonies using a tetrazolium salt. Cancer Lett. 1976 May;1(5):259–262. doi: 10.1016/s0304-3835(75)97506-0. [DOI] [PubMed] [Google Scholar]
- Scholzen T., Solursh M., Suzuki S., Reiter R., Morgan J. L., Buchberg A. M., Siracusa L. D., Iozzo R. V. The murine decorin. Complete cDNA cloning, genomic organization, chromosomal assignment, and expression during organogenesis and tissue differentiation. J Biol Chem. 1994 Nov 11;269(45):28270–28281. [PubMed] [Google Scholar]
- Vogel K. G., Paulsson M., Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984 Nov 1;223(3):587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. W. Gene inhibition using antisense oligodeoxynucleotides. Nature. 1994 Nov 24;372(6504):333–335. doi: 10.1038/372333a0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Mann D. M., Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990 Jul 19;346(6281):281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Ruoslahti E. Expression of human proteoglycan in Chinese hamster ovary cells inhibits cell proliferation. Nature. 1988 Nov 17;336(6196):244–246. doi: 10.1038/336244a0. [DOI] [PubMed] [Google Scholar]