Abstract
To examine the possible correlation of aberrant Wnt signaling and pathological changes in Alzheimer's disease, we established a rat model of Alzheimer's disease and measured axin and β-catenin expression in the hippocampus. Rats were pretreated with moxibustion or electroacupuncture, or both, at Baihui (GV20) and Shenshu (BL23). Axin expression was lower, β-catenin expression was greater, and neuronal cytoplasmic edema was visibly prevented in the rats that had received the pretreatments. Our results suggest that the mechanism underlying the neuroprotective effect of acupuncture and moxibustion in Alzheimer's disease is associated with axin and β-catenin expression in the Wnt signal transduction pathway.
Keywords: nerve regeneration, brain injury, acupuncture and moxibustion, pretreatment, Alzheimer's disease, axin, β-catenin, NSFC grant, neural regeneration
Introduction
Wnt signaling is a cellular regulatory system, and plays an important role in cell differentiation and proliferation, as well as in tumor development. Wnt proteins are highly expressed in brain areas involved in learning and memory and vulnerable to damage in Alzheimer's disease, such as the entorhinal cortex and hippocampus. Aberrant Wnt signaling may be associated with pathological alterations seen in Alzheimer's disease. Axin, the product of the Fused locus in mice (Zeng et al., 1997), is a negative regulator of the Wnt signaling pathway and is involved in body axis development, neuronal differentiation and cell death. Axin forms a complex with adenomatous polyposis coli, glycogen synthase kinase-3β and β-catenin. Amongst its roles, axin regulates the phosphorylation and stability of β-catenin and promotes its degradation, and negatively regulates tau phosphorylation by glycogen synthase kinase-3β. Axin also has binding sites for protein phosphatase 2A and can block the formation of neurofibrillary tangles in Alzheimer's disease.
β-Catenin is an adhesion molecule which forms a complex with E-cadherin in the cytoplasm of cells in normal tissue. Cytoplasmic β-catenin concentrations are normally low, and form a crutch-like structure mediating intercellular adhesion (Clements et al., 2003). β-Catenin accumulates in the cytoplasm and localizes to the nucleus in a complex with TCF/LEF transcription factors, where it initiates transcription and expression of downstream target genes and controls cell growth. If there is an excess of β-catenin, then a complex of adenomatous polyposis coli, axin and glycogen synthase kinase-3 will appear. The complex contributes to the phosphorylation of β-catenin by glycogen synthase kinase-3β. Phosphorylated β-catenin is identified and degraded by ubiquitin hydrolase. Under the stimulus of the Wnt signaling pathway or other extracellular signals, Wnt binds to Frizzled family proteins, antagonizes the formation of the β-catenin degradation complex (adenomatous polyposis coli-axin) through Dishevelled protein, and ultimately blocks the phosphorylation and ubiquitination of β-catenin. In the absence of Wnt signaling, high levels of cytoplasmic β-catenin stimulate the expression of negative regulatory molecules such as the adenomatous polyposis coli anti-oncogene, glycogen synthase kinase-3β and axin, forming multiple compounds and promoting the phosphorylation of β-catenin by glycogen synthase kinase-3β kinase. Phosphorylated β-catenin binds to β-TRCP protein, covalently binds to ubiquitin, and is degraded by β-TRCP/SLMB proteolytic enzymes until cytoplasmic β-catenin concentrations are low enough to ensure normal cellular physiological and biochemical functions (Nakamura et al., 1998; Burchett, 2000; De Robertis et al., 2000; Clements et al., 2003).
To explore the mechanism underlying the neuroprotective action of acupuncture and moxibustion in Alzheimer's disease, we established a rat model of Alzheimer's disease by injecting amyloid-beta peptide (25–35) into the hippocampus of Sprague-Dawley rats. Before model establishment, we performed acupuncture and moxibustion at the Baihui (GV20) and Shenshu (BL23) acupoints. Immunohistochemistry, western blot and transmission electron microscopy were used to investigate the effects of pretreatment with acupuncture and moxibustion on axin and β-catenin expression and neuronal ultrastructure in the hippocampus of Alzheimer's disease rats.
Materials and Methods
Experimental animals
A total of 24 healthy male Sprague-Dawley rats, aged 22–24 months, weighing 480 ± 20 g, were provided by the Experimental Animal Center, Tongji Medical College, Huazhong University of Science and Technology, China. The experiments were approved by the Animal Ethics Committee, Tongji Medical College, Huazhong University of Science and Technology in China.
Experimental groups and model establishment
A total of 24 rats were equally and randomly divided into 6 groups: normal controls, sham surgery, model, moxibustion pretreatment, electroacupuncture pretreatment, electroacupuncture + moxibustion pretreatment (n = 4 per group). Left and right hemispheres were obtained, resulting in a total of eight hippocampi in each group.
In the moxibustion, electroacupuncture and electroacupuncture + moxibustion groups, the model of Alzheimer's disease was established in accordance with previously published methods (Cui et al., 2009; Ke et al., 2009). Each rat was intraperitoneally anesthetized with 10% chloral hydrate (300 mg/kg) and the head was secured in a stereotaxic frame. The parietal area was shaved, the skin sterilized, and the superficial fascia peeled away to expose the parietal bone. Bilateral holes were drilled posterior to the coronal suture. Bone chips were removed, leaving the cerebral dura mater intact. Rat hippocampi (3.5 mm posterior to the anterior fontanelle, 2.0 mm lateral to the median line and 2.7 mm below the cerebral dura mater) were injected with amyloid-beta peptide (25–35) (Sigma, St. Louis, MO, USA) diluted to 5 μg/μL with 0.1% trifluoroacetic acid and incubated at 37°C for 1 week. One microliter (5 μg) of diluted sample was injected into each side within 5 minutes. The needle was maintained in place for 5 minutes to limit backflow upon removal. After surgery, the holes in the skull were filled with denture powder and the skin was sutured. Penicillin-G, 100,000 units, was injected intramuscularly daily for the first 3 days after surgery, to prevent infection.
In the sham surgery group, surgical and drug injection methods were identical to the model group, except an equivalent volume of physiological saline was injected instead of amyloid-beta peptide (25–35).
The model group did not receive any pretreatment before model induction. The normal control group did not undergo any treatment or surgery.
In the electroacupuncture group, the rats were subjected to electroacupuncture at Baihui (middle of the parietal bone) and Shenshu (on both sides below the second lumbar vertebra, alternating daily between left and right Shenshu) using a 0.5 cun filiform needle (Huatuo Brand). The needle was inserted forward horizontally 3–5 mm at Baihui and inward obliquely 5 mm at Shenshu. A G6805-II electroacupuncture therapeutic apparatus (Shanghai, China) was used. A negative and a positive electrode was connected to Baihui and Shenshu, respectively. Continuous wave was used at a frequency of 2 Hz, with slight shaking of the needle handle. The needle was maintained in place for 20 minutes and the procedure was performed once a day for 6 days (one course of treatment). Three courses of treatment were performed, with 1 day of rest between each course. Surgery for amyloid-beta peptide (25–35) injection was performed immediately after the final session of electroacupuncture. Seven days after model induction, electroacupuncture was conducted for further 7 consecutive days.
In the moxibustion group, rats received moxibustion before model induction. A moxa stick approximately 0.6 cm in diameter was utilized. The acupoints were the same as in the electroacupuncture group. Suspended moxibustion was carried out with a mounting bracket (constructed by the laboratory) 2–3 cm above Baihui and bilateral Shenshu. Acupoint skin temperature was 43 ± 1°C under the same treatment schedule as the electroacupuncture. Surgery for amyloid-beta peptide (25–35) injection was carried out immediately after the last session of moxibustion. Seven days after model induction, moxibustion was conducted for further 7 consecutive days.
In the electroacupuncture + moxibustion group, rats received the same therapeutic procedures as the electroacupuncture and moxibustion groups but electroacupuncture and moxibustion were performed for 10 minutes each. Surgery for amyloid-beta peptide (25–35) injection was carried out immediately after the last pretreatment session. Seven days after model induction, electroacupuncture and moxibustion were conducted for further 7 consecutive days.
Immunohistochemical staining
The rats were sacrificed under chloral hydrate anesthesia. Bilateral hippocampi were dissected out and subjected to immunohistochemical staining using a kit, in accordance with the manufacturer's instructions (Boster, Wuhan, Hubei Province, China). The rats were perfused with 4% paraformaldehyde. Immunohistochemistry was carried out on frozen tissue. Primary antibodies were axin (H-98) rabbit polyclonal IgG (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and β-catenin (H-102) rabbit polyclonal IgG (1:1,000; Santa Cruz Biotechnology), with horseradish peroxidase-labeled anti-rabbit secondary antibody (1:500) were used in strict accordance with the instruction of the kit. Histomorphology of stained sections and the number of target protein-positive cells in each group were observed under a light microscope (Olympus, Tokyo, Japan).
Western blot assay
Rats were sacrificed by anesthesia. Bilateral hippocampi were obtained, homogenized in homogeneous buffer in an electric homogenizer, and then centrifuged. The supernatant was collected. Residual protein and other tissues were washed away. The samples were stored at −20°C for further use. Centrifugation was performed at 4°C. Total protein precipitates were mixed with 20 μL of precooled homogeneous buffer. 10 μL of sample was obtained from each tube and diluted to 60 μL with double-distilled water. Protein content was determined using a bicinchoninic acid assay kit (Boster). The samples underwent polyacrylamide gel electrophoresis and were transferred to a nitrocellulose membrane for 1 hour. The membrane was stained with ponceau red for 10 minutes, and washed with distilled water. Protein bands were observed as a loading control. The membrane containing the proteins was placed in blocking buffer containing 1% bovine serum and shaken gently at room temperature for 30 minutes, before being rinsed in 1.5% non-fat milk 3 times for 5 minutes each time. The membrane was then incubated in anti-axin antibody (1:500; Santa Cruz Biotechnology) and β-catenin antibody (1:1,000; Santa Cruz Biotechnology) at 4°C overnight, before being washed with Tris-buffered saline with Tween containing 1.5% non-fat milk powder, 3 times for 5 minutes each, and incubated with horseradish peroxidase-labeled secondary antibody (1:500; Santa Cruz Biotechnology) at 37°C for 2 hours. The nitrocellulose membrane was washed with Tris-buffered saline with Tween 3 times for 5 minutes each, and then visualized by enhanced chemiluminescence. After X-ray film exposure, the film was scanned to digitize the bands for quantification. β-Actin was the internal control in accordance with the instruction of the kit. Gelbase/Gelplot software in the Gel Document Analysis System (UVP, UK) was used to analyze absorbance of the target bands. Relative protein expression was calculated as the ratio of absorbance of target protein to that of β-actin.
Transmission electron microscopy
The rats were perfused with 60 mL of physiological saline via the left ventricle, and then fixed with 50 mL of lanthanum. The brain was obtained rapidly by craniotomy. The forebrain was cut into 1 mm-thick coronal sections from the optic chiasma. Two blocks of brain tissues (about 1.0 mm × 1.0 mm × 0.5 mm) were separately obtained from the cortex and basal ganglia of ischemic side and contralateral side, and fixed in 2.5% glutaral for at least 24 hours. Prefixed tissues were washed with 0.075 mol/L Tris-hydrochloric acid, 3 times for 10 minutes each. Brain tissues were postfixed in 1% osmic acid for 1.5 hours, dehydrated with alcohol and acetone, immersed and embedded in Epon 812, sliced into semithin sections using a LPT ultramicrotome, and stained with methylene blue and fuchsin. The samples were then sliced into 50–70 nm ultrathin sections, which underwent uranium-lead double staining. Brain tissue ultrastructure was observed with a transmission electron microscope (model H600; Hitachi, Tokyo, Japan).
Statistical analysis
Measurement data were expressed as mean ± SD, and analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Group differences were compared using a two-sample t-test. A value of P < 0.05 was considered statistically significant.
Results
Effects of acupuncture and moxibustion pretreatment on axin and β-catenin protein expression in the hippocampus of Alzheimer's disease rats
In the normal and sham surgery groups, axin expression was regular shape, clear nucleus and cytoplasm, large and round nucleus, visible nucleolus and abundant cytoplasm. In the model group, intense brown staining revealed extensive expression of axin in the nuclear and cell membranes (Figure 1C). In the electroacupuncture and moxibustion groups, axin expression was lower than in the model group (Figure 1D, E), and lower still in the electroacupuncture + moxibustion group (Figure 1F), where expression of axin was similar to that in the normal control and sham surgery groups.
Figure 1.
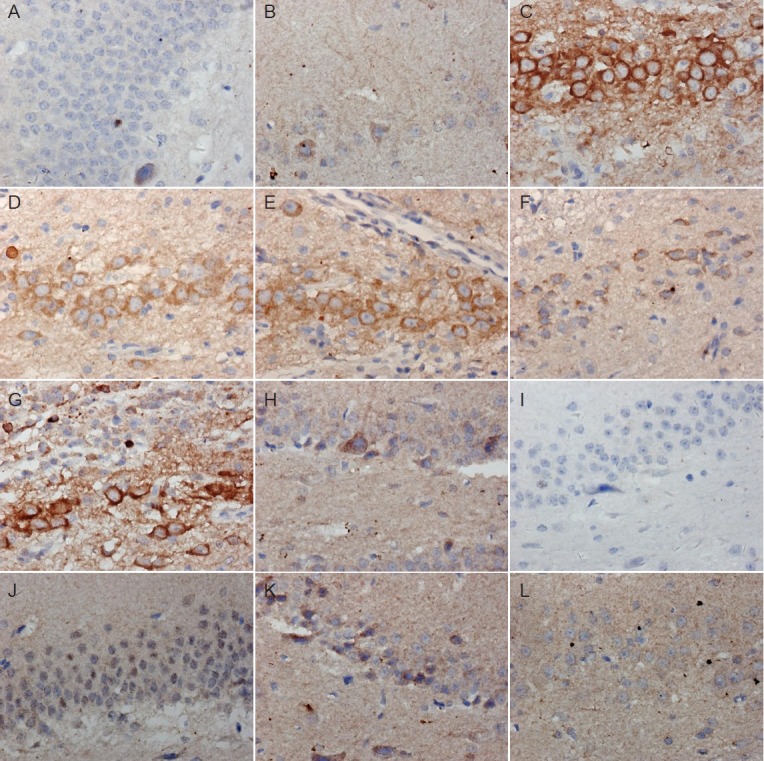
Immunohistochemical staining of axin (A–F) and β-catenin (G–L) in the hippocampal dentate gyrus of Alzheimer's disease rats with or without pretreatment with acupuncture and/or moxibustion (× 400).
Normal group (A: axin; G: β-catenin) and sham surgery group (B: axin; H: β-catenin) show few axin-positive cells but extensive β-catenin-posi-tive staining. Model group shows many axin-positive cells (C) but few β-catenin-positive cells (I). Electroacupuncture (D, J), moxibustion (E, K) and electroacupuncture + moxibustion (F, L) groups show similar numbers of axin- and β-catenin-positive cells to the normal and sham surgery groups.
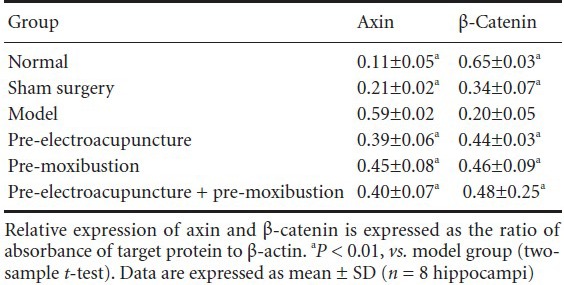
The western blot (Figure 2) revealed a low level of axin protein expression, but a high level of β-catenin expression, in control and sham-operated rats. The model group had significantly higher axin expression (P < 0.01), but lower β-catenin expression (P < 0.01) than the control group (Table 1). Compared with the model group, axin protein expression was lower (P < 0.01), but β-catenin protein expression was higher (P < 0.01) in the electroacupuncture, moxibustion, and electroacupuncture + moxibustion groups (Figure 2, Table 1).
Figure 2.
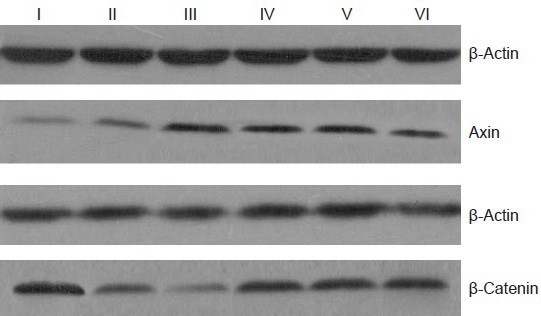
Western blot showing axin and β-catenin protein expression (and β-actin internal control) in the hippocampus of Alzheimer's disease rats with or without pretreatment with acupuncture and/or moxibustion.
Compared with the model group, axin protein expression was lower, but β-catenin protein expression was higher, in the electroacupuncture, moxibustion, and electroacupuncture + moxibustion groups. I: Normal group; II: sham surgery group; III: model group; IV: moxibustion group; V: electroacupumcture group; VI: electroacupuncture + moxi-bustion group.
Table 1.
Changes in axin and β-catenin protein expression in the hippocampus of Alzheimer's disease rats with or without pretreatment with acupuncture and/or moxibustion
Effects of acupuncture and moxibustion pretreatment on hippocampal ultrastructure in Alzheimer's disease rats
In the normal control and sham surgery groups, endoplasmic reticulum, mitochondria, microtubules and actin filaments were observed in neurons (Figure 3A, B). The nucleus mainly contained euchromatin, which was uniformly distributed. In the model group, neuronal cytoplasmic swelling was evident, showing as loose, empty and bright cytoplasm (Figure 3C). Organelle disintegration, nuclear swelling and pyknosis were obvious. Mitochondria, ribosomes and euchromatin were visibly reduced. Heterochromatin was notably increased, condensed into a massive, granular shape. Compared with the model group, neuronal cell injury was markedly reduced in all three pretreatment groups; organelles were visible in the cytoplasm, and chromatin appeared normal (Figure 3D–F).
Figure 3.
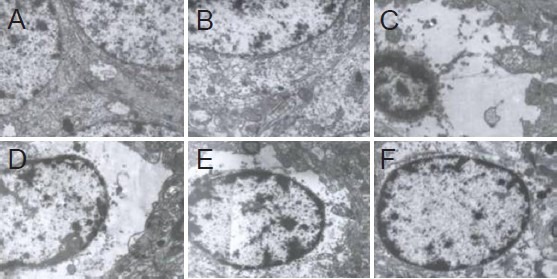
Effects of acupuncture and/or moxibustion pretreatment on ultrastructure of hippocampal neurons in Alzheimer's disease rats (transmission electron microscope, × 8,000).
Normal group (A) and sham surgery group (B): normal ultrastructure. Model group (C) and electroacupuncture group (D): severe swelling surrounding neurons, dark heterochromatin. Moxibustion group (E) and electroacupuncture + moxibustion group (F): mild swelling edema in neurons.
Discussion
Previous studies have shown that Shenshu improves impaired learning and memory and it has been suggested as the main acupoint for Alzheimer's disease treatment. The combined use of Shenshu and Baihui could replenish the kidney essence and marrow, and promote resuscitation. Modern studies have demonstrated that acupuncture and moxibustion at Baihui and Shenshu regulate acetylcholinesterase activity, elevate cortical excitability and cerebral blood flow, improve cerebral circulation, protect against free radical oxidation and neurotoxic injury, suppress inflammation and cell apoptosis, improve learning and memory functions, and effectively treat senile dementia (Mao et al., 2012; Luo et al., 2013).
Numerous clinical and experimental studies confirmed that electroacupuncture dredged meridians, regulated balance, improved and enhanced internal compensation of neural networks, and in particular regulated nervous system disease. Moxibustion noticeably prevents and treats senile dementia (Wu et al., 2008; Yin et al., 2008). The present study demonstrates that electroacupuncture pretreatment has a neuroprotective function in the hippocampus, suggesting that this technique could be used to protect learning and memory functions, and prevent senile dementia. Electroacu-puncture has good repeatability, each parameter being objective and exact, and the technique is easy to implement in the clinic. The G6805-II electroacupuncture therapeutic apparatus used in our study (variable waveform and frequency) has been shown to have stable functions and good therapeutic efficacy in the clinic. The combination of moxibustion and electroacupuncture obtained a better neuroprotective effect than either technique alone, and can be applied in the clinic.
Amyloid-beta peptide injection used in the present study can be applied extensively in Alzheimer's disease models. The materials are economical and readily available, experimental operation is relatively simple, the model is easily replicated, the survival rate of the animals is high after model induction, and the model closely resembles clinical manifestations of the disease. The present study showed that the neurological function was severely damaged in the model rats. However, it has been reported that animal models established by amyloid-beta peptide injection did not have neurofibrillary tangles; this deserves further study. Our results demonstrated that pretreatment with electroacupuncture and moxibustion visibly regulated axin and β-catenin expression in the rat hippocampus. Axin expression was significantly higher in the model group than in the normal and sham surgery groups. Electroacupuncture and moxibustion noticeably diminished axin expression. β-catenin expression was significantly lower in the model group than in the normal and sham surgery groups, but electroacupuncture and moxibustion increased its expression. Axin and β-catenin interact with each other and are strongly associated with Wnt signaling. We therefore propose that the mechanisms of action underlying the potential benefits of acupuncture and moxibustion in the prevention and treatment of Alzheimer's disease involve the regulation of Wnt signaling via alterations in axin and β-catenin expression.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 30772837; the Wuhan Municipal “Morning Sun” Science and Technology Plan, No. 200850731347.
Copyedited by Slone-Murphy J, Stow A, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Burchett SA. Regulators of G Protein Signaling. J Neurochem. 2000;75:1335–1351. doi: 10.1046/j.1471-4159.2000.0751335.x. [DOI] [PubMed] [Google Scholar]
- 2.Clements WM, Lowy AM, Groden J. Adenomatous polyposis coli/β-catenin interaction and downstream targets: altered cene expression in gastrointestinal tumors. Clin Colorectal Cancer. 2003;3:113–120. doi: 10.3816/ccc.2003.n.018. [DOI] [PubMed] [Google Scholar]
- 3.Cui L, Sun G, Zhou H, Du Y. Influence of pre-stimulation with acupuncture and moxibustion on learning and memory ability and the activity of SOD, NOS in hippocampal area of Alzheimer disease model rats. Hubei Zhongyi Xueyuan Xuebao. 2009;3:6–8. [Google Scholar]
- 4.De Robertis E, Larrain J, Oelgeschläger M, Wessely O. The establishment of Spemann's organizer and patterning of the vertebrate embryo. Nat Rev Genet. 2000;1:171–181. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ke H, Sun G, Zhou H. Experimental research of acupunctural pre-stimulation on changing the level of cerebral radicals of AD rats. Hubei Zhongyi Xueyuan Xuebao. 2009;2:14–16. [Google Scholar]
- 6.Luo L, Sun GJ, Du YJ. Effects of acupuncture and moxibustion on energy metabolism-related protein of hippocampai neuron mitochondria in Alzheimer's disease rats. Zhongguo Zhenjiu. 2013;33:913–918. [PubMed] [Google Scholar]
- 7.Mao X, Zhu XD, Jiang XC, Zhang Y. Effect of electroacupuncture treatment on behaviors in a rat model of Alzheimer's disease. Shanghai Zhenjiu Zazhi. 2012;31:764–766. [Google Scholar]
- 8.Nakamura T, Hamada F, Ishidate T, Anai K, Kawahara K, Toyoshima K, Akiyama T. Axin an inhibitor of the Wnt signalling pathway, interacts with β-catenin GSK-3β and APC and reduces the β-catenin level. Genes Cells. 1998;3:395–403. doi: 10.1046/j.1365-2443.1998.00198.x. [DOI] [PubMed] [Google Scholar]
- 9.Wu Q, Yin H, Zeng F. Hippocampal neurogenesis increased in aged cognitive decline rats by marrow-supplementing therapy of moxibustion. Zhongguo Laonianxue Zazhi. 2008;21:2081–2083. [Google Scholar]
- 10.Yin H, Wu Q, Zeng F. The effect of moxibustion on hippocampus stem cell related genes in aged cognitive decline rats. Zhongguo Laonianxue Zazhi. 2008;10:937–939. [Google Scholar]
- 11.Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, Iii, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse fused locus encodes Axin, an inhibitor of the wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]


