Abstract
Ginsenoside Rb1 has been reported to exert anti-aging and anti-neurodegenerative effects. In the present study, we investigate whether ginsenoside Rb1 is involved in neurite outgrowth and neuroprotection against damage induced by amyloid beta (25–35) in cultured hippocampal neurons, and explore the underlying mechanisms. Ginsenoside Rb1 significantly increased neurite outgrowth in hippocampal neurons, and increased the expression of phosphorylated-Akt and phosphorylated extracellular signal-regulated kinase 1/2. These effects were abrogated by API-2 and PD98059, inhibitors of the signaling proteins Akt and MEK. Additionally, cultured hippocampal neurons were exposed to amyloid beta (25–35) for 30 minutes; ginsenoside Rb1 prevented apoptosis induced by amyloid beta (25–35), and this effect was blocked by API-2 and PD98059. Furthermore, ginsenoside Rb1 significantly reversed the reduction in phosphorylated-Akt and phosphorylated extracellular signal-regulated kinase 1/2 levels induced by amyloid beta (25–35), and API-2 neutralized the effect of ginsenoside Rb1. The present results indicate that ginsenoside Rb1 enhances neurite outgrowth and protects against neurotoxicity induced by amyloid beta (25–35) via a mechanism involving Akt and extracellular signal-regulated kinase 1/2 signaling.
Keywords: nerve regeneration, ginsenoside Rb1, hippocampal neurons, neurite outgrowth, apoptosis, amyloid beta protein (25–a35), growth-associated protein-43, Hoechst-33258 staining, PD98059, API-2, Akt and ERK1/2 signaling, NSFC grant, neural regeneration
Introduction
The world population is rapidly aging. In aging-related diseases, neurons and neurites become dystrophic and undergo progressive degeneration, resulting in neuronal loss. However, the underlying mechanisms are poorly understood, and clinical treatments show only limited effectiveness. Recently, it has been reported that oral administration of ginsenosides significantly reduces the levels of amyloid beta peptide in the brains of mouse models of Alzheimer's disease (Chen et al., 2006). Ginsenoside Rb1 is one of the active components of ginseng, and accumulating evidence shows that ginsenoside Rb1 possesses neurotrophic and neuroprotective properties in many different cell lines, as well as having anti-amnestic and anti-aging effects (Cheng et al., 2005). For example, it facilitates memory acquisition and retrieval, and ameliorates memory loss and cognitive deficits under various pathological conditions such as cerebral ischemia and dementia (Wen et al., 1996; Mook-Jung et al., 2001; Wang et al., 2001; Wang et al., 2010). Studies in vivo have also demonstrated that ginsenoside Rb1 improves spatial learning and increases hippocampal synaptophysin levels in mice (Mook-Jung et al., 2001), and upregulates plasticity-related proteins in the hippocampus of the senescence accelerated mouse-prone 8 (SAMP8) model (Zhao et al., 2001). In addition, pretreatment of ginsenoside Rb1 can attenuate amyloid beta-induced phosphorylation of tau protein (Li et al., 2005; Xie et al., 2007; Chen et al., 2008; Jia et al., 2011). However, the mechanisms underlying these actions are poorly understood.
Cellular signaling is involved in many physiological and pathological processes such as neurite outgrowth, cellular survival and death. There are two distinct and separable signaling pathways: the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and the mitogen-activated protein kinase (MAPK) pathway. The PI3K/Akt signaling pathway is well-known for its role in cell survival and anti-apoptosis. The MAPK pathway has been implicated in hippocampal synaptic plasticity and hippocampus-dependent memory formation (Liu et al., 2011). There are three major MAPK groups: extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinases, and p38 MAPK (Peti et al., 2013). Recently, the neuroprotective effects of ginsenoside Rb1 and tau hyperphosphorylation were blocked by LY294002, a PI3K inhibitor, in cultured cortical neurons (Zhao et al., 2011). Furthermore, ginsenoside Rb1 was shown to attenuate amyloid beta (25–35)-induced tau hyperphosphorylation in cortical neurons via inhibition of cyclin-dependent kinase-5 activity and through the c-Jun N-terminal kinase/p38 MAPK pathway (Chen et al., 2008; Song et al., 2008).
Hippocampal neurons are vulnerable under various pathological conditions. The hippocampus plays a major role in learning and memory, and is the region where senile plaques generate in patients with Alzheimer's disease. However, the mechanisms underlying the effects of ginsenoside Rb1 on hippocampal neurons are unclear. In the present study, we investigated the effects of ginsenoside Rb1 on neurite outgrowth and amyloid beta (25–35)-induced neurotoxicity in cultured hippocampal neurons and explored whether the Akt and ERK1/2 signaling pathways were involved in these biological processes.
Materials and Methods
Animals
Sixty newborn Sprague-Dawley rats (30 female, 30 male, average 2 g, less than 24 hours old), were purchased from the Shanghai Institute of the Chinese Academy of Science in China (license No. SCXK (Hu) 2007–0003). Animal care and experimental protocols were approved by the Chinese Academy of Sciences, China, ensuring that animal numbers and suffering were kept to a minimum. All experiments were performed according to the Guidelines laid down by the National Institutes of Health in the US regarding the care and use of animals for experimental procedures, and were approved by the Ethics Committee of Tongji University in China.
Primary culture of hippocampal neurons
Primary hippocampal neurons were prepared as described previously (Banker et al., 1997), with some modifications. In brief, hippocampi from newborn rats were dissected in cold modified Krebs-Ringer solution. After removal of meninges, the tissue was roughly minced by chopping with a scalpel blade, and trypsinized (0.25% bovine trypsin; Sigma Aldrich, St. Louis, MO, USA) for 9–10 minutes at 37°C. The dissociated cells were then washed in the presence of DNAse I and soybean trypsin inhibitor (Sigma Aldrich) and gently triturated through a series of fire-polished constricted Pasteur pipettes. The cells were cultured in Neurobasal™ medium and supplemented with 2% B27 supplement, 10 μL/mL penicillin-streptomycin, 1% glutamax, 0.4% bovine serum albumin and 20 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid at 37°C in a humidified 5% CO2 incubator (Thermo Fisher Scientific Inc, Waltham, MA, USA) for 24 hours.
Neurite outgrowth of hippocampal neurons in vitro
To observe neurite outgrowth by immunocytochemical staining, primary hippocampal neurons were plated at a density of 1 × 104/cm2 onto coverslips precoated with 10 mg/mL poly-L-lysine in 24-well plates. After 24 hours of culture, the medium was refreshed and the test cells were treated with 50 μmol/L ginsenoside Rb1 in the presence or absence of 10 μmol/L API-2 (Akt inhibitor; Tocris Cookson Inc., Ellisville, MO, USA) or 10 μmol/L PD98059 (MAPK kinase (MEK) inhibitor; Tocris Cookson Inc.) for 24 hours.
The cells were divided into groups: (1) untreated group (normal control): no treatments for 48 hours; (2) ginsenoside Rb1 group: treated with 50 μmol/L ginsenoside Rb1 for the second 24 hours of incubation; (3) ginsenoside Rb1 and PD98059 group: treated with 50 μmol/L ginsenoside Rb1 + 10 μmol/L PD98059 for the second 24 hours of incubation; (4) ginsenoside Rb1 and API-2 group: treated with 50 μmol/L ginsenoside Rb1 + 10 μmol/L API-2 for the second 24 hours of incubation. Ginsenoside Rb1 was dissolved in 0.9% NaCl at a concentration of 2 mmol/L as stock solution and stored at –80°C. PD98059 and API-2 were dissolved in dimethyl sulfoxide to a concentration of 25 mmol/L and stored at –80°C. Ginsenoside Rb1 and inhibitors were added to the culture medium at corresponding final concentrations. The inhibitors were added to the medium immediately after ginsenoside Rb1 treatment.
For western blot assay and enzyme-linked immunosorbent assay analyses, the primary hippocampal neurons were seeded in 60 mm diameter flasks precoated with 10 mg/mL poly-L-lysine at 2 × 105/cm2 for 24 hours. Then the medium was refreshed and cultures were treated as described above. After a further 24 hours of culture, the supernatants were collected and stored at −80°C for enzyme-linked immunosorbent assay, and the cell pellets were collected for western blot assay.
Growth-associated protein-43 immunocytochemical staining
After primary neurons were treated with ginsenoside Rb1 and inhibitors for 24 hours in 24-well plates as described above, the cells were fixed in 4% paraformaldehyde for 30 minutes at room temperature and incubated with blocking solution (5% bovine serum albumin) for further 30 minutes, at 37°C in a moisture chamber, to block nonspecific binding. The cells were then incubated with anti-rat growth-associated protein-43 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA; 1:1,000 dilution) overnight at 4°C. After washing with PBS three times for 5 minutes each time, cells were then incubated with peroxidase-conjugated rabbit anti-mouse IgG secondary antibody (Beyotime Institute of Biotechnology Co., Ltd., Haimen, China; 1:100 dilution) at 37°C for 2 hours. Finally, the cells were incubated with avidin-biotin complex (Beyotime; 1:100 dilution) at 37°C for 1.5 hours. Diaminobenzidine (Sigma Aldrich) was used as a chromogen for light microscopy. A negative control was carried out using the same procedures without primary antibody. Four separate experiments were performed.
Neurite outgrowth in cell cultures was analyzed under a light microscope (Nikon, Tokyo, Japan) by an investigator blinded to the groups. The neuronal cell bodies were counted (n = 100 each well) and the lengths of neurites from the cell bodies were measured. The ratio between the lengths of neurites and the numbers of cell bodies was used as an estimate of the average neurite length.
Hippocampal neurons exposed to amyloid beta (25–35) in vitro
The primary hippocampal neurons were prepared as described above, and plated onto poly-L-lysine-coated coverslips at 4 × 104/cm2 in 24-well plates. After 24 hours of culture, the medium was refreshed, and the cells were divided into groups: (1) untreated group (normal control); (2) amyloid beta group: cells treated with 20 μmol/L amyloid beta (25–35) (Tocris Cookson Inc.) for 48 hours; (3) insulin-like growth factor-1-positive group: 50 ng/mL insulin-like growth factor-1 (Tocris Cookson Inc.) was added to the medium after treatment with 20 μmol/L amyloid beta (25–35) for 30 minutes; (4) ginsenoside Rb1 group: 50 μmol/L ginsenoside Rb1 (The National Institute For the Control of Pharmaceutical and Biological Products, Beijing, China) was added to the medium after treatment with 20 μmol/L amyloid beta (25–35) for 30 minutes; (5) ginsenoside Rb1 + PD98059 group: 50 μmol/L ginsenoside Rb1 and 10 μmol/L PD98059 were added to the medium after treatment with 20 μmol/L amyloid beta (25–35) for 30 minutes; (6) ginsenoside Rb1 + API-2 group: 50 μmol/L ginsenoside Rb1 and 10 μmol/L API-2 were added to the medium after treatment with 20 μmol/L amyloid beta (25–35) for 30 minutes. API-2 and PD98059 were added immediately after ginsenoside Rb1 treatment. All cultures were then incubated for an additional 48 hours. The cells were fixed by 4% paraformaldehyde for 30 minutes at room temperature for Hoechst-33258 staining. IGF-1 was dissolved in sterile ionized water at a concentration of 100 μg/mL and 3 mmol/L as stock solution and stored at −20°C.
For the western blot assay, the primary hippocampal neurons were plated onto poly-L-lysine coated coverslips at 2 × 105/cm2 in 60 mm flasks. After 24 hours, the cells were treated with amyloid beta (25–35) and then treated with ginsenoside Rb1 and inhibitors as described above. After 48 hours, the supernatants were collected and stored at −80°C for enzyme-linked immunosorbent assay; the cell precipitates were collected for western blot assay.
Hoechst-33258 staining
For nuclear staining, Hoechst-33258 (Beyotime) was added to the medium and incubated at 37°C for 15 minutes. Fluorescence images were obtained with an inverted fluorescence microscope (Nikon). Apoptotic and viable cells (n = 200 each well) were counted blindly and the percentage of apoptotic neurons was calculated, i.e., apoptotic rate = apoptotic cells/(apoptotic cells + viable cells) × 100%. Four separate experiments were performed.
Western blot assay
Cells were washed in ice-cold PBS and lysed in radioimmunoprecipitation assay lysis buffer containing 50 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, 2 mmol/L ethylenediaminetetraacetic acid, 25 mmol/L NaF, 0.1% sodium dodecyl sulphate, 1% NP-40, 0.5% Na-deoxycholate, 1 mmol/L Na3 VO4 1 mmol/L phenylmethyl sulfonylfluoride and 1 μg/mL leupeptin, aprotinin, pepstatin each for 30 minutes at 4°C followed by centrifugation at 18,514 × g for 30 minutes at 4°C. The supernatant was collected and protein content was determined with a bicinchoninic acid protein assay kit (Beyotime). Samples (50 μg protein) were dissolved in sample buffer and boiled for 5 minutes prior to loading onto polyacrylamide gels. The concentrations of separation gel and stacking gel were 12% and 5%, respectively. Proteins were then transferred to polyvinylidene fluoride membranes (Beyotime), and blocked with 5% non-fat dry milk in Tris-buffered saline/0.05% Tween-20. The membranes were incubated with primary antibodies (1:1,000 dilution) against phospho-ERK1/2 (Thr202/Tyr204), ERK1/2 (rabbit anti-rat phospho-ERK1/2, rabbit anti-rat ERK1/2; Cell Signaling Technology, Beverly, MA, USA), phospho-Akt (Ser473) and Akt (rat anti-human phospho-Akt, rat anti-human Akt; Cell Signaling Technology), followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit or anti-rat IgG (Cell Signaling Technology; dilution 1:3,000) for 1 hour at room temperature and visualized using an enhanced chemiluminescence kit (Beyotime). Quantification of protein bands was achieved by densitometric analysis using Gelpro 32 image analysis software. The ratio of phospho-ERK1/2 and ERK1/2, phospho-Akt and Akt to the total absorbance of the respective proteins of GAPDH was normalized to 1. Three separate experiments were performed.
Enzyme-linked immunosorbent assay
The concentrations of nerve growth factor, neurotrophin-3 and brain-derived neurotrophic factor in the above supernatants were detected using biotinylated goat anti-rat nerve growth factor, biotinylated goat anti-human neurotrophin-3 and mouse monoclonal anti-brain-derived neurotrophic factor antibodies, respectively (all 1:1,000 dilution; all from R&D Systems Inc., Minneapolis, MN, USA) in a double antibody sandwich ABC-enzyme-linked immunosorbent assay kit (Beyotime) according to the manufacturer's instructions. The absorbance value of the colored product was read at 450 nm in a microplate reader (Bio-Rad Laboratories, Richmond, CA, USA) within 30 minutes. The concentrations of nerve growth factor, neurotrophin-3, and brain-derived neurotrophic factor were calculated based on a standard curve. Four separate experiments were performed.
Statistical analysis
Results were expressed as mean ± SD. Data were analyzed using SPSS 18.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance was conducted followed by Student-Newman-Keuls test to assess the differences between the relevant control and each experimental group. A value of P < 0.05 was considered statistically significant.
Results
Ginsenoside Rb1 promoted neurite outgrowth in hippocampal neurons, and this effect was reversed by PD98059 and API-2
Ginsenoside Rb1 treatment enlarged cell bodies and increased the number of neurites and the length of neurites compared with untreated cells (Figure 1). After treatment with ginsenoside Rb1 plus PD98059 for 24 hours, cells showed irregular margins and condensed cell bodies, consistent with nutritional deficiency. Moreover, after treatment with ginsenoside Rb1 plus API-2 for 24 hours, the majority of hippocampal neurons were fragmented into debris, with very few viable cells. The small number of viable cells that were detected had short neurites. Statistical analysis showed that the length of neurites of cells treated with ginsenoside Rb1 was significantly greater than those of untreated cells, and this effect was completely reversed by API-2 (P < 0.01), and partly reversed by PD98059 (P < 0.05).
Figure 1.
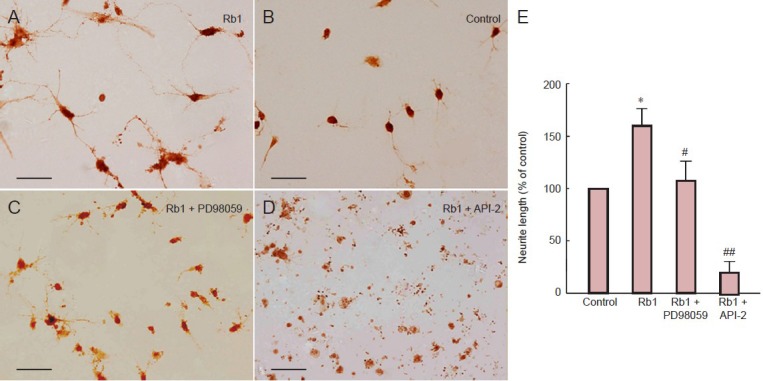
Ginsenoside Rb1 (Rb1) promotes neurite outgrowth in hippocampal neurons.
(A–D) Hippocampal neurons immunostained with growth associated protein-43 antibody. (A) Untreated primary hippocampal neurons cultured for 48 hours. (B) Primary hippocampal neurons cultured for 24 hours then incubated with Rb1 for 24 hours show enlarged cellular bodies and more numerous and longer neurites. (C) Primary hippocampal neurons cultured for 24 hours then incubated with Rb1 plus PD98059 (MEK1/2 inhibitor; 10 μmol/L) for 24 hours show irregular margins and condensed bodies, consistent with nutritional deficiency. (D) Primary hippocampal neurons cultured for 24 hours then incubated with Rb1 plus API-2 (Akt inhibitor; 10 L) for 24 hours show very few viable cells with few and short neurites. Scale bars: 200 μm. (E) Neurite length in primary hippocampal neurons after incubation with Rb1 in the presence or absence of 10 L API-2 or 10 L PD98059, immunostained for growth associated protein-43. The ratio between the lengths of neurites and the number of cell bodies was used as an estimate of the average neurite length. Relative value of neurite length was calculated by the average neurite length of the treatment group divided by that of the control group. Data are expressed as mean ± SD (n = 9; one-way analysis of variance and Student-Newman-Keuls test). *P < 0.05, vs. control group (untreated cells); #P < 0.05, ##P < 0.01, vs. Rb1.
Ginsenoside Rb1 increased phosphorylation of Akt and ERK1/2 in cultured hippocampal neurons
To further confirm whether ginsenoside Rb1-induced neurite outgrowth involved the Akt and ERK1/2 signaling pathways, phosphorylation levels of Akt and ERK1/2, including phosphorylated-Akt (p-Akt) and phosphorylated extracellular signal-regulated kinase 1/2 (p-ERK1/2) were measured by western blot assay. p-Akt and p-ERK1/2 levels in hippocampal neurons were significantly greater in cells treated with ginsenoside Rb1 for 24 hours than in control (untreated) cells (P < 0.01; Figure 2). By contrast, p-Akt levels were significantly lower after treatment with ginsenoside Rb1 and API-2 than after ginsenoside Rb1 alone, and even lower than those in untreated cells. In addition, after treatment with ginsenoside Rb1 and PD98059 for 24 hours, p-ERK1/2 levels were notably decreased compared with those after ginsenoside Rb1 alone (P < 0.01), suggesting that ginsenoside Rb1 activated Akt and ERK1/2 signaling.
Figure 2.
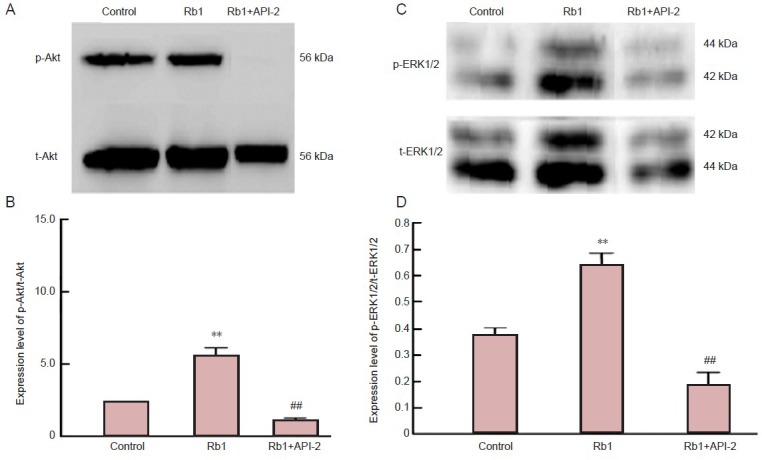
Ginsenoside Rb1 (Rb1) increases expression of phosphorylated Akt (p-Akt; A, B) and extracellular signal-regulated kinase 1/2 (p-ERK1/2; C, D) in cultured hippocampal neurons.
Primary hippocampal neurons were cultured for 24 hours, then 50 μmol/L Rb1 with or without 10 L API-2 (Akt inhibitor) or 10 L PD98059 (MEK1/2 inhibitor) was added to the medium for culture for an additional 24 hours. Absorbance ratio of p-ERK1/2/total ERK1/2 (t-ERK1/2) and of p-Akt /total Akt (t-Akt) were detected by western blot assay. **P < 0.01, vs. control group; ##P < 0.01, vs. Rb1. Data are expressed as mean ± SD (n = 9; one-way analysis of variance and Student-Newman-Keuls test).
Ginsenoside Rb1 prevented apoptosis of hippocampal neurons after exposure to amyloid beta (25–35) and this effect was blocked by PD98059 and API-2
After hippocampal neurons were treated with amyloid beta (25–35) for 2 days, fewer viable cells and fewer or no neurites were observable in the culture using inverted phase contrast microscopy; in contrast, viable cells with longer and more numerous neurites were observed in the untreated group (Figure 3A, B). Apoptotic and viable nuclei were visualized using Hoechst-33258 staining (Figure 3C). Viable cells exhibited regular and round nuclei with pallid blue fluorescence, whereas apoptotic cells were characterized by condensation and fragmentation of nuclei. The number of apoptotic cells after amyloid beta treatment was significantly greater than that of untreated cells (Figure 3D; P < 0.01). There were significantly fewer apoptotic cells after treatment with ginsenoside Rb1 than after amyloid beta (Figure 3D; P < 0.05). However, this protective effect of ginsenoside Rb1 against amyloid beta exposure was reversed by PD98059 and API-2 (Figure 3D; P < 0.01).
Figure 3.
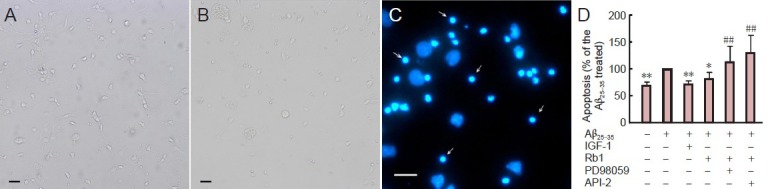
Ginsenoside Rb1 (Rb1) prevents apoptosis of hippocampal neurons and involves Akt and ERK1/2 signaling.
Primary hippocampal neurons were cultured for 24 hours and amyloid beta (25–35) (Aβ25–35) was added to the cultures for 30 minutes, then Rb1 with or without 10 μmol/L API-2 (Akt inhibitor) or 10 μmol/L PD98059 (MEK1/2 inhibitor) was added to the cultures for 48 hours. Insulin-like growth factor-1 (IGF-1) was added as a positive control. Cells without Aβ25–35 treatment were considered normal controls. Hoechst-33258 staining was used to evaluate the apoptotic (condensed bright fluorescence, indicated by arrows) and viable (pallid blue fluorescence) nuclei. (A) Hippocampal neurons cultured for 3 days under inverted phase contrast microscope. (B) Hippocampal neurons cultured for 24 hours followed by exposure to Aβ25–35 for 48 hours under inverted phase contrast microscope. (C) Hoechst 33258 staining shows apoptotic (arrows) and viable cells. (A-C) Scale bars: 200 μm. (D) Percentage of apoptotic cells. Data expressed as mean ± SD (n = 9; one-way analysis of variance and Student-Newman-Keuls test). *P < 0.05, **P < 0.01, vs. Aβ25–35; ##P < 0.01, vs. Rb1. Rb1 prevented apoptosis of hippocampal neurons after exposure to Aβ25–35. This effect was blocked by PD98059 and API-2.
Ginsenoside Rb1 reversed amyloid beta (25–35)-induced inactivation of Akt and ERK1/2 in hippocampal neurons in vitro
As reported above, ginsenoside Rb1 treatment protected against amyloid beta-induced apoptosis and this effect was blocked by PD98059 and API-2. To confirm the possible link between the Akt and ERK1/2 signaling pathways and ginsenoside Rb1-mediated neuroprotection, we measured p-Akt and p-ERK1/2 expression by western blotting. Amyloid beta treatment significantly decreased p-Akt and p-ERK1/2 levels compared with untreated (control) cells (Figure 4; P < 0.01), indicating that amyloid beta inhibits the activation of ERK1/2 and Akt. Insulin-like growth factor-1 is a neuronal survival factor and prevents amyloid beta-induced apoptosis (Jimenez et al., 2006). As a positive control in the present study, insulin-like growth factor-1 increased p-Akt and p-ERK1/2 levels compared to amyloid beta treatment (Figure 4; P < 0.01). Ginsenoside Rb1 treatment significantly increased p-Akt and p-ERK1/2 levels compared with amyloid beta treatment (P < 0.01), but these effects were completely blocked by API-2 (Figure 4; P < 0.01).
Figure 4.
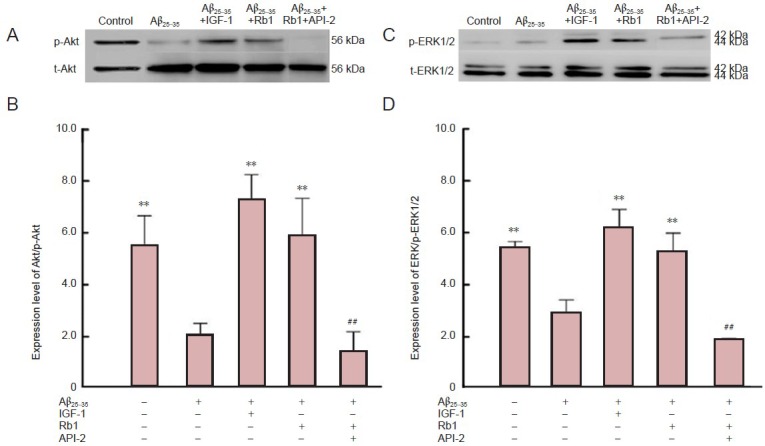
Ginsenoside Rb1 (Rb1) reverseds amyloid beta (25–35) (Aβ25–35)-induced inactivation of Akt and extracellular signal-regulated kinase 1/2 (ERK1/2) in hippocampal neurons.
Expression levels of Akt/phosphorylated Akt (p-Akt; A, B) and ERK1/2/phosphorylated ERK1/2 (p-ERK1/2; C, D) detected by western blot assay in injured hippocampal neurons. After primary hippocampal neurons were cultured for 24 hours, Aβ25–35 was added to the cultures for 30 minutes. Rb1 in the presence or absence of API-2 or PD98059 was added for 48 hour culture. In addition, insulin-like growth factor-1 (IGF-1) was added to a further group as a positive control. Cells without Aβ25–35 treatment were considered normal controls. Relative expression of p-ERK1/2 and p-Akt were detected by western blot assay. Data are expressed as mean ± SD (n = 9; one-way analysis of variance and Student-Newman-Keuls test). **P < 0.01, vs. Aβ25–35; ##P < 0.01, vs. Rb1.
Neurotrophins may not mediate the effects of ginsenoside Rb1 on cultured hippocampal neurons
Neurotrophins, mainly comprising nerve growth factor, brain-derived neurotraphic factor and neurotrophin-3, are crucial for neurite outgrowth and survival of neurons. Thus, to determine whether the observed effects of ginsenoside Rb1 on hippocampal neurons are implicit in increasing neurotrophin secretion in these neurons, we used enzyme-linked immunosorbent assay to measure the content of neurotrophins in the supernatants from the neurite outgrowth and amyloid beta (25–35) exposure experiment. Interestingly, no significant differences in neurotrophin-3, nerve growth factor or brain-derived neurotraphic factor were detected among the groups (data not shown).
Discussion
Here, we demonstrate, for the first time, that ginsenoside Rb1 promotes neurite outgrowth of cultured hippocampal neurons and exerts a neuroprotective effect against amyloid beta (25–35) exposure in hippocampal neurons through Akt and ERK1/2 signaling.
Previous reports have shown that ginsenoside Rb1 induces significant neurite outgrowth in human neuroblastoma SK-N-SH cells and potentiates nerve growth factor-induced neurite outgrowth in cultured chick embryonic dorsal root ganglia (Zou et al., 2002; Nishiyama et al., 1994). Our findings that ginsenoside Rb1 promotes neurite outgrowth of hippocampal neurons in vitro suggest a role for ginseng as a preventative therapy for aging-related diseases such as Alzheimer's. Contrary to our results, Radad et al. (2004a) reported that ginsenoside Rb1 could not promote neurite outgrowth of mesencephalic dopaminergic cells in vitro. This difference may arise from different kinds of neurons. We observed that API-2 not only abrogated ginsenoside Rb1-induced neurite outgrowth, but also induced apoptosis of many cells, and that PD98059 only neutralized ginsenoside Rb1-induced neurite outgrowth. Moreover, ginsenoside Rb1 significantly enhanced p-Akt and p-ERK1/2 and this effect was completely blocked by API-2. However, PD98059 reversed ginsenoside Rb1-induced ERK1/2 activation. Taken together, these results indicate that ERK1/2 and Akt signaling are involved in ginsenoside Rb1-induced neurite outgrowth in hippocampal neurons.
There are differing reports concerning the neuroprotective effect of ginsenoside Rb1 against various insults. Liao et al. (2002) reported that ginsenoside Rb1 protected spinal cord neurons from the excitotoxicity induced by glutamate and kainic acid, as well as oxidative stress induced by H2O2 (Liao et al., 2004). Lee et al. (2002) also showed in vivo that ginsenoside Rb1 inhibited cell death caused by kainic acid in both the CA1 and CA3 regions of rat hippocampus and protected hippocampal neurons from ischemia (Lee et al., 2004). By contrast, Radad et al. (2004a, b) demonstrated that ginsenoside Rb1 (pre-treated or post-treated) could not prevent the dopaminergic cell loss produced by glutamate and MPP+. The failure of these ginsenosides to antagonize cell loss by MPP+ may be attributed to the inability of these ginsenosides to block the selective uptake of MPP+ by dopamine neurons via a high-affinity dopamine transporter or to overcome its inhibitory effects on mitochondria and production of overt free radicals or due to their inability to antagonize N-Methyl-D-aspartic acid receptor over-activation by glutamate particularly at the early stage. In the present study, we have shown that ginsenoside Rb1 reverses amyloid beta-induced apoptosis, and that this effect is blocked by PD98059 and API-2. All of this showed that different cells would be led to different biological effects through different pathways. Other researchers showed that ginsenoside Rb1 significantly decreased cortical neuron apoptosis induced by amyloid beta (25–35) or amyloid beta (1–42) in a dose-dependent manner (Qian et al., 2009; Xie et al., 2010; Zhao et al., 2011). Additionally, other reports have shown that ginsenoside Rb1 protection against amyloid beta neurotoxicity appears to have an anti-neuroinflammation effect and that this effect is likely mediated by cyclin-dependent kinase 5 in rat models of Alzheimer's disease (Chen et al., 2008; Wang et al., 2011). Taken together, these results suggest that ginsenoside Rb1 may prevent hippocampal neuronal apoptosis induced by amyloid beta (25–35) and that the Akt and ERK1/2 signaling pathways may be involved in ginsenoside Rb1 neuroprotection against amyloid beta (25–35) neurotoxicity.
Notably, there are differing reports about the effects of amyloid beta exposure on p-Akt and p-ERK1/2 (Takashima et al., 1996; Takashima et al., 1998; Lesné et al., 2005; Zhao et al., 2011). Zhao et al. (2011) reported that Akt activation is inhibited in cells exposed to amyloid beta compared with untreated cells. Lesné et al. (2005) showed that early activation of Akt and ERK1/2 was initially induced by amyloid beta (25–35), followed by a rapid decrease of p-Akt to basal levels, but levels of ERK1/2 were not significantly different from those in untreated cells after exposure for 12 and 24 hours, suggesting that longer amyloid beta (25–35) exposure inhibits activation of Akt, but does not affect activation of ERK1/2. By contrast, Rapoport and Ferreira reported that amyloid beta (1–40) activates ERK1/2 and sustains activated ERK1/2 in mature hippocampal neurons (Rapoport et al., 2000). Amyloid beta peptide, a 39–43 amino acid β-sheet peptide, is the major protein component of senile plaques in the Alzheimer's disease brain. Amyloid beta (25–35) is a short amyloid beta fragment that exhibits large β-sheet fibrils and retains the same neurotoxicity as the full-length peptide (Benseny-Cases et al., 2012). Our present results show that amyloid beta treatment inhibits p-Akt and p-ERK1/2 to levels below those in untreated cells, consistent with the majority of previous reports. This difference observed by Rapoport and Ferreira may result from characteristics of mature hippocampal neurons. Together, these results suggest that amyloid beta (25–35) exposure may lead to inactivation of Akt and may produce different effects on ERK1/2 signaling depending on exposure time and different kinds of cells.
In addition, ginsenoside Rb1 can increase p-Akt levels, an effect blocked by LY294002 (Zhao et al., 2011), also suggesting that ginsenoside Rb1 protection against amyloid beta-induced neurotoxicity is mediated by PI3K/Akt signaling. Consistent with these results, we have demonstrated that ginsenoside Rb1 reverses inactivation of Akt and ERK1/2 induced by amyloid beta (25–35), and that these effects are completely abolished by API-2. Along with the increase in p-Akt and p-ERK1/2 observed after ginsenoside Rb1 treatment, we also found that ginsenoside Rb1 could prevent the apoptosis induced by amyloid beta (25–35) exposure. Therefore, we speculate that ginsenoside Rb1 prevents apoptosis of hippocampal neurons by activating the Akt and ERK1/2 signaling pathways. In our experiment, IGF-1 was used as a positive control because it is a neuronal survival factor in the treatment of Alzheimer's disease and prevents amyloid beta (25–35)-induced apoptosis (Jimenez et al., 2006). As expected, IGF-1 increased survival of hippocampal neurons and, in parallel, increased p-Akt and p-ERK1/2 levels in hippocampal neurons after amyloid beta (25–35) exposure.
However, how ginsenoside Rb1 affects Akt and ERK1/2 signaling remained unclear. Neurotrophins are thought to be crucial for neurite outgrowth and for the survival of neurons in the developing nervous system and under various types of insult (Koh et al., 1995). They act at a set of high-affinity tyrosine kinase (Trk) receptors (Skaper, 2012). Upon activation, Trk receptors are autophosphorylated within tyrosine residues located at the cytoplasmic tails, which serve as docking sites for kinases such as PI3K. Therefore, we asked whether the effects of ginsenoside Rb1 on hippocampal neurons occur by an increase in neurotrophin secretion. However, no significant difference was detected, indicating that the observed ginsenoside Rb1-induced effects are not mediated by increases in neurotrophins in hippocampal neurons. Our previous experiments demonstrated that ginsenoside Rb1 did increase brain-derived neurotraphic factor levels in rats following cerebral ischemia (Gao et al., 2010). In addition, ginsenoside Rb1 is known to increase TrkA mRNA in the basal forebrain and nerve growth factor mRNA in the hippocampus (Salim et al., 1997). Therefore, our present results suggest that the increased neurotrophins induced by ginsenoside Rb1 in previous studies might derive from glial cells in rats. To date, there is no report indicating that ginsenoside Rb1 can increase secretion of neurotrophins in neurons in vitro. Together, these results indicate that the mechanism underlying the neurotrophic and neuroprotective effects of ginsenoside Rb1 does not involve an increase in secretion of neurotrophins in hippocampal neurons.
In summary, the present study is the first to reveal that ginsenoside Rb1 promotes neurite outgrowth in primary hippocampal neurons and protects these neurons against amyloid beta exposure via the Akt and ERK1/2 signaling pathways. Activated Akt may be required for neurite outgrowth as well as survival of hippocampal neurons. Whether ginsenoside Rb1 crosses the cellular membrane and acts intracellularly or triggers a signaling cascade from the cell membrane remains to be explored.
Footnotes
Conflicts of interest: None declared.
Funding: This work was supported by grants from the National Natural Science Foundation of China, No. 30971531, 81070987.
Copyedited by Slone-Murphy J, Frenchman B, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1997;126:397–342. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- 2.Benseny-Cases N, Klementieva O, Cladera J. In vitro oligomerization and fibrillogenesis of amyloid-beta peptides. Subcell Biochem. 2012;65:53–74. doi: 10.1007/978-94-007-5416-4_3. [DOI] [PubMed] [Google Scholar]
- 3.Chen F, Eckman EA, Eckman CB. Reductions in levels of the Alzheimer's amyloid beta peptide after oral administration of ginsenosides. FASEB J. 2006;20:1269–1271. doi: 10.1096/fj.05-5530fje. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Huang T, Zhang J, Song J, Chen L, Zhu Y. Involvement of calpain and p25 of CDK5 pathway in ginsenoside Rb1's attenuation of beta-amyloid peptide 25-35-induced tau hyperphosphorylation in cortical neurons. Brain Res. 2008;1200:99–106. doi: 10.1016/j.brainres.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y, Shen LH, Zhang JT. Anti-amnestic and anti-aging effects of ginsenoside Rb1 and Rg1 and its mechanism of action. Acta Pharmacol Sin. 2005;26:143–149. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 6.Gao XQ, Yang CX, Chen GJ, Wang GY, Chen B, Tan SK, Liu J, Yuan QL. Ginsenoside Rb1 regulates the expressions of brain-derived neu rotrophic factor and caspase-3 and induces neurogenesis in rats with experimental cerebral ischemia. J Ethnopharmacol. 2010;132:393–399. doi: 10.1016/j.jep.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 7.Jia LY, Pan XH, Liu J, Cui X, Wang ML. Vol. 49. Yi Xue Ban: Shan Dong Da Xue Xue Bao; 2011. Protective effects of ginsenoside Rb1 and Re on SK-N-SH cells injuried by Aβ 25-35; pp. 33–37. [Google Scholar]
- 8.Jimenez Del Rio M, Velez-Pardo C. Insulin-like growth factor-1 prevents Abeta[25-35]/(H2O2)-induced apoptosis in lymphocytes by reciprocal NF-kappa B activation and p53 inhibition via PI3K-dependent pathway. Growth Factors. 2006;24:67–78. doi: 10.1080/08977190500361788. [DOI] [PubMed] [Google Scholar]
- 9.Koh JY, Gwag BJ, Lobner D, Lobner D, Choi DW. Potentiated necrosis of cultured cortical neurons by neurotrophins. Science. 1995;268:573–575. doi: 10.1126/science.7725105. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Kim SR, Bae CS, Kim D, Hong H, Nah S. Protective effect of ginsenosides, active ingredients of panax ginseng, on kainic acid-induced neurotoxicity in rat hippocampus. Neurosci Lett. 2002;325:129–133. doi: 10.1016/s0304-3940(02)00256-2. [DOI] [PubMed] [Google Scholar]
- 11.Lesné S, Gabriel C, Nelson DA, White E, Mackenzie ET, Vivien D, Buisson A. Akt-dependent expression of NAIP-1 protects neurons against amyloid-{beta} toxicity. J Biol Chem. 2005;280:24941–24947. doi: 10.1074/jbc.M413495200. [DOI] [PubMed] [Google Scholar]
- 12.Liao B, Newmark H, Zhou R. Neuroprotective effect of ginseng total saponin and ginsenosides Rb1 and Rg1 on spinal cord neurons in vitro. Exp Neurol. 2002;173:224–234. doi: 10.1006/exnr.2001.7841. [DOI] [PubMed] [Google Scholar]
- 13.Li YK, Chen XC, Zhu YQ, Peng XS, Zeng YQ, Sheng J, Huang TW. Ginsenoside Rb1 attenuates okadaic acid-induced Tau protein hyperphosphorylation in rat hippocampal neurons. Sheng Li Xue Bao. 2005;57:154–160. [PubMed] [Google Scholar]
- 14.Liu MG, Wang RR, Chen XF, Zhang FK, Cui XY, Chen J. Differential roles of ERK, JNK and p38 MAPK in pain-related spatial and temporal enhancement of synaptic responses in the hippocampal formation of rats: multi-electrode array recordings. Brain Res. 2011;1382:57–69. doi: 10.1016/j.brainres.2011.01.076. [DOI] [PubMed] [Google Scholar]
- 15.Mook-Jung I, Hong HS, Boo JH, Lee KH, Yun SH, Cheong MY, Joo I, Huh K, Jung MW. Ginsenoside Rb1 and Rg1 improve spatial learning and increase hippocampal synaptophysin level in mice. J Neurosci Res. 2001;63:509–515. doi: 10.1002/jnr.1045. [DOI] [PubMed] [Google Scholar]
- 16.Nishiyama N, Cho SI, Kitagawa I, Saito H. Malonylginsenoside Rb1 potentiates nerve growth factor (NGF)-induced neurite outgrowth of cultured chick embryonic dorsal root ganglia. Biol Pharm Bull. 1994;17:509–513. doi: 10.1248/bpb.17.509. [DOI] [PubMed] [Google Scholar]
- 17.Peti W, Page R. Molecular basis of MAP kinase regulation. Protein Sci. 2013;22:1698–1710. doi: 10.1002/pro.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian YH, Han H, Hu XD, Shi LL. Protective effect of ginsenoside Rb1 on beta-amyloid protein(1-42)-induced neurotoxicity in cortical neurons. Neurol Res. 2009;31:663–667. doi: 10.1179/174313209X385572. [DOI] [PubMed] [Google Scholar]
- 19.Radad K, Gille G, Moldzio R, Saito H, Ishige K, Rausch WD. Ginsenosides Rb1 and Rg1 effects on survival and neurite growth of MPP+ -affected mesencephalic dopaminergic cells. J Neural Transm. 2004a;111:37–45. doi: 10.1007/s00702-003-0063-1. [DOI] [PubMed] [Google Scholar]
- 20.Radad K, Gille G, Moldzio R, Saito H, Rausch WD. Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004b;1021:41–53. doi: 10.1016/j.brainres.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 21.Rapoport M, Ferreira A. PD98059 prevents neurite degeneration induced by fibrillar beta-amyloid in mature hippocampal neurons. J Neurochem. 2000;74:125–133. doi: 10.1046/j.1471-4159.2000.0740125.x. [DOI] [PubMed] [Google Scholar]
- 22.Salim KN, McEwen BS, Chao HM. Ginsenoside Rb1 regulates ChAT, NGF and trkA mRNA expression in the rat brain. Mol Brain Res. 1997;47:177–182. doi: 10.1016/s0169-328x(97)00042-9. [DOI] [PubMed] [Google Scholar]
- 23.Skaper SD. The neurotrophin family of neurotrophic factors: an overview. Methods Mol Biol. 2012;846:1–12. doi: 10.1007/978-1-61779-536-7_1. [DOI] [PubMed] [Google Scholar]
- 24.Song JQ, Chen XC, Zhang J, Huang TW, Zeng YQ, Shen J, Chen LM. JNK/p38 MAPK involves in ginsenoside Rb1 attenuating beta-amyloid peptide (25-35)-induced tau protein hyperphosphorylation in embryo rat cortical neurons. Yao Xue Xue Bao. 2008;43:29–34. [PubMed] [Google Scholar]
- 25.Takashima A, Noguchi K, Michel G, Mercken M, Hoshi M, Ishiguro K, Imahori K. Exposure of rat hippocampal neurons to amyloid beta peptide (25-35) induces the inactivation of phosphatidyl inositol-3 kinase and the activation of tau protein kinase I/glycogen synthase kinase-3 beta. Neurosci Lett. 1996;203:33–36. doi: 10.1016/0304-3940(95)12257-5. [DOI] [PubMed] [Google Scholar]
- 26.Takashima A, Honda T, Yasutake K, Michel G, Murayama O, Murayama M, Ishiguro K, Yamaguchi H. Activation of tau protein kinase I/glycogen synthase kinase-3 beta by amyloid beta peptide (25-35) enhances phosphorylation of tau in hippocampal neurons. Neurosci Res. 1998;31:317–323. doi: 10.1016/s0168-0102(98)00061-3. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Sun LH, Jia W, Jia W, Liu XM, Dang HX, Mai WL, Wang N, Steinmetz A, Wang YQ. Comparison of ginsenosides Rg1 and Rb1 for their effects on improving scopolamine-induced learning and memory impairment in mice. Phytother Res. 2010;24:1748–1754. doi: 10.1002/ptr.3130. [DOI] [PubMed] [Google Scholar]
- 28.Wang XY, Chen J, Zhang JT. Effects of total ginsenoside on learning and memory impairment induced by beta-amyloid peptide (25-35) and its mechanism of action. Yao Xue Xue Bao. 2001;36:1–4. [PubMed] [Google Scholar]
- 29.Wang Y, Liu J, Zhang Z, Bi P, Qi Z, Zhang C. Anti-neuroinflammation effect of ginsenoside Rbl in a rat model of Alzheimer disease. Neurosci Lett. 2011;487:70–72. doi: 10.1016/j.neulet.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 30.Wen TC, Yoshimura H, Matsuda S, Lim JH, Sakanaka M. Ginseng root prevents learning disability and neuronal loss in gerbils with 5-minute forebrain ischemia. Acta Neuropathol. 1996;91:15–22. doi: 10.1007/s004010050387. [DOI] [PubMed] [Google Scholar]
- 31.Xie X, Wang HT, Li CL, Gao XH, Ding JL, Zhao HH, Lu YL. Ginsenoside Rb1 protects PC12 cells against β-amyloid-induced cell injury. Mol Med Report. 2010;3:635–639. doi: 10.3892/mmr_00000308. [DOI] [PubMed] [Google Scholar]
- 32.Xie YH, Chen XC, Zhang J, Huang TW, Song JQ, Fang YX, Pan XD, Lin ZY. Ginsenoside Rb1 attenuates beta-amyloid peptide(25-35)-induced hyperphosphorylation of tau protein through CDK5 signal pathway. Yao Xue Xue Bao. 2007;42:828–432. [PubMed] [Google Scholar]
- 33.Zhao H, Li Q, Zhang Z, Pei X, Wang J, Li Y. Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res. 2009;1256:111–122. doi: 10.1016/j.brainres.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 34.Zhao R, Zhang Z, Song Y, Song Y, Wang D, Qi J, Wen S. Implication of phosphatidylinositol-3 kinase/Akt/glycogen synthase kinase-3β pathway in ginsenoside Rb1's attenuation of beta-amyloid-induced neurotoxicity and tau phosphorylation. J Ethnopharmacol. 2011;133:1109–1116. doi: 10.1016/j.jep.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 35.Zou K, Zhu S, Meselhy MR, Tohda C, Cai S, Komatsu K. Dammarane-type Saponins from Panax japonicus and their neurite outgrowth activity in SK-N-SH cells. J Nat Prod. 2002;65:1288–1292. doi: 10.1021/np0201117. [DOI] [PubMed] [Google Scholar]


