Keywords: neural regeneration, brain injury, Rho-associated protein kinase, neurite outgrowth, microtubule, remodeling, vinculin, neuron, hippocampus, lysophosphatidic acid, Y-27632, grants-supported paper, neuroregeneration
Abstract
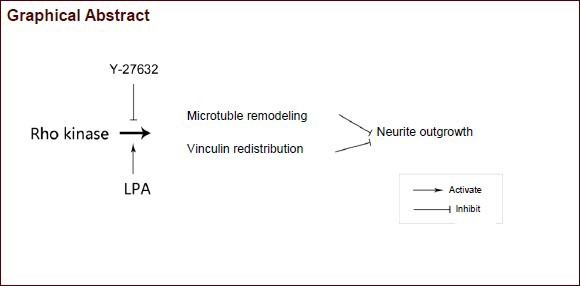
Rho-associated protein kinase is an essential regulator of cytoskeletal dynamics during the process of neurite extension. However, whether Rho kinase regulates microtubule remodeling or the distribution of adhesive proteins to mediate neurite outgrowth remains unclear. By specifically modulating Rho kinase activity with pharmacological agents, we studied the morpho-dynamics of neurite outgrowth. We found that lysophosphatidic acid, an activator of Rho kinase, inhibited neurite outgrowth, which could be reversed by Y-27632, an inhibitor of Rho kinase. Meanwhile, reorganization of microtubules was noticed during these processes, as indicated by their significant changes in the soma and growth cone. In addition, exposure to lysophosphatidic acid led to a decreased membrane distribution of vinculin, a focal adhesion protein in neurons, whereas Y-27632 recruited vinculin to the membrane. Taken together, our data suggest that Rho kinase regulates rat hippocampal neurite growth and microtubule formation via a mechanism associated with the redistribution of vinculin.
INTRODUCTION
The morphology of a neuron is critical to its function. Neurons extend a long axon and several shorter dendrites to form neuronal circuits that transmit neuronal signals in the nervous system. Current knowledge indicates that the cytoskeleton shapes the morphology of a neuron. However, the cytoskeleton is a very dynamic and closely regulated system[1]. The cytoskeleton regulates neuronal polarity by not only forming the structural scaffold underlying cell shape, but also by organizing the polarized cell interior and intracellular transport[2,3]. Actin filaments regulate the shape and directed growth of the growth cone while microtubules provide structure to an axon shaft, both of which are essential for neurite extension[4].
The Rho GTPase family members mainly include the Rho, Rac and Cdc42 subgroups[5,6]. These members function as binary molecular switches by cycling between GTP- and GDP-bound states, and play important roles in neuronal differentiation, migration and polarity[7,8]. In particular, Rho- associated protein kinase (ROCK), a serine/ threonine kinase and one of the major downstream effectors of the small GTPase, RhoA, is involved in many aspects of neuronal function, including neurite outgrowth and retraction[9,10]. As a negative regulator of neurite outgrowth, ROCK activation impairs neurite outgrowth whereas ROCK inhibition promotes neurite outgrowth[11,12]. Although ROCK is mainly associated with actin cytoskeleton dynamics, the involvement of microtubules cannot be excluded during neurite formation and outgrowth[13]. Stabilized microtubules are important for maintaining neuronal morphology, and an appropriate level of microtubule dynamics is critical for neurite formation and outgrowth[13]. In response to external signals, ROCK induces microtubule polymerization and depolymerization, thus impacting neuronal polarity and neurite outgrowth[14,15].
Microtubules are important in the formation and maintenance of neuronal polarity, and their rapid turnover (microtubule dynamics) facilitates remodeling of the cytoskeleton in response to environmental cues[16]. A variety of studies have shown that Rho signaling regulates microtubules through the Rho effector, mDia1, to generate stabilized microtubules[17,18].
Vinculin is an adhesion protein that plays a central role in the mechanical coupling of integrins to the cytoskeleton, as well as in the control of cytoskeletal mechanics, cell shape, protrusion amplitude and cell motility[19,20]. Vinculin interacts with other cytoskeletal proteins, including talin and actin, to mediate cell adhesion[21,22]. Moreover, vinculin has been reported to correlate with ROCK activity in regulating parietal endoderm migration[20] and focal adhesions[23]. Based on this knowledge, we hypothesized that the modulation of ROCK activity would impact neurite outgrowth by inducing the remodeling of microtubules and terminal anchoring proteins. Here, by using the ROCK inhibitor, Y-27632, and the agonist, lysophosphatidic acid, we observed that neurite outgrowth, microtubule remodeling and vinculin redistribution were tightly regulated by ROCK.
RESULTS
Modulation of ROCK activity changed neurite outgrowth behavior in cultured hippocampal neurons
To study the effect of ROCK on neurite outgrowth, hippocampal neurons were observed under phase contrast time-lapse microscopy on 24-well culture plates. Three days after plating, hippocampal neurons gradually extended several unequal processes, one of which had an enlarged growth cone at the leading edge (Figure 1A). The neurites shortened following treatment with lysophosphatidic acid for 1 hour, which persisted for several hours after switching to normal culture medium without lysophosphatidic acid (Figure 1B). Following lysophosphatidic acid treatment, we added the ROCK inhibitor, Y-27632, in the absence of lysophosphatidic acid for an additional 1 hour.
Figure 1.
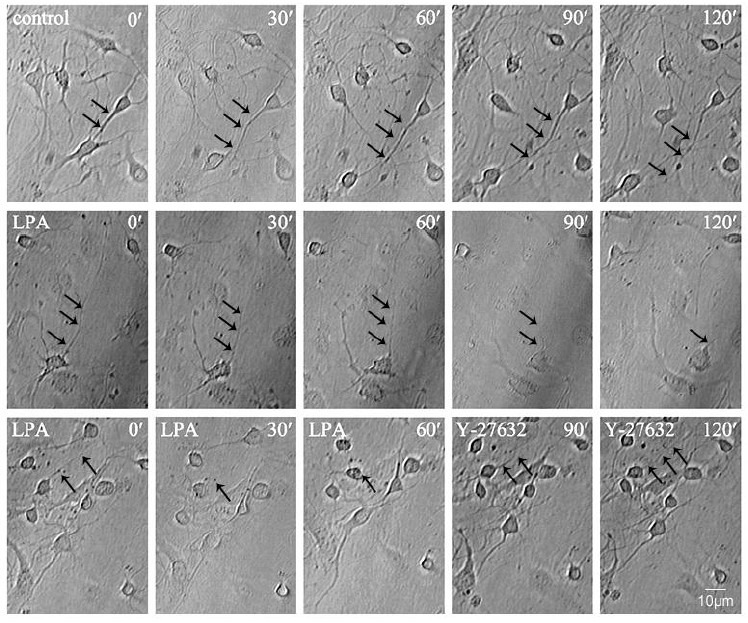
Modulation of Rho-associated protein kinase (ROCK) activity during neurite outgrowth in hippocampal neurons (arrows).
DIV5 neurons were treated with the indicated pharmacological agents and images were captured using a LEICA DFC300 CCD camera driven by LEICA-Qwin software with a 10 × objective lens, with constant filming at an interval of 30 minutes as shown by the time points. Representative phase contrast time-lapse images of cells treated with vehicle (control), lysophosphatidic acid (LPA) or Y-27632 (the ROCK inhibitor) after LPA were obtained using Leica DMIRE2 microscope equipment. At least 50 cells were counted in each group and the experiments were independently repeated at least three times. Scale bar: 10 μm.
We found that the neurites shortened by lysophosphatidic acid treatment became re-activated and started to grow (Figure 1C). All of these results suggest that ROCK negatively regulates neurite outgrowth.
Quantification of the impact of ROCK on neurite outgrowth
To quantify neurite outgrowth, hippocampal neurons were cultured on the permeable, polycarbonate membranes of transwell cell culture inserts. The pores separated the neurites from the cell bodies, and purified neurites were harvested at various time points and then quantified as described in the methods section. As shown in Figure 2, neurons were treated with lysophosphatidic acid or lysophosphatidic acid followed by Y-27632 for 1, 2, 3 or 5 hours, and untreated neurons were used as controls. Neurite outgrowth was significantly lower in the lysophosphatidic acid group than in the control group at 1 hour (P < 0.05 or P < 0.01). Neurite outgrowth was significantly increased after treatment with Y-27632 for an additional 1, 2 or 4 hours and became higher than in the control group (P < 0.05 or P < 0.01). Taken together, these results suggest that lysophosphatidic acid inhibits neurite growth, and Y-27632 reverses the inhibitory effect of lysophosphatidic acid treatment to promote neurite outgrowth. These results were consistent with phase contrast observations (Figure 2).
Figure 2.
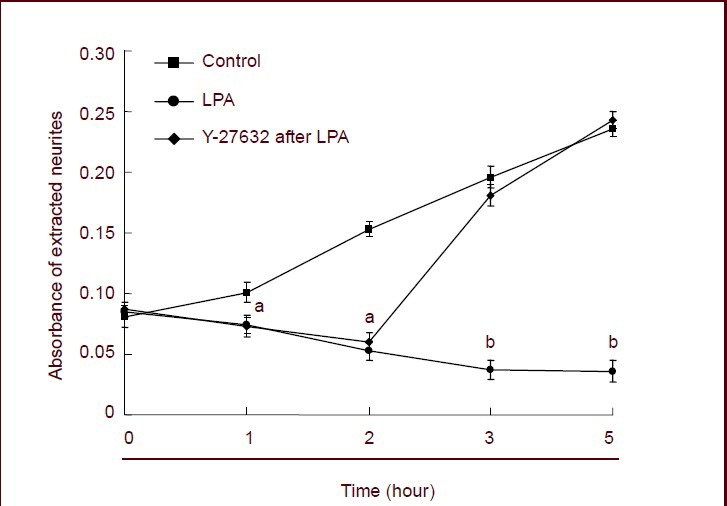
Quantification of neurite outgrowth regulated by Rho-associated protein kinase.
Neurons were treated with vehicle, lysophosphatidic acid (LPA) or Y-27632 (the ROCK inhibitor) after treatment with LPA for 1 hour at the indicated time points. The cells were then treated with neurite extraction buffer, and neurite outgrowth was quantified spectrophotometrically at 562 nm. Total protein in the neuronal cell bodies was used to normalize the data, which are presented as mean ± SEM of three independent experiments. aP < 0.05; bP < 0.01. One-way analysis of variance and Dunnett's test were used.
ROCK regulated microtubule remodeling to mediate neurite outgrowth
To further investigate the relationship between ROCK, microtubule rearrangement and neurite outgrowth, immunofluorescence staining was used to reveal the remodeling of microtubules. As shown in Figure 3A, in control neurons, microtubules in the cell bodies distributed around the nucleus and intertwined to form a mesh-like network. Some microtubules stretched from the nucleus to the front cell membrane and distributed along the inner membrane surface, and some stretched with free ends. Microtubules within the neurite were mainly bundled into fascicles along the long axis, and stretched deep into the neurite. Microtubules within the growth cone showed a fan-shaped distribution. Microtubules in the center of the growth cone were longer and stretched straight forward, while those on the sides of the growth cones were shorter and curved. Furthermore, the majority of microtubules in the growth cone possessed free ends. In lysophosphatidic acid-treated cultures, the neurites were shortened and only faint microtubule staining was observed in the distal part of the neurite. In the cell body, no clear mesh-like microtubule network was seen, but rather irregular microtubules of uneven thickness and unstructured arrangement were observed. At the root of some neurites, some microtubules disappeared and did not stretch into the neurite. Inside the neurite, microtubules were arranged in a braid-like formation, with large gaps appearing between the bundles of microtubules. The ends of neurites were small with no typical growth cones, and most of the microtubules disappeared (Figure 3B). Treatment with Y-27632 following lysophosphatidic acid treatment enabled the damaged microtubules to largely recover. Mesh-like microtubule networks of comparable thickness were seen, except around the nucleus where microtubules stretched deeply into the neurite with free ends. Compared with the lysophosphatidic acid-treated neurons, the arrangement of microtubules was much more highly ordered in Y-27632-treated neurons. In particular, the presence of growing microtubules was maintained inside growth cones and longer microtubules stretched to the leading edges (Figure 3C).
Figure 3.
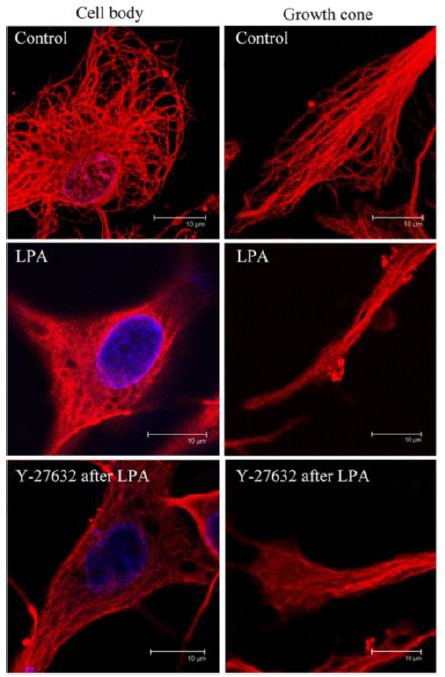
Rho-associated protein kinase-regulated microtubule remodeling in rat hippocampus (immunofluorescence staining).
Neurons were treated with vehicle (control), lysophosphatidic acid (LPA) or Y-27632 (the ROCK inhibitor) after LPA as in Figure 1. Inside the neurites, microtubules were arranged in a disorderly manner, and no typical growth cones were observed. Following treatment with Y-27632 after LPA, the damaged microtubules largely recovered, and growth cones with visible microtubules were observed. The microtubules are shown in red (Cy3). Nuclei were stained with Hoechst 33258 (blue). Scale bars: 10 μm.
ROCK regulated the localization of vinculin in rat hippocampus
To investigate the role of vinculin in ROCK-regulated neurite outgrowth, immunostaining was performed to reveal the membrane distribution of vinculin. In control neurons, vinculin was distributed in the membrane around the cell body. After lysophosphatidic acid treatment, the membrane distribution disappeared. By using Y-27632 after lysophosphatidic acid treatment, vinculin was restored in the membrane with brighter and aggregated signals (Figure 4).
Figure 4.
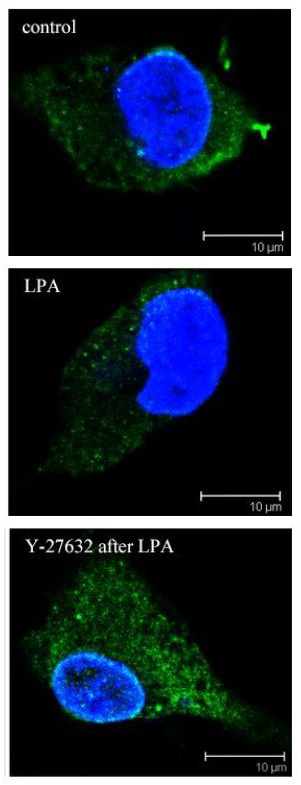
Regulation of vinculin distribution by Rho-associated protein kinase (ROCK) in rat hippocampal neurons (immunofluorescence staining).
Neurons were treated with vehicle (control), lysophosphatidic acid (LPA) or Y-27632 (the ROCK inhibitor) after LPA. Vinculin was distributed in the membrane. After LPA treatment, the membrane distribution disappeared. Following ROCK inhibition with Y-27632, vinculin gathered at the edge of cell body, particularly at the roots of processes. Vinculin, red (fluorescein isothiocyanate). Nuclei were stained with Hoechst 33258 (blue). Scale bars: 10 μm.
DISCUSSION
In this study, we demonstrated that activation of ROCK by lysophosphatidic acid impaired neurite outgrowth, and inhibition of ROCK by Y27623 rescued the inhibitory effect of lysophosphatidic acid on neurite outgrowth in cultured hippocampal neurons. Therefore, ROCK regulates microtubule remodeling and vinculin distribution to regulate neurite outgrowth.
Neurons grow from initially non-polar cells into polarized cells with dendrites and axons by continuous neurite outgrowth and differentiation, and the core event of this process is reorganization of the cytoskeleton[1]. Neurotrophins are believed to be critical for neurite outgrowth[24,25], which reshape neurons by activating membrane receptors such as G protein-coupled receptors[26,27]. Rho GTPases activate a cascade of signals that regulate distinct aspects of cytoskeletal reorganization, including actin and microtubule polymerization and depolymerization[28,29]. ROCK, one of the downstream effectors of the small GTPase, Rho, regulates cytoskeletal reorganization via many molecular signaling pathways that mediate neurite initiation and outgrowth[30,31].
Lysophosphatidic acid and Y-27632 activate and inhibit ROCK kinase activity, respectively, and are often used as tools to study the functional role of ROCK[32,33]. First, we showed that lysophosphatidic acid induced neurite collapse and Y-27632 promoted neurite outgrowth, even after lysophosphatidic acid treatment. Inhibition of ROCK not only reversed the inhibitory effect of lysophosphatidic acid treatment, it also further promoted neurite outgrowth. Quantification of neurite outgrowth by transwell culture further confirmed the results from phase contrast time-lapse images. We have previously shown that the Rho kinase pathway is closely associated with neurite development at different branch levels in hippocampal neurons, as observed by atomic force microscopy[34]. ROCK regulates not only the length of neurites, but also the number of branches at different levels, suggesting that ROCK is involved throughout the process of neurite outgrowth.
Although ROCK is not the only effector of RhoA, its role in neurite outgrowth cannot be ignored. ROCK regulates many signaling molecules, such as LIMK and MLCK, to reorganize the cytoskeleton via the regulation of actin filaments[35,36], and the microtubule-associated protein[37], CRMPs, to regulate microtubules[38,39]. Whatever ROCK controls, its final target is the cytoskeleton. By regulating the assembly and disassembly of the cytoskeleton, ROCK modulates cytoskeletal movement to reshape the morphologies of neurons.
Microtubules, the main component of the cytoskeleton, are important for maintaining neuronal polarity[40]. Once the membrane is anchored by adhesion proteins, actin filaments are gathered, resulting in membrane protrusion to form the initial neurite. Next, microtubules stretch into the protrusion to determine and stabilize the direction of growth[41,42]. In addition, microtubules with free ends, which are considered to be dynamic microtubules, stretch toward the cell membrane, while other microtubules bend and fold at the inner surface of the membrane[43,44]. Dynamic microtubules play a key role in both neurite initiation and branch formation.
Is ROCK involved in the movement of dynamic microtubules? We found that in control neurons, most of the immunofluorescence-labeled microtubules were curled and formed a mesh-like structure in the cell body, and microtubules with free ends were abundant near the membrane or in the growth cones. In contrast, treatment with lysophosphatidic acid led to neurite retraction and the depolymerization of microtubules. As a result, the mesh-like structure disappeared in the cell body, uniform fascicles assumed a braid-like formation inside the neurite, large gaps appeared between microtubules, and the growth cone lost its original shape with only tiny protrusions and no typical growth cone structure. Y-27632 reversed the neurite collapsing effect of lysophosphatidic acid. As a result, neurites regrew with clear microtubules inside the cell body and their free ends stretched to the membrane, microtubules at the roots of neurites were maintained and rearranged into an ordered formation with no obvious gaps, and growth cones retained their original shape and contained microtubules with free ends. All of these data suggest that ROCK regulates the remodeling of microtubules, particularly those with free ends.
It has been proposed that the first step in neuritogenesis in the central nervous system is the breaking of the neuronal sphere by the formation of lamellipodia as a result of cytoskeletal movements. Then lamellipodia extend into neurites and the growth cone guides the dendrites along the proper pathways to form complex and accurate neuronal circuits via synaptic structures. The initial breakdown of symmetry and the outgrowth of a neurite are strongly associated with cytoskeletal dynamics, including polymerization, depolymerization and reorganization of microtubules. How would ROCK inhibition lead to microtubule stability? ROCK has been reported to regulate PAR-3 phosphorylation, which disrupts the PAR complex that is required for front-rear polarization of migrating cells[45]. The location of an active PAR complex may stabilize microtubules by promoting the interaction of adenomatous polyposis coli with the plus-ends of microtubules[46,47]. Alternatively, other modulators such as microtubule-associated proteins may play a role in this cascade. Regulating the phosphorylation of microtubule-associated proteins is important for controlling the dynamics of the leading edge in neuronal growth cones[48]. Further, it has been reported that ROCK phosphorylation of Tau and microtubule-associated protein-2 promotes their dissociation from microtubules, thereby enhancing microtubule destabilization[37].
Vinculin plays a critical role in the mechanical coupling of integrins to the cytoskeleton, as well as in regulating cell shape, protrusion amplitude and cell motility[19,20]. The tips of dynamic microtubules are bound to many adhesion proteins, and the interaction of adhesions with the matrix surface is key to the formation and outgrowth of a protrusion. By anchoring the focal adhesions in the membrane to the matrix, a neurite grows in the direction determined by the actin cytoskeleton. If these focal complexes cannot be formed, actin is depolymerized and the neurite retracts[49]. We found that vinculin was mainly distributed at the membrane. Lysophosphatidic acid treatment weakened the fluorescent vinculin signals whereas treatment with Y-27632 after LPA treatment increased the vinculin signals, indicating that vinculin may be recruited to the front of a neurite by dynamic microtubules. Microtubules interact with the actin network via vinculin. The coupling movement of these three elements elicits a series of processes such as the movement of growth cones and lamellipodia, attachment to the extracellular matrix (ECM), growth direction determination and neurite extension[50]. During the early stages of neurite outgrowth, the movement and rearrangement of the actin cytoskeleton makes microtubules stretch into the actin network, stabilizing the neurite structure. This study is still unable to determine the time series of the linkage processes of these cytoskeletal proteins regulated by ROCK, but provides some evidence that ROCK modulates neurite outgrowth by regulating microtubule remodeling and redistribution of the adhesion protein, vinculin. Confirmation of these results obtained with small molecule compounds by knockout and overexpression studies would be very useful to further substantiate the author's claims.
MATERIALS AND METHODS
Design
A pharmacological study.
Time and setting
Experiments were performed in the Laboratory of Medical College of Jinan University in China from January 2011 to December 2012.
Materials
One-day-old, specific-pathogen-free Sprague-Dawley rat pups were purchased from the Experimental Animal Center of Southern Medical University in China (SYXK 2011-0074). The experimental procedures were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[51].
Methods
Hippocampal neuron culture and treatment
Hippocampi were dissected from postnatal pups, and dissociated hippocampal neurons were obtained by digestion with 0.125% trypsin and plated at a density of 1 × 104 cells/cm2 onto coverslips. Cultured hippocampal neurons were maintained in Neurobasal A medium containing 2% B27 and 0.5 mmol/L glutamine supplement at 37°C in a 5% CO2 humidified incubator (Thermo Scientific, Fremont, CA, USA), and half of the culture medium was replaced every 3 days. To study the effect of ROCK on neurite extension and microtubule and vinculin remodeling, neurons were treated with 200 ng/mL lysophosphatidic acid for 1 hour and then maintained in culture medium with or without 10 μmol/L Y-27632 for an additional period, as indicated in the Results.
Phase contrast time-lapse microscopy of rat hippocampal neurons
All time-lapse microscopy images were obtained using Leica DMIRE2 microscope equipment (Leica microsystems, Wetzlar, Germany). Briefly, cells were cultured in the aforementioned supplemented Neurobasal™ medium (Gibco, Grand Island, NY, USA) in a humidified 37°C, 5% CO2 incubator or in a custom-made microscope stage incubator (Thermo Scientific). A Plexiglas enclosure around the microscope's body was heated to 37°C using a silent air blower to maintain the optimal temperature for neuronal growth and minimize thermal drifts during imaging. Inside the enclosure, the cell culture dish was placed on a thin, glass microscope stage insert. The dish was covered with a small box, which was perfused with 5% CO2 air heated and humidified in a packed-bed humidification column present inside the enclosure. DIV5 neurons were treated with the indicated pharmacological agents and images were captured using a Leica DFC300 CCD camera (Leica microsystems) driven by Leica-Qwin software (Leica microsystems) with a 10 × objective lens, with constant filming at an interval of 30 minutes.
Neurite extraction and quantification
Neurons were treated according to the Neurite Outgrowth Quantification Assay Kit (Chemicon, Temecula, CA, USA). Prior to starting the neurite outgrowth assay, the transwell membrane surface for each individual cell line/primary culture was carefully prepared. In an empty well of the 24-well plate, 500 μL of the preferred ECM protein solution was added to coat the membrane on the underside of the chamber. The membranes were coated for 2 hours at 37°C. Prior to plating, the cells were removed from the culture dishes with trypsin-free detachment buffer and a cell viability assay was performed using trypan blue. The cells were resuspended in differentiation medium at 1 × 105 cells/mL. The transwell membranes were removed from the ECM protein coating solution (without rinsing) and placed into the wells of 24-well plates containing 500 μL of differentiation medium. A cell suspension volume of 100 μL (containing 1.0–2.0 × 105 cells) was added on top of the membranes (upper chamber) and the cells were allowed to extend neurites into the lower chamber for 4–24 hours at 37°C. After performing the pharmacological treatments at the indicated times, the membrane inserts were removed and rinsed. The inserts were then stained with 500 μL of neurite staining solution for 5–15 minutes at room temperature. Following a brief rinse with distilled water, cell bodies on the upper membrane surface were removed by wiping with a cotton swab to extract stained neurite extensions for quantification (Figure 4). For quantification, a 100–200 μL drop of neurite stain extraction buffer was placed onto a flat piece of Parafilm and the underside of the membrane (containing stained neurites) was positioned onto the drop of extraction buffer such that it covered the entire membrane surface. The underside of the membrane was incubated with extraction buffer for 5 minutes at room temperature. Thereafter, 100–200 μL of extraction buffer was quantified by reading on a spectrophotometer (Dunedin, FL, USA) at 562 nm. The total protein level in each group was measured to normalize the data.
Immunofluorescence staining
Immunofluorescence staining was performed according to a standard protocol[52]. The cells were fixed in 4% paraformaldehyde for 20 minutes, blocked with 5% goat serum for 30 minutes, and permeabilized with 0.2% Triton X-100 for 30 minutes. The fixed cells were incubated with mouse monoclonal anti-α-tubulin (1:1 000; Sigma, St. Louis, MO, USA) antibody and mouse monoclonal anti-vinculin primary antibody (Sigma) overnight at 4°C. The cells were then washed three times in PBS and incubated with Cy3-conjugated or fluorescein isothiocyanate (FITC)-conjugated donkey anti-mouse IgG (1: 5 000; Invitrogen, Carlsbad, CA, USA) for 40 minutes at 37°C in the dark. Hoechst 33258 was used to stain the nuclei. Immunofluorescence staining results were visualized using a LSM710 confocal microscope (Carl Zeiss, Oberkochen, Germany).
Statistical analysis
All values were presented as mean ± SEM. Data were collected and analyzed using SPSS for Windows 13.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance was employed to examine differences among groups. Dunnett's t-test was used for pairwise comparisons. Alpha was set to 0.05, and P < 0.05 was considered statistically significant.
Research background: ROCK is an essential regulator of cytoskeletal dynamics during the process of neurite extension. However, whether ROCK regulates microtubule remodeling and the distribution of adhesive proteins to mediate neurite outgrowth remains unclear.
Research frontiers: In response to external signals, ROCK induced microtubule polymerization and depolymerization, thus impacting neuronal polarity and neurite outgrowth. To further understand how ROCK regulates neurite extension, whether modulation of ROCK activity induces microtubule remodeling and further leads to redistribution of the anchoring protein, vinculin, should be explored.
Clinical significance: This study demonstrated that ROCK regulates microtubule remodeling and vinculin distribution, thus regulating neurite outgrowth. Illustrating the mechanisms of neurite extension provides new insights into neuronal regeneration.
Academic terminology: ROCK is a downstream effector of Rho A kinase. ROCK responds to extracellular signals and phosphorylates many downstream targets such as LIMK1 and LIMK2 to induce cytoskeletal remodeling and vinculin redistribution, which finally contributes to neurite outgrowth.
Peer review: This study provides new evidence for understanding how the downstream targets of ROCK regulate neurite outgrowth. The results strongly indicate that ROCK is involved in neurite outgrowth by regulating microtubule dynamics and vinculin distribution, thus providing novel information on how ROCK mediates neurite outgrowth.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 31170941; the Fundamental Research Funds for the Central Universities, No. 21612424; and the Science and Technology Planning Project of Guangdong Province, No. 2010B031600102.
Conflicts of interest: None declared.
Ethical approval: This experiment was approved by the Experimental Animal Center of Southern Medical University in China.
(Reviewed by Lodge A, Raye W, Cai WJ, Yuan DF)
(Edited by Wang J, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Stiess M, Bradke F. Neuronal polarization: the cytoskeleton leads the way. Dev Neurobiol. 2011;71(6):430–444. doi: 10.1002/dneu.20849. [DOI] [PubMed] [Google Scholar]
- [2].Nelson WJ. Remodeling epithelial cell organization: transitions between front-rear and apical-basal polarity. Cold Spring Harb Perspect Biol. 2009;1(1):a000513. doi: 10.1101/cshperspect.a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Margolin W. Sculpting the bacterial cell. Curr Biol. 2009;19(17):R812–822. doi: 10.1016/j.cub.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol cell Biol. 2009;10(5):332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11(18):2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- [6].Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116(2):167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- [7].Auer M, Hausott B, Klimaschewski L. Rho GTPases as regulators of morphological neuroplasticity. Ann Anat. 2011;193(4):259–266. doi: 10.1016/j.aanat.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Govek EE, Hatten ME, Van Aelst L. The role of Rho GTPase proteins in CNS neuronal migration. Dev Neurobiol. 2011;71(6):528–553. doi: 10.1002/dneu.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tan HB, Zhong YS, Cheng Y, et al. Rho/ROCK pathway and neural regeneration: a potential therapeutic target for central nervous system and optic nerve damage. Int J Ophthalmol. 2011;4(6):652–657. doi: 10.3980/j.issn.2222-3959.2011.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19(1):1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- [11].Ahmed Z, Berry M, Logan A. ROCK inhibition promotes adult retinal ganglion cell neurite outgrowth only in the presence of growth promoting factors. Mol Cell Neurosci. 2009;42(2):128–133. doi: 10.1016/j.mcn.2009.06.005. [DOI] [PubMed] [Google Scholar]
- [12].Causeret F, Hidalgo-Sanchez M, Fort P, et al. Distinct roles of Rac1/Cdc42 and Rho/Rock for axon outgrowth and nucleokinesis of precerebellar neurons toward netrin 1. Development. 2004;131(12):2841–2852. doi: 10.1242/dev.01162. [DOI] [PubMed] [Google Scholar]
- [13].Schmandke A, Strittmatter SM. ROCK and Rho: biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist. 2007;13(5):454–469. doi: 10.1177/1073858407303611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Grigoriev I, Borisy G, Vorobjev I. Regulation of microtubule dynamics in 3T3 fibroblasts by Rho family GTPases. Cell Motil Cytoskeleton. 2006;63(1):29–40. doi: 10.1002/cm.20107. [DOI] [PubMed] [Google Scholar]
- [15].Wittmann T, Waterman-Storer CM. Cell motility: can Rho GTPases and microtubules point the way? J Cell Sci. 2001;114(Pt 21):3795–3803. doi: 10.1242/jcs.114.21.3795. [DOI] [PubMed] [Google Scholar]
- [16].Kadir S, Astin JW, Tahtamouni L, et al. Microtubule remodelling is required for the front-rear polarity switch during contact inhibition of locomotion. J Cell Sci. 2011;124(Pt 15):2642–2653. doi: 10.1242/jcs.087965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Palazzo AF, Cook TA, Alberts AS, et al. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat Cell Biol. 2001;3(8):723–729. doi: 10.1038/35087035. [DOI] [PubMed] [Google Scholar]
- [18].Yamana N, Arakawa Y, Nishino T, et al. The Rho-mDia1 pathway regulates cell polarity and focal adhesion turnover in migrating cells through mobilizing Apc and c-Src. Mol Cell Biol. 2006;26(18):6844–6858. doi: 10.1128/MCB.00283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mierke CT. The role of vinculin in the regulation of the mechanical properties of cells. Cell Biochem Biophys. 2009;53(3):115–126. doi: 10.1007/s12013-009-9047-6. [DOI] [PubMed] [Google Scholar]
- [20].Mills E, LaMonica K, Hong T, et al. Roles for Rho/ROCK and vinculin in parietal endoderm migration. Cell Commun Adhes. 2005;12(1-2):9–22. doi: 10.1080/15419060500305948. [DOI] [PubMed] [Google Scholar]
- [21].Burridge K, Mangeat P. An interaction between vinculin and talin. Nature. 1984;308(5961):744–746. doi: 10.1038/308744a0. [DOI] [PubMed] [Google Scholar]
- [22].Menkel AR, Kroemker M, Bubeck P, et al. Characterization of an F-actin-binding domain in the cytoskeletal protein vinculin. J Cell Biol. 1994;126(5):1231–1240. doi: 10.1083/jcb.126.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dumbauld DW, Shin H, Gallant ND, et al. Contractility modulates cell adhesion strengthening through focal adhesion kinase and assembly of vinculin-containing focal adhesions. J Cell Physiol. 2010;223(3):746–756. doi: 10.1002/jcp.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol. 2006;66(3):236–242. doi: 10.1002/neu.20215. [DOI] [PubMed] [Google Scholar]
- [25].Yamashita T, Tucker KL, Barde YA. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24(3):585–593. doi: 10.1016/s0896-6273(00)81114-9. [DOI] [PubMed] [Google Scholar]
- [26].Ng J, Luo L. Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron. 2004;44(5):779–793. doi: 10.1016/j.neuron.2004.11.014. [DOI] [PubMed] [Google Scholar]
- [27].Kalil K, Dent EW. Touch and go: guidance cues signal to the growth cone cytoskeleton. Curr Opin Neurobiol. 2005;15(5):521–526. doi: 10.1016/j.conb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- [28].Zheng Y. G protein control of microtubule assembly. Annu Rev Cell Dev Biol. 2004;20:867–894. doi: 10.1146/annurev.cellbio.20.012103.094648. [DOI] [PubMed] [Google Scholar]
- [29].Hall A, Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb Perspect Biol. 2010;2(2):a001818. doi: 10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bashour AM, Fullerton AT, Hart MJ, et al. IQGAP1, a Rac- and Cdc42-binding protein, directly binds and cross-links microfilaments. J Cell Biol. 1997;137(7):1555–1566. doi: 10.1083/jcb.137.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67(9):545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang XF, Schaefer AW, Burnette DT, et al. Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron. 2003;40(5):931–944. doi: 10.1016/s0896-6273(03)00754-2. [DOI] [PubMed] [Google Scholar]
- [33].Sakata H, Sakabe M, Matsui H, et al. Rho kinase inhibitor Y27632 affects initial heart myofibrillogenesis in cultured chick blastoderm. Dev Dyn. 2007;236(2):461–472. doi: 10.1002/dvdy.21055. [DOI] [PubMed] [Google Scholar]
- [34].Chen J, Hao H, Guo G, et al. Effect of Rho-kinase pathway on neurite outgrowth of rat hippocampal neurons under atomic force microscopy. Neural Regen Res. 2012;7(7):496–500. doi: 10.3969/j.issn.1673-5374.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ishibashi F. High glucose increases phosphocofilin via phosphorylation of LIM kinase due to Rho/Rho kinase activation in cultured pig proximal tubular epithelial cells. Diabetes Res Clin Pract. 2008;80(1):24–33. doi: 10.1016/j.diabres.2007.11.004. [DOI] [PubMed] [Google Scholar]
- [36].Kaneko-Kawano T, Takasu F, Naoki H, et al. Dynamic regulation of myosin light chain phosphorylation by Rho-kinase. PLoS One. 2012;7(6):e39269. doi: 10.1371/journal.pone.0039269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Amano M, Kaneko T, Maeda A, et al. Identification of Tau and MAP2 as novel substrates of Rho-kinase and myosin phosphatase. J Neurochem. 2003;87(3):780–790. doi: 10.1046/j.1471-4159.2003.02054.x. [DOI] [PubMed] [Google Scholar]
- [38].Hall C, Brown M, Jacobs T, et al. Collapsin response mediator protein switches RhoA and Rac1 morphology in N1E-115 neuroblastoma cells and is regulated by Rho kinase. J Biol Chem. 2001;276(46):43482–43486. doi: 10.1074/jbc.C100455200. [DOI] [PubMed] [Google Scholar]
- [39].Arimura N, Inagaki N, Chihara K, et al. Phosphorylation of collapsin response mediator protein-2 by Rho-kinase. Evidence for two separate signaling pathways for growth cone collapse. J Biol Chem. 2000;275(31):23973–23980. doi: 10.1074/jbc.M001032200. [DOI] [PubMed] [Google Scholar]
- [40].Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- [41].Gundersen GG, Gomes ER, Wen Y. Cortical control of microtubule stability and polarization. Curr Opin Cell Biol. 2004;16(1):106–112. doi: 10.1016/j.ceb.2003.11.010. [DOI] [PubMed] [Google Scholar]
- [42].Palazzo AF, Gundersen GG. Microtubule-actin cross-talk at focal adhesions. Sci STKE. 2002;2002(139):pe31. doi: 10.1126/stke.2002.139.pe31. [DOI] [PubMed] [Google Scholar]
- [43].Dent EW, Kalil K. Axon branching requires interactions between dynamic microtubules and actin filaments. J Neurosci. 2001;21(24):9757–9769. doi: 10.1523/JNEUROSCI.21-24-09757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Buck KB, Zheng JQ. Growth cone turning induced by direct local modification of microtubule dynamics. J Neurosci. 2002;22(21):9358–9367. doi: 10.1523/JNEUROSCI.22-21-09358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nakayama M, Goto TM, Sugimoto M, et al. Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev Cell. 2008;14(2):205–215. doi: 10.1016/j.devcel.2007.11.021. [DOI] [PubMed] [Google Scholar]
- [46].Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421(6924):753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- [47].Zumbrunn J, Kinoshita K, Hyman AA, et al. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Curr Biol. 2001;11(1):44–49. doi: 10.1016/s0960-9822(01)00002-1. [DOI] [PubMed] [Google Scholar]
- [48].Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9(4):309–322. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- [49].Bailly M. Connecting cell adhesion to the actin polymerization machinery: vinculin as the missing link? Trends Cell Biol. 2003;13(4):163–165. doi: 10.1016/s0962-8924(03)00030-8. [DOI] [PubMed] [Google Scholar]
- [50].Janssen ME, Kim E, Liu H, et al. Three-dimensional structure of vinculin bound to actin filaments. Mol Cell. 2006;21(2):271–281. doi: 10.1016/j.molcel.2005.11.020. [DOI] [PubMed] [Google Scholar]
- [51].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [52].Tan M, Li Z, Ma S, et al. Heroin activates Bim via c-Jun N-terminal kinase/c-Jun pathway to mediate neuronal apoptosis. Neuroscience. 2013;233:1–8. doi: 10.1016/j.neuroscience.2012.12.005. [DOI] [PubMed] [Google Scholar]


