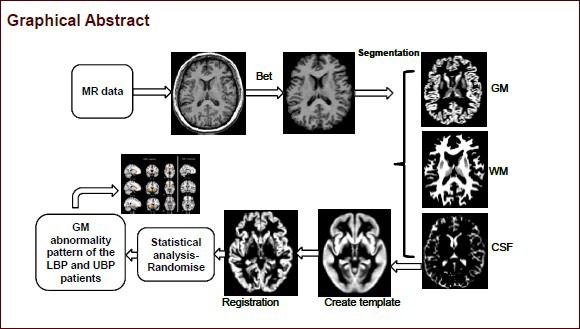
Keywords: neural regeneration, brain injury, chronic low back pain, upper back pain, voxel-based morphometry, gray matter, magnetic resonance imaging, basal ganglia, atrophy, chronic pain, grants-supported paper, neuroregeneration
Abstract
A reduction in gray matter volume is common in patients with chronic back pain, and different types of pain are associated with gray matter abnormalities in distinct brain regions. To examine differences in brain morphology in patients with low back pain or neck and upper back pain, we investigated changes in gray matter volume in chronic back pain patients having different sites of pain using voxel-based morphometry. A reduction in cortical gray matter volume was found primarily in the left postcentral gyrus and in the left precuneus and bilateral cuneal cortex of patients with low back pain. In these patients, there was an increase in subcortical gray matter volume in the bilateral putamen and accumbens, right pallidum, right caudate nucleus, and left amygdala. In upper back pain patients, reduced cortical gray matter volume was found in the left precentral and left postcentral cortices. Our findings suggest that regional gray matter volume abnormalities in low back pain patients are more extensive than in upper back pain patients. Subcortical gray matter volume increases are found only in patients with low back pain.
INTRODUCTION
Accumulating evidence indicates that chronic pain of different etiologies is often associated with distinct gray matter volume reductions in multiple brain regions associated with acute pain processing. Gray matter volume reductions have been observed in chronic back pain[1,2,3,4], various types of headache[5,6,7], fibromyalgia[8,9], complex regional pain syndrome[10], various chronic visceral pains[11,12], chronic pelvic pain syndrome[13], rheumatoid arthritis[14], and other pain disorders[15,16,17]. Brain regions commonly affected in the different subtypes of chronic pain include the cingulate cortex, prefrontal cortex, insular cortex and thalamus[18,19].
While gray matter atrophy critically affects the perception and modulation of chronic pain, distinct gray matter abnormalities are found in the various types of pain. A previous study showed that the impact of low back pain (LBP) on the brain may vary according to the pain type (i.e., neuropathic vs. non-neuropathic)[1], and suggested that structural changes occur more easily in neuropathic pain conditions. However, it remains to be clarified how the pain site contributes to brain structural abnormalities in patients.
Cortical and subcortical reorganization plays an important role in the chronification process of LBP[20]. Although previous studies have suggested that gray matter changes in cortical structures are similar in different chronic pain types, conflicting findings have been reported for subcortical structures, and correlations with pain duration, pain intensity, personality traits, and medications have been inconsistent. Some studies suggested that gray matter volume is decreased in subcortical regions[21,22], while others showed gray matter volume increases[4,6,14,23,24]. Voxel-based morphometry has been widely used to identify morphological changes in patients with various pain disorders. In this study, we performed an anatomical MRI study in patients with LBP or upper back pain (UBP) and their healthy controls to (1) identify whether there are different brain morphological changes between LBP and UBP patients, and (2) to identify correlations between gray matter volume changes and disease-related clinical indices such as pain intensity, pain duration, and psychometric state.
RESULTS
Demographic and psychometric information on the subjects
A total of 30 healthy controls, 30 LBP patients and 15 UBP patients were included in this study. The Hamilton Depression Rating Scale and Hamilton Anxiety Scale scores were significantly higher in LBP patients than in controls (P < 0.01). In UBP patients, the Hamilton Depression Rating Scale and Hamilton Anxiety Scale scores were significantly higher than in controls (P < 0.001, P < 0.01). In both groups, the Montreal Cognitive Assessment scores in patients were lower than in controls (P < 0.01). Detailed psychometric data are shown in Table 1.
Table 1.
Demographic and clinical data on the participants
Gray matter volume reductions in LBP and UBP patients
The results from whole brain voxel-based morphometry analysis showed that, compared with healthy controls, gray matter volume was decreased in several cortical structures in LBP patients, including the left precentral and postcentral cortices, and the bilateral cuneal and left precuneal cortices (P < 0.001, uncorrected; Figure 1). In UBP patients, cortical gray matter volume reductions were found in the right precentral and right postcentral cortices (P < 0.001, uncorrected; Figure 1). No gray matter volume changes were found in subcortical structures in LBP or UBP patients. These gray matter reductions did not withstand correction for multiple comparisons.
Figure 1.
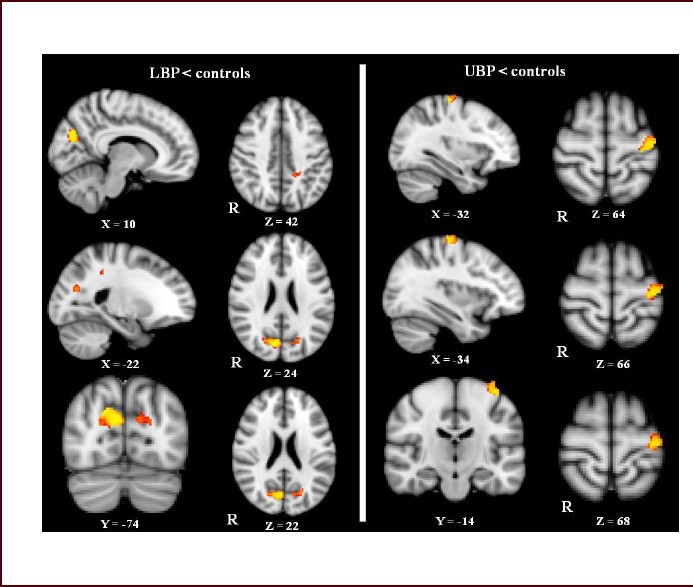
Gray matter volume reductions in cortical structures in low back pain (LBP) and upper back pain (UBP) patients revealed by whole-brain analysis.
In LBP patients, gray matter volume was decreased in the left postcentral cortex and the left precuneus and bilateral cuneal cortex (the upper left side of the picture) after controlling for the effects of age, sex and total intracranial volume.
In UBP patients, a reduction in gray matter volume was only found in the left precentral cortex and part of the left postcentral cortex (the right side of the upper picture).
Between-group differences are represented as statistical maps color-coded on a red-yellow scale, with brighter (more yellow) regions corresponding to more significant differences. Images are presented with right hemisphere structures shown on the right. R: Right.
Region of interest (ROI) voxel-based morphometric analysis revealed gray matter volume reductions in cortical structures in the left postcentral gyrus, left precuneus and bilateral cuneal cortex of LBP patients (P < 0.05, family wise error corrected; Figure 1).
However, in subcortical structures in LBP patients, gray matter volume increases were observed in the bilateral putamen and accumbens, and in the right pallidum and caudate nucleus, as well as in the amygdala in the left hemisphere (P < 0.05, family wise error corrected; Figure 2). In UBP patients, gray matter decreases were mainly in the left precentral cortex, and in part of the left postcentral cortex (Figure 1). No significant gray matter changes were found in subcortical structures (Figure 2). Detailed coordinate data is shown in Table 2.
Figure 2.
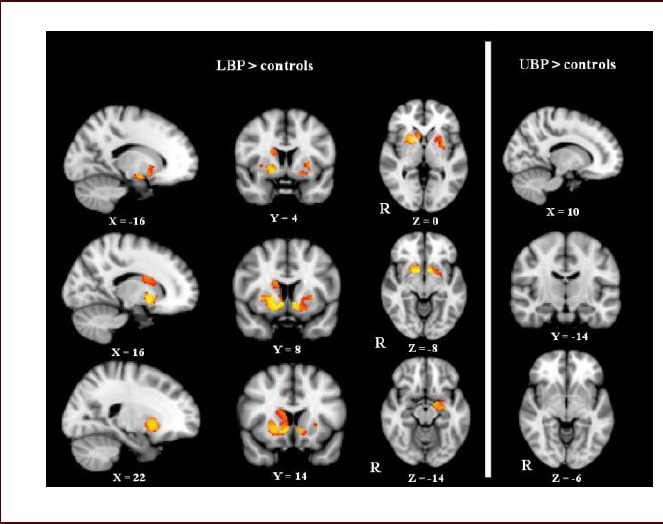
Gray matter volume increases in low back pain (LBP) and upper back pain (UBP) patients revealed by region of interest voxel-based morphometry analyses.
In LBP patients, increased gray matter volume was observed in the bilateral putamen and nucleus accumbens, the left amygdala, the right caudate nucleus and the pallidum (the upper left side of the picture).
In UBP patients, no significant gray matter increases were found in subcortical regions. Between-group differences are represented as statistical maps color-coded on a red-yellow scale, with brighter (more yellow) regions corresponding to more significant differences. R: Right.
Table 2.
Gray matter changes in low back pain (LBP) and upper back pain (UBP) patients revealed by voxel-based morphometry
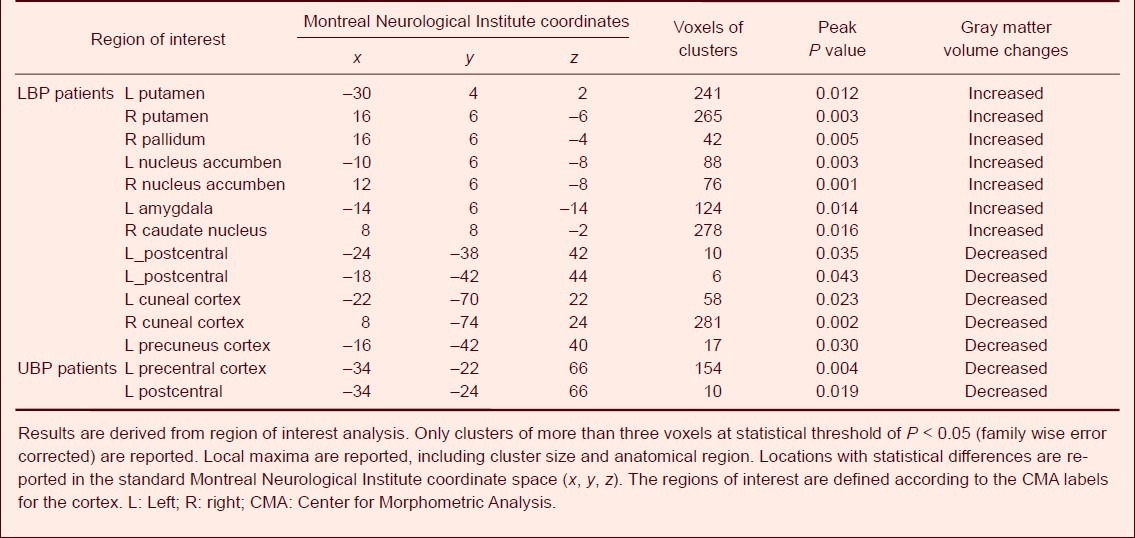
Correlations between gray matter abnormalities and psychometric variables in LBP and UBP patients
For LBP patients, no significant correlations were found between gray matter abnormalities and psychometric variables. For UBP patients, gray matter density in the bilateral insular cortex was negatively correlated with clinical pain intensity (short-form McGill pain questionnaire scores, Pearson correlation: r = −0.195, P < 0.05) and Hamilton Anxiety Scale scores (Pearson correlation: r = −0.436, P < 0.05; Figure 3A, B). Meanwhile, the pain intensity in UBP patients was positively correlated with Hamilton Depression Rating Scale scores (Pearson correlation: r = 0.691, P < 0.001; Figure 3C) and Hamilton Anxiety Scale scores (Pearson correlation: r = 0.612, P < 0.05; Figure 3D). In both LBP and UBP patients, a positive correlation was found between Hamilton Depression Rating Scale and Hamilton Anxiety Scale scores (Spearman rank correlation: r = 0.803, P = 0.000, Figure 4A; Pearson correlation: r = 0.792, P = 0.000, Figure 4B). Furthermore, a positive correlation was found between the Mini-Mental State examination and Montreal Cognitive Assessment scores in LBP patients (Spearman rank correlation: r = 0.645, P = 0.000; Figure 4C), but not in UBP patients (Pearson correlation: r = 0.365, P = 0.181; Figure 4D).
Figure 3.
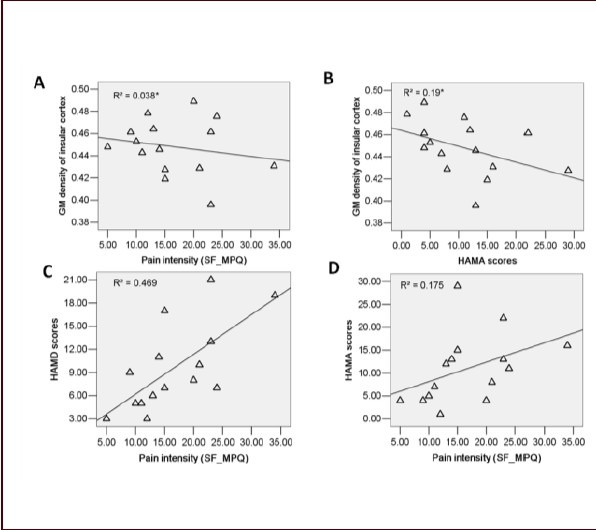
Correlation between gray matter density in the bilateral insular cortex, pain intensity and psychometric variables in upper back pain (UBP) patients (Pearson correlation).
(A) Negative correlation between clinical pain intensity and gray matter density in the bilateral insular cortex is observed in UBP patients (r = −0.195, P < 0.05). (B) Negative correlation between HAMA scores and gray matter density in the insular cortex is observed in UBP patients (r = −0.436, P < 0.05). (C) Positive correlation between clinical pain intensity and HAMD scores is observed in UBP patients (r = 0.691, P < 0.001). (D) Positive correlation between pain intensity and HAMA scores is observed in UBP patients (r = 0.612, P < 0.05).
R2: Calculated from multivariate linear regression; r: correlation coefficient; SF_MPQ: short-form McGill pain questionnaire; HAMD: Hamilton Depression Rating Scale; HAMA: Hamilton Anxiety Scale.
Figure 4.
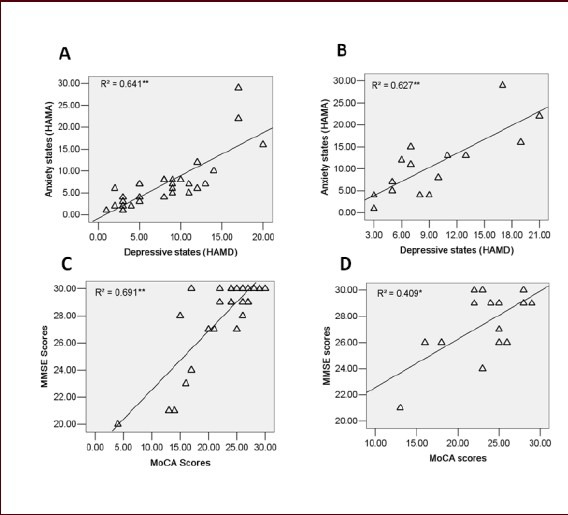
Correlations between psychological variables in low back pain (LBP) and upper back pain (UBP) patients.
Positive correlation between affective and cognitive scores was revealed by correlation analyses.
(A) Positive correlation between HAMD scores and HAMA scores in LBP patients (Spearman rank test: r = 0.803, P < 0.001). (B) Positive correlation between HAMD scores and HAMA scores in UBP patients (Spearman rank test: r = 0.792, P < 0.001). (C) Positive correlation between MMSE and MoCA scores in LBP patients (Pearson coefficient: r = 0.645, P < 0.001). (D) The correlations between MMSE and MoCA scores are not significant in UBP patients (Spearman rank test: r = 0.365, P > 0.05).
R2: Calculated from multivariate linear regression; r: correlation coefficient; MMSE: Mini-Mental State examination; HAMD: Hamilton Depression Rating Scale; HAMA: Hamilton Anxiety Scale; MoCA: Montreal Cognitive Assessment.
DISCUSSION
Our results indicate that gray matter volume alterations are more extensive in LBP patients than in UBP patients. The LBP patients showed reduced gray matter volume in cortical structures, and increased gray matter volume in subcortical structures. In contrast, chronic neck and UBP patients had decreased gray matter volumes only in cortical brain areas. In UBP patients, the gray matter density of the insular cortex displayed a negative correlation with the short-form McGill pain questionnaire and Hamilton Anxiety Scale scores. Robust gray matter volume abnormalities in the basal ganglia were observed in LBP patients, but not in UBP patients.
In LBP patients, cortical gray matter volume reductions were found in the bilateral cuneal and left precuneal cortices, and in the left precuneal and left postcentral cortices. In UBP patients, reduced gray matter volumes were found in the somatic motor and somatosensory cortices. No gray matter losses were found in the thalamus, insular or prefrontal cortex, although they were often observed in previous studies[1,2,25,26,27]. These inconsistencies may be related to subject variability and differences in analytical technique. Abnormalities in the cuneal cortex have been rarely reported in previous studies[21]. The precuneus has widespread connections with the thalamus, caudate nucleus and putamen[28]. The precuneus is a central hub within the default mode network. Chronic back pain-induced disruption of the default-mode network has been found previously[29]. We suspect that changes in the default mode network might contribute to the observed gray matter abnormalities. Gray matter volume decreases in the somatic motor and somatosensory cortices (precentral and postcentral cortices) in LBP/UBP patients might be related to motor dysfunction.
Subcortical gray matter volume increases in LBP patients were mainly located in the basal ganglia (bilateral putamen and pallidum, right pallidum and right caudate nucleus) and left amygdala. The basal ganglia, thalamus, hippocampus and amygdala are major subcortical structures[27]. Preclinical and clinical data implicate these regions in pain processing[30]. The basal ganglia are located close to the thalamus and have intimate and highly specific afferent and efferent connections with the cerebral cortex[31]. The thalamo-cortico-basal ganglia loop is the main loop affected by pain[32,33]. Gray matter increases in the posterior putamen and globus pallidus have been found in other studies[34,35,36]. The asymmetry of the subcortical nuclei increases with age[34,35], which might help to explain why changes in subcortical nuclei are not always bilateral. In UBP patients, no significant structural abnormalities were found in the subcortical brain regions. However, it is possible that subtle differences in some pain-related brain areas between UBP patients and healthy controls might be beyond the sensitivity of voxel-based morphometry after threshold-free cluster enhancement correction.
The amygdala is an important site of interaction between pain and negative affective states. A reciprocal relationship has been found between persistent pain and negative affective states such as fear, anxiety and depression (i.e., plastic changes in nociceptive neurons in the amygdala can be induced by pain-related sensitization)[37]. Synaptic plasticity and increased neuronal excitability in the amygdala in neuropathic pain has been demonstrated by Ikeda et al[37,38]. The reversibility of gray matter volume increases has been observed in patients with osteoarthritis, and the normalization of amygdala volume to control levels parallels reductions in depression and anxiety scores[39]. We hypothesize that structural abnormalities in the left amygdala in LBP patients might be associated with synaptic plasticity, and may be related to a longer-lasting chronic pain state. The caudate and putamen are the main recipients of input to the basal ganglia, and are targeted by axonal projections from nearly all parts of the cortex. The caudate nucleus might play a critical role in the planning and execution of strategies and behavior required for achieving complex goals[40]. In comparison, the putamen appears to subserve cognitive functions primarily associated with stimulus-response, or habit, learning. We found increased gray matter volumes in the right caudate of LBP patients. Similar results have been found in chronic vulvar pain[41]. We conjecture that the gray matter alterations in the caudate and putamen might be related to the learning of chronic pain.
Reports from functional imaging studies suggest that the basal ganglia may be involved in most aspects of pain processing, including the sensory-discriminative, emotional/affective and cognitive dimension of pain and pain modulation[30,42]. A magnetic resonance spectroscopy study showed that levels of choline-containing compounds are increased in the basal ganglia in chronic fatigue syndrome[43,44]. The authors argued that this might be an indicator of higher cell membrane turnover due to gliosis or altered intramembrane signaling.
The cellular mechanisms underlying the gray matter morphological changes remain unclear. An increased number of glial cells might account for the increased gray matter volume in the basal ganglia[6]. We hypothesize that an increase in the number of glial cells might contribute to the gray matter volume increases in the basal ganglia in LBP patients. Another factor contributing to the MRI-detectable increases in gray matter volume might be neuroinflammation[45,46]. Microglial activation might be triggered and modulated by Toll-like receptors, which could underlie both abnormal gray matter increases and the maintenance of gray matter volume. Another hypothesis is that the gray matter abnormalities in chronic pain patients might represent neuronal plasticity[47]. Diffusion tensor imaging can provide useful information about the axonal properties in human brain. Combined voxel-based morphometry and diffusion tensor imaging has helped to demonstrate regional decreased gray mater density and regional white matter abnormalities, manifested as a change in fractional anisotropy, in the patients.
The pathophysiology and pathoanatomy of LBP and UBP remain unclear. However, heavy physical workload, sitting for a long time, extended computer use, and elevated body mass index are all relevant factors[48]. Compared with LBP, UBP is not a major public health problem. LBP has a greater impact on operational capability than neck or upper back pain. This might account for the different patterns of gray matter abnormalities among these conditions.
The biopsychosocial approach has also furthered our understanding and treatment of chronic pain disorders. It is becoming clear that not only pain, but also psychological and social factors, can interact with brain processes[34,49,50,51]. In the future, more attention should be placed on cognitive, affective, behavioral, and homeostatic factors.
Pain intensity might also be a factor affecting gray matter density in the insular cortex. Although in previous studies, specific morphological alterations in brain structures may have correlated with the intensity and unpleasantness of pain, no similar correlations were found between voxel-based morphometry data and pain characteristics in LBP patients in this study. This might be because the reporting of pain intensity relies on subjective memories of past pain, rather than on medical records. Cognition and emotion might be another contributing factor to the gray matter abnormalities because chronic back pain is associated with cognitive deficits, which may impact everyday behavior, particularly in emotional situations[52].
Although increasing evidence supports the association of chronic pain with gray matter abnormalities, the cause-effect relationships have not been established for chronic back pain and increased gray matter volumes in the subcortical areas. Consequently, longitudinal studies using a large group of patients, ideally of single etiology, are necessary to explore the potential reversibility of gray matter changes in the basal ganglia.
A major limitation of this study is the unequal number of subjects in the LBP and UBP groups. The group of UBP patients was rather small (n = 15), which might partly influence our results. Secondly, medication, including the use of antinociceptive drugs, and confounding psychosocial factors (depression and anxiety) might also have affected our results. Thirdly, we did not differentiate between neuropathic and non-neuropathic pain. Thus, future studies should dissect the impact of the various pain types on brain structures. Moreover, the gray matter increases and decreases in this study need to be considered in light of previous reports and the cellular mechanisms underlying these neuroanatomic changes.
In summary, the present study suggests that regional gray matter volume abnormalities in LBP patients are more extensive than in UBP patients, and that the distinct etiologies of the various types of chronic pain might underlie this difference. Gray matter volume reductions occurred in both UBP and LBP patients, while subcortical gray matter volume increases occurred only in the patients with LBP. The gray matter volume increase in the basal ganglia of LBP patients might be a reflection of the adaptation of neurons, not necessarily an atrophy, reflecting a potential dysfunction of execution and motor function. Negative affective states and concomitant symptoms of LBP/UBP might also be potential factors impacting gray matter abnormalities. Longitudinal studies with a large sample size are necessary to investigate the potential reversibility of gray matter changes in LBP and UBP patients.
SUBJECTS AND METHODS
Design
A case-control imaging study.
Time and setting
The study was performed at the First Affiliated Hospital, School of Medicine, Xi’an Jiaotong University, China. MRI data collection was done from March 2012 to June 2013.
Subjects
All patients were recruited from the Outpatient Clinic, Department of Pain, the First Affiliated Hospital, School of Medicine, Xi’an Jiaotong University in China. They were diagnosed by experienced clinicians according to the International Association for the Study of Pain criteria for chronic pain[53]. The duration of pain ranged from 3 months to 40 years. All patients continued their normal medication process for pain. All the healthy controls were recruited through advertised poster.
Inclusion criteria: (1) aged 20–65 years; (2) education ≥ 6 years, subjects are required to be able to complete the questionnaires; (3) could remain stationary in the MR scanner; (4) be free of contraindications for MRI study (e.g., metal implants or claustrophobia).
Exclusion criteria: (1) have contraindications for MRI study (e.g., metal implants or claustrophobia); (2) have dentures; or (3) have severe concomitant neurological or psychiatric disorders, or other diseases, such as hypertension, diabetes or coronary disease.
Three subgroups of right-handed participants were included: 30 patients with chronic LBP (aged 34–65 years); 15 patients with neck and UBP (aged 30–70 years); and 30 pain-free, age- and sex-matched healthy volunteers (aged 38–63 years). Fifteen age- and sex-matched healthy controls (aged 38–62 years) selected from the 30 healthy subjects were used as the control group for UBP patients. All patients gave written informed consent.
Methods
Assessment of clinical pain and cognitive state
The intensity of pain was assessed using the Short-Form McGill Pain Questionnaire[54], in which the intensity of pain was rated on a visual analog scale ranging from 0–10 (0: no pain; 10: maximum imaginable pain) on scan day. The duration of pain was measured in years. The affective and cognitive conditions in all groups were evaluated using the following questionnaires: Mini-Mental State examination[55], Montreal Cognitive Assessment[56], Hamilton Depression Rating Scale and Hamilton Anxiety Scale[57].
MRI data acquisition
MRI was performed on a 3.0 T MRI system (General Electric Signa HDXT, Milwaukee, WI, USA) using a three-dimensional T1-weighted fast spoiled gradient echo sequence with the following parameters: repetition time = 10.8 ms, echo time = 4.8 ms, matrix = 256 × 256, field of view = 256 mm × 256 mm, slice thickness = 1 mm, space between slices = 0, 140 axial slices, voxel size = 1 mm × 1 mm × 1 mm, scan duration = 5 minutes. Routine T2-weighted images: repetition time = 4 680.0 ms, echo time = 105.2 ms, matrix size = 256 × 256, field of view = 256 mm × 256 mm, slice thickness = 5 mm. T1 weighted imaging: repetition time = 2 360.4 ms, echo time = 21.9 ms, matrix = 256 × 256, field of view = 256 mm × 256 mm, slice thickness = 5 mm. Routine T1- and T2-weighted images were analyzed for the presence of lesions. All images were visually inspected by two neuroradiologists, and those with excessive motion artifacts were excluded.
MRI data processing
Structural MRI data of the brain were analyzed with FMRIB Software Library's voxel-based morphometry function (version 5.0; http://www.fmrib.ox.ac.uk/fsl/fslvbm/index.html; Oxford University, Oxford, United Kingdom)[58,59,60]. First, structural images were brain-extracted using BET[61]. Next, tissue-type segmentation was carried out using FAST4[36]. The resulting gray-matter partial volume images were then aligned to MNI152 standard space using the affine registration tool FLIRT[62,63], followed optionally by nonlinear registration using FNIRT[64,65], which uses a b-spline representation of the registration warp field. The resulting images were averaged to create a study-specific template, to which the native grey matter images were then non-linearly re-registered. The registered partial volume images were then modulated (to correct for local expansion or contraction) by dividing by the Jacobian of the warp field. The modulated segmented images were then smoothed with an isotropic Gaussian kernel with a sigma of 3 mm.
Group differences in whole-brain gray matter between LBP/UBP patients and corresponding healthy controls were examined using permutation-based nonparametric testing (5 000 permutations) within the general linear model, with age, sex and total intracranial volume as covariates, correcting for multiple comparisons by implimenting threshold-free cluster enhancement. Total intracranial volume was calculated as the sum of gray matter, white matter and cerebrospinal fluid volumes from FSL Brain Extraction Tool segmentations. After whole-brain voxel-based morphometry analysis, specific regions of interest (ROIs) analyses were performed on the brain cortical regions containing eight ROIs: bilateral frontal lobe, anterior cingulate cortex, temporal lobe, precentral and postcentral cortices, and the insular, cuneal, and precuneal cortices, as defined by the Harvard-Oxford cortical structure probabilistic atlas (www.fmrib.ox.ac.uk/fsl/fslview/). For subcortical structures, ROIs included the bilateral caudate nucleus, putamen, accumbens, pallidum, thalamus, amygdala and hippocampus, as defined by the Harvard-Oxford subcortical structure probabilistic atlas (www.fmrib.ox.ac.uk/fsl/fslview/), based on an adaptation of the MNI atlases by the Center for Morphometric Analysis[66]. Results were considered to be significant at P < 0.05 (5 000 permutations, family wise error corrected). The clusters of significance were localized using an adaptation of the MNI and Talairach atlases. In this study, only the results from ROI voxel-based morphometry analysis were provided.
Finally, we extracted the regional gray matter density of the upper ROIs from the gray matter clusters identified in the cohort analyses, for both LBP and UBP patients, which yielded an average gray matter density for that region for each person. We compared the differences in gray matter density between LBP/UBP patients and their corresponding healthy controls.
Statistical analysis
Statistical analysis was performed with SPSS 13.0 software (SPSS, Chicago, IL, USA). Demographic, clinical, and MR imaging differences between the groups were tested using Student's t-test and the Mann-Whitney rank sum test, as appropriate. Results were considered significant at P < 0.05. Two independent-samples t-test was used to compare differences between groups for demographic data, and Mann-Whitney rank sum test for Mini-Mental State examination, Montreal Cognitive Assessment and Hamilton Depression Rating Scale scores. Pearson, Spearman rank correlation and multi-variate linear regression were used to assess the relationships between the pain characteristics, psychometric variables and the voxel-based morphometry data in both LBP and UBP patients, according to the normality of the relative data. The psychometric data included: the duration of disease; the pain intensity (scores of visual analog scale and short-form McGill pain questionnaire); the Mini-Mental State examination, Montreal Cognitive Assessment, Hamilton Depression Rating Scale and Hamilton Anxiety Scale scores. For all the non-FSL analysis a significance level of P < 0.05 was considered statistically significant.
Research background: Different subtypes of chronic pain produce different response patterns in the brain.
Research frontiers: Different subtypes of chronic pain present with different alterations in brain structure. Although previous studies compared abnormalities found in neuropathic pain and non-neuropathic pain, the relationship between structural differences and the position of the pain on the body had not been analyzed.
Clinical significance: To provide insight into the neural processing mechanisms among the different subtypes of chronic pain, and to provide the empirical foundation for comprehensive and individualized therapy for chronic pain based on image analysis.
Academic terminology: Voxel-based morphometry can reflect anatomical differences through quantitative calculation of voxel numbers, white or gray matter density or volume. It is an automatic, comprehensive, objective analysis technology based on structural brain magnetic resonance imaging. Voxel-based morphometry can accurately perform morphological analysis on the brain in vivo.
Peer review: This report is unique in that it attempts to observe regional differences in brain structure between two types of back pain using voxel-based morphometry, thus providing the basis for understanding the underlying pathological mechanisms and for the longitudinal monitoring of chronic back pain patients.
Acknowledgments
We thank all the colleagues in the Department of Radiology, the First Affiliated Hospital, College of Medicine, Xi'an Jiaotong University, for their kind help in data acquisition and data processing, and we thank Professor Yang XL from the Department of Pain, the First Affiliated Hospital, College of Medicine, Xi'an Jiaotong University, for his kind advice on experimental design.
Footnotes
Funding: This work was supported partially by two grants from the National Natural Science Foundation of China, No. 30870686 and 81371530.
Conflicts of interest: None declared.
Ethical approval: The study was approved by the Local Research Ethics Committee of the First Affiliated Hospital, School of Medicine, Xi’an Jiaotong University, China.
(Reviewed by Patel B, Raye W, Zhang L, Jia DL)
(Edited by Yu J, Yang Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Holm JW, Hartvigsen J, Lings S, et al. Modest associations between self-reported physical workload and neck trouble: a population-based twin control study. Int Arch Occup Environ Health. 2013;86(2):223–231. doi: 10.1007/s00420-012-0755-7. [DOI] [PubMed] [Google Scholar]
- [2].Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24(46):10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Buckalew N, Haut MW, Morrow L, et al. Chronic pain is associated with brain volume loss in older adults: preliminary evidence. Pain Med. 2008;9(2):240–248. doi: 10.1111/j.1526-4637.2008.00412.x. [DOI] [PubMed] [Google Scholar]
- [4].Ivo R, Nicklas A, Dargel J, et al. Brain structural and psychometric alterations in chronic low back pain. Eur Spine J. doi: 10.1007/s00586-013-2692-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schmidt-Wilcke T, Leinisch E, Gänssbauer S, et al. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125(1-2):89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- [6].Kim JH, Suh SI, Seol HY, et al. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008;28(6):598–604. doi: 10.1111/j.1468-2982.2008.01550.x. [DOI] [PubMed] [Google Scholar]
- [7].Schmidt-Wilcke T, Gänssbauer S, Neuner T, et al. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia. 2008;28(1):1–4. doi: 10.1111/j.1468-2982.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- [8].Schmidt-Wilcke T, Leinisch E, Straube A, et al. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65(9):1483–1486. doi: 10.1212/01.wnl.0000183067.94400.80. [DOI] [PubMed] [Google Scholar]
- [9].Kuchinad A, Schweinhardt P, Seminowicz DA, et al. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27(15):4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Robinson ME, Craggs JG, Price DD, et al. Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. J Pain. 2011;12(4):436–443. doi: 10.1016/j.jpain.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Geha PY, Baliki MN, Harden RN, et al. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60(4):570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Agostini A, Benuzzi F, Filippini N, et al. New insights into the brain involvement in patients with Crohn's disease: a voxel-based morphometry study. Neurogastroenterol Motil. 2013;25(2):147–e82. doi: 10.1111/nmo.12017. [DOI] [PubMed] [Google Scholar]
- [13].Farmer MA, Chanda ML, Parks EL, et al. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2011;186(1):117–124. doi: 10.1016/j.juro.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006–1014. doi: 10.1016/j.pain.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wartolowska K, Hough MG, Jenkinson M, et al. Structural changes of the brain in rheumatoid arthritis. Arthritis Rheum. 2012;64(2):371–379. doi: 10.1002/art.33326. [DOI] [PubMed] [Google Scholar]
- [16].Moayedi M, Weissman-Fogel I, Salomons TV, et al. Abnormal gray matter aging in chronic pain patients. Brain Res. 2012;1456:82–93. doi: 10.1016/j.brainres.2012.03.040. [DOI] [PubMed] [Google Scholar]
- [17].Ruscheweyh R, Deppe M, Lohmann H, et al. Pain is associated with regional grey matter reduction in the general population. Pain. 2011;152(4):904–911. doi: 10.1016/j.pain.2011.01.013. [DOI] [PubMed] [Google Scholar]
- [18].Valet M, Gündel H, Sprenger T, et al. Patients with pain disorder show gray-matter loss in pain-processing structures: a voxel-based morphometric study. Psychosom Med. 2009;71(1):49–56. doi: 10.1097/PSY.0b013e31818d1e02. [DOI] [PubMed] [Google Scholar]
- [19].May A. Chronic pain may change the structure of the brain. Pain. 2008;137(1):7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- [20].Absinta M, Rocca MA, Colombo B, et al. Selective decreased grey matter volume of the pain-matrix network in cluster headache. Cephalalgia. 2012;32(2):109–115. doi: 10.1177/0333102411431334. [DOI] [PubMed] [Google Scholar]
- [21].Gustin SM, Peck CC, Wilcox SL, et al. Different pain, different brain: thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes. J Neurosci. 2011;31(16):5956–5964. doi: 10.1523/JNEUROSCI.5980-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schmidt-Wilcke T, Luerding R, Weigand T, et al. Striatal grey matter increase in patients suffering from fibromyalgia--a voxel-based morphometry study. Pain. 2007;132(Suppl 1):S109–116. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- [23].Younger JW, Shen YF, Goddard G, et al. Chronic myofascial temporomandibular pain is associated with neural abnormalities in the trigeminal and limbic systems. Pain. 2010;149(2):222–228. doi: 10.1016/j.pain.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Davis KD, Pope G, Chen J, et al. Cortical thinning in IBS: implications for homeostatic, attention, and pain processing. Neurology. 2008;70(2):153–154. doi: 10.1212/01.wnl.0000295509.30630.10. [DOI] [PubMed] [Google Scholar]
- [25].Geuze E, Westenberg HG, Heinecke A, et al. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage. 2008;41(3):675–681. doi: 10.1016/j.neuroimage.2008.03.007. [DOI] [PubMed] [Google Scholar]
- [26].Jovicich J, Czanner S, Han X, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46(1):177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- [28].Baliki MN, Geha PY, Apkarian AV, et al. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28(6):1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Borsook D, Upadhyay J, Chudler EH, et al. A key role of the basal ganglia in pain and analgesia--insights gained through human functional imaging. Mol Pain. 2010;6:27. doi: 10.1186/1744-8069-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst. 2002;18(8):386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- [31].Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- [32].Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- [33].Greenberg DL, Messer DF, Payne ME, et al. Aging, gender, and the elderly adult brain: an examination of analytical strategies. Neurobiol Aging. 2008;29(2):290–302. doi: 10.1016/j.neurobiolaging.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yamashita K, Yoshiura T, Hiwatashi A, et al. Volumetric asymmetry and differential aging effect of the human caudate nucleus in normal individuals: a prospective MR imaging study. J Neuroimaging. 2011;21(1):34–37. doi: 10.1111/j.1552-6569.2009.00403.x. [DOI] [PubMed] [Google Scholar]
- [35].Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- [36].Neugebauer V, Li W, Bird GC, et al. The amygdala and persistent pain. Neuroscientist. 2004;10(3):221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- [37].Ikeda R, Takahashi Y, Inoue K, et al. NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain. 2007;127(1-2):161–172. doi: 10.1016/j.pain.2006.09.003. [DOI] [PubMed] [Google Scholar]
- [38].Gwilym SE, Filippini N, Douaud G, et al. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum. 2010;62(10):2930–2940. doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- [39].Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86(3):141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- [40].Schweinhardt P, Kuchinad A, Pukall CF, et al. Increased gray matter density in young women with chronic vulvar pain. Pain. 2008;140(3):411–419. doi: 10.1016/j.pain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- [41].May A. Structural brain imaging: a window into chronic pain. Neuroscientist. 2011;17(2):209–220. doi: 10.1177/1073858410396220. [DOI] [PubMed] [Google Scholar]
- [42].Bingel U, Gläscher J, Weiller C, et al. Somatotopic representation of nociceptive information in the putamen: an event-related fMRI study. Cereb Cortex. 2004;14(12):1340–1345. doi: 10.1093/cercor/bhh094. [DOI] [PubMed] [Google Scholar]
- [43].Chaudhuri A, Condon BR, Gow JW, et al. Proton magnetic resonance spectroscopy of basal ganglia in chronic fatigue syndrome. Neuroreport. 2003;14(2):225–228. doi: 10.1097/00001756-200302100-00013. [DOI] [PubMed] [Google Scholar]
- [44].Grachev ID, Fredrickson BE, Apkarian AV. Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. Pain. 2000;89(1):7–18. doi: 10.1016/S0304-3959(00)00340-7. [DOI] [PubMed] [Google Scholar]
- [45].DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10(1):40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- [46].Guo LH, Schluesener HJ. The innate immunity of the central nervous system in chronic pain: the role of Toll-like receptors. Cell Mol Life Sci. 2007;64(9):1128–1136. doi: 10.1007/s00018-007-6494-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- [48].Heuch I, Heuch I, Hagen K, et al. Body mass index as a risk factor for developing chronic low back pain: a follow-up in the Nord-Trøndelag Health Study. Spine (Phila Pa 1976) 2013;38(2):133–139. doi: 10.1097/BRS.0b013e3182647af2. [DOI] [PubMed] [Google Scholar]
- [49].Gatchel RJ, Peng YB, Peters ML, et al. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133(4):581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- [50].Grachev ID, Fredrickson BE, Apkarian AV. Brain chemistry reflects dual states of pain and anxiety in chronic low back pain. J Neural Transm. 2002;109(10):1309–1334. doi: 10.1007/s00702-002-0722-7. [DOI] [PubMed] [Google Scholar]
- [51].Moayedi M, Weissman-Fogel I, Crawley AP, et al. Contribution of chronic pain and neuroticism to abnormal forebrain gray matter in patients with temporomandibular disorder. Neuroimage. 2011;55(1):277–286. doi: 10.1016/j.neuroimage.2010.12.013. [DOI] [PubMed] [Google Scholar]
- [52].Apkarian AV, Sosa Y, Krauss BR, et al. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108(1-2):129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- [53].Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1–226. [PubMed] [Google Scholar]
- [54].Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- [55].Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- [56].Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- [57].Endicott J, Cohen J, Nee J, et al. Hamilton Depression Rating Scale. Extracted from Regular and Change Versions of the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry. 1981;38(1):98–103. doi: 10.1001/archpsyc.1981.01780260100011. [DOI] [PubMed] [Google Scholar]
- [58].Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- [59].Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- [60].Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- [61].Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- [63].Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- [64].Andersson JLR, Jenkinson M, Smith S, et al. Oxford (UK): FMRIB Centre; 2007. Non-linear optimisation. FMRIB Technical Report TR07JA1. [Google Scholar]
- [65].Andersson JL, Jenkinson M, Smith S. Oxford: FMRIB Analysis Group of the University of Oxford; 2007. Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. [Google Scholar]
- [66].Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]


