Abstract
The prevalence of neurodegenerative diseases and neural injury disorders is increasing worldwide. Research is now focusing on improving current neurogenesis techniques including neural stem cell therapy and other biochemical drug-based approaches to ameliorate these disorders. Unfortunately, we are still facing many obstacles that are rendering current neurotherapies ineffective in clinical trials for reasons that are yet to be discovered. That is why we should start by fully understanding the complex mechanisms of neurogenesis and the factors that affect it, or else, all our suggested therapies would fail since they would not be targeting the essence of the neurological disorder but rather the symptoms. One possible paradigm shift is to switch from neuroprotectant therapies towards neurodegeneration/neurorestorative approaches. In addition, other and our laboratories are increasingly focusing on combining the use of pharmacological agents (such as Rho-associated kinase (ROCK) inhibitors or other growth factors (such as brain-derived neurotrophic factor (BDNF)) and stem cell treatment to enhance the survivability and/or differentiation capacity of transplanted stem cells in neurotrauma or other neurodegeneration animal models. Ongoing stem cell research is surely on the verge of a breakthrough of multiple effective therapeutic options for neurodegenerative disorders. Once, we fully comprehend the process of neurogenesis and its components, we will fully be capable of manipulating and utilizing it. In this work, we discuss the current knowledge of neuroregenerative therapies and their associated challenges.
Keywords: neural stem cells, neurodegeneration, neuroinjury, TBI, rho-associated kinase (ROCK) inhibitor, BDNF, growth factors, stem cell therapy
Introduction
The prevalence of neurodegenerative diseases is increasing worldwide. That is not only due to increased incidences of direct or indirect injury to the central nervous system (CNS) but also due to the increase in the percentage of the aging population. Incurable neurodegenerative disorders such as Alzheimer's disease (AD), traumatic brain injury (TBI), Parkinson's disease (PD) and multiple sclerosis (MS) have reached a staggering prevalence of around 5.2 million (Hebert et al., 2013), 1.7 million (Faul et al., 2010), 500 thousand (National Institute of Neurological Disorders and Stroke, 2014a) and 250-350 thousand (National Institute of Neurological Disorders and Stroke, 2014b) cases, respectively, in the USA alone. With these relatively high numbers of cases and our current deficit in effective therapeutic modalities that cure those disorders, it is imperative that we find new neurotherapeutic targets to prevent the increasing morbidities or at least ameliorate them. For the past few decades, researchers have been interested in the realm of stem cells and the prospect of using them in an attempt of curing diseases that entail organ, tissue and cellular degeneration. Even with the exponential increase in our knowledge of stem cell formation and manipulation, we are still far from fully understanding the intricate mechanisms in which these intriguing cells function. That is apparent in studies that do not get a 100% remission rate, or encounter relapses or side effects in animal models or human subjects in clinical trials following a failure of stem cells transplant. Yet, this field remains to be promising, and with the progress we are at, we are not far from a major breakthrough in the field of stem cells in general and neuronal stem cells in particular.
The endogenous pool of neural stem cells
The greatest challenge of stem cell research is to be able to apply stem cell therapy in neurodegenerative diseases, due to several reasons. For one, the nervous system remains till today the least understood system of all, even with most research concentrating on it for the past few decades. Another factor is that neuronal cells are one of the very few body cells that remain almost post-mitotic. A third reason is the scarcity and restriction of the endogenous pool of neuronal stem cells (Maldonado-Soto et al., 2014). Until recently, it was believed that neurogenesis is a distinct characteristic of embryogenesis and very early life and that neurons of adulthood are post-mitotic cells with no endogenous pool of neuronal and glial stem cells. Earlier studies have shown that through human surgical samples, in vitro neurons could be generated (Kirschenbaum et al., 1994; Pincus et al., 1998). These were later followed by post-mortem human studies that detected the presence of neuroblast markers and migration indices (Bedard and Parent, 2004; Curtis et al., 2007). Now, we know that adult neurogenesis is possible via a pool of progenitor stem cells. There are stem cells in the subventricular zone (SVZ) of the lateral ventricles, which propagate to the olfactory bulb, and in the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG), which aid in the maintenance of spatial memory formation and cognition (Kempermann, 2012; Kohman and Rhodes, 2013; Sawada and Sawamoto, 2013).
Through the incorporation of reagents such as doublecortin and bromo-deoxy-brimidine, it was found that during adult neurogenesis, the stem cell pool undergoes extensive proliferation before transforming into glial and neural progenitor cells, which mature within 3–4 weeks (van Praag et al., 2002). It has been determined that during neurogenesis, quiescent progenitor cells are activated and asymmetrically divide into amplifying neural progenitor cells, which would in turn transform into post-mitotic, migratory neuroblasts or glioblasts (Encinas et al., 2011). It is the alterations in this delicate process that underlie or augment the pathogenesis of many of the neurodegenerative diseases where replacement of diseased or injured neurons is reduced or even totally blocked. Furthermore, it has been found that cognitive decline may start during the second decade of human life (Salthouse, 2009) and, with aging, the proliferation rate of the endogenous neural stem cell population of rodents decreases by 50–80% (Ahlenius et al., 2009) and they may even reach a terminal astrocytic differentiation of their neural progenitors (Encinas et al., 2011), indicating that there is some kind of biological clock controlling neurogenesis.
Aging, environmental factors and neurogenesis
What scientists are trying to do now is to find an effective neuronal replacement therapy, but what we really need to do is to better understand the mechanism of aging and disease progression. Cell replacement therapy is not working since the replenished stem cells are being destroyed via unknown disease mechanisms, and thus the only way to prevent this is by understanding these mechanisms in order to know how to protect our endogenous pool and the administered cells. It has been discovered that, with aging, the number of migrating neuroblasts decreases in the SVZ and periventricular white matter of humans (Taylor et al., 2013). Moreover, the culprits behind the aging process of neuronal stem cells include cyclin-dependent kinase (CDK) inhibitors and telomere shortening (Mandal et al., 2011), and a dysregulation of certain factors, or their receptors, such as tumor necrosis factor-α (TNF-α) (Tropepe et al., 1997), epidermal growth factor (EGF) (Pastrana et al., 2009), fibroblast growth factor (FGF) (Frinchi et al., 2008) and Notch delta (Imayoshi et al., 2010). Thus, one interesting way of preserving the potential of our endogenous pool of neural stem cells is by inhibiting CDK inhibitors and telomere shortening (by enhancing telomerase activity) and maybe even finding a way to prevent the dysregulation of the different factors that are negatively affecting neurogenesis. Yet, the downside of inhibiting CDK inhibitors and enhancing telomerase activity is the increased possibility of developing an oncogenic phenotype within the stem cell population (Mandal et al., 2011). Nevertheless, the process of aging remains to be the highlight of continuous investigation in the hopes that, one day, we could reach a breakthrough in reversing, inhibiting or at least diminishing this process.
When studying stem cells, there are many factors to consider, and undoubtedly, more factors will emerge as we delve deeper into this newly explored realm. One of the most important factors affecting neurogenesis are environmental factors, and that could be translated into daily life style activities (smoking, diabetes, stress and exercise) in which many studies have established their pivotal role in controlling neurogenesis (Bruijnzeel et al., 2011; Farioli-Vecchioli et al., 2014; Loi et al., 2014; Ramos-Rodriguez et al., 2014). These factors could contribute to epigenetic alterations that would translate into differential signaling pathways (such as activated NFkB), which may in turn recede the process of neural regeneration (Koo et al., 2010).
Microglia and neurogenesis
We are still unable to fully conceive the mechanisms employed by microglial cells; different studies have shown that microglia may have trophic, toxic, phagocytic and developmental functions within the brain, as they alternate between an activated and deactivated state (Marin-Teva et al., 2004; Nakajima and Kohsaka, 2004; Monier et al., 2006; Sierra et al., 2010; Verney et al., 2010; Antony et al., 2011; Cunningham et al., 2013; Ueno et al., 2013). To better understand the way microglia function, recent studies have ablated certain genes, such as Cx3cr1-cre and Cx3cr1-creER, in order to inhibit individual functions and investigate their potential role (Kim et al., 2010; Goldmann et al., 2013; Wolf et al., 2013). Such methods of ablating genes, via small interfering RNA (siRNA) for example, may present promising techniques for better understanding the interrelation of microglia and neurogenesis.
RNA and neurogenesis
Another domain to consider for improving our neural stem cell therapeutic modalities is the field of microRNAs (miRNA). Studies have shown that these few-base pair molecules may have a huge impact on neuronal development, such as the miR-29b that represses several pro-apoptotic genes during early neural development and differentiation (Kole et al., 2011) and miR-124 that enhances neuronal differentiation during embryonic development (Makeyev et al., 2007; Visvanathan et al., 2007). Furthermore, a new set of non-encoding RNA, called piRNA, was recently discovered to be responsible for suppressing transposable elements and it has been recently shown to be expressed in the CNS (Lee et al., 2011). In addition to that, it has been shown that piRNAs are up-regulated by serotonin, and in turn, lead to the down-regulation of the CREB2 gene, thereby enhancing memory formation (Rajasethupathy et al., 2012). Another class of RNAs is the long non-coding RNA (lncRNA), which was also recently discovered to be present in the brain and responsible for maintaining neural self-renewal by binding to sites correlated with transcription factors of pluripotency (Ng et al., 2012).
Umbilical cord blood transplants and neurogenesis
Whether it may be a neurodegenerative disease or a TBI, it is important for us to devise an effective way to replace the damaged neurons which are way too much for the relatively small endogenous stem cell population to replenish. In one study, it was found that it is possible to generate in vitro phenotypically layer-specific neurons through passaged neurospheres, which are designed to heal damaged areas with functionally compatible neuron subtypes (Cramer et al., 2014). Another method of healing is using umbilical cord blood (UCB), which contains a mixture of stem and progenitor cells. UCB transplants were able to restore sensorimotor abilities of hypoxic rat brains (Geissler et al., 2011) and enhance tissue repair, cognition (de Paula et al., 2012) and neural stem cell proliferation (Wang et al., 2013). Furthermore, it has been shown that autologous intravenous UCB transplantation is a safe technique to use in children with acquired neurological disorders (Buzanska et al., 2006). That is why there are several ongoing clinical trials to test for the safety and efficacy of UCB transplants in curing various neurological disorders. What remains is to devise a successful way to incorporate the neural grafts into the damaged CNS, restore the neural circuitry and ensure the graft's survival. Furthermore, it is important that we become better capable of expanding fetal derived tissue, or UCB, in vitro and the transplanted tissue itself, along with promoting the differentiation of the transplanted stem cells. We could even employ deep brain stimulation (DBS), where Encinas et al. (2011) have shown that it increases the number of cells by 69% in the DG of mice by inducing the proliferation of the endogenous pool of neural stem cells. It could be possible that DBS induces the same effect on the endogenous human neural stem cells and even better activate and maintain the transplanted neural tissue.
Topical administration of mesenchymal stem cells for treating TBI
In an experiment in our lab, we devised a new method to utilize mesenchymal stem cells to treat TBI in rats (Lam et al., 2013). Previous methods to administer mesenchymal stem cells led to a limited number of stem cells reaching the site of brain injury (Harting et al., 2009; Walker et al., 2009). These methods included intravenous infusions that scatter cells in various organs, intra-arterial administration that causes microemboli and cerebral ischemia, and direct intracerebral implantation that is invasive and may be fatal. After inducing TBI on the right parietal cortex of rats, we topically administered green fluorescent protein (GFP)-expressing mesenchymal cells, which were isolated from the adipose tissue of rats, along with fibrin for fixation to the left parietal cortex (Lam et al., 2013). Five days later, GFP cells were detected proliferating at the site of application and the site of injury, and in rats without administered fibrin, very few cells were detected at the application site. Furthermore, no GFP-expressing mesenchymal cells were detected in other somatic organs in both control rats and rats with TBI. Moreover, transplanting cells into an uninjured site prevents the death of the transplanted cells due to inflammation following TBI, and provides the cells with the appropriate environment or settling in and proliferating before migrating to the site of injury. Thus, our neurotherapeutic method provides a new and effective way for inducing neurogenesis following TBI, but which needs further research and experimentation for optimization.
ROCK inhibitors and possible combination of stem cell therapy
In our lab, we investigated the possibility of inducing neuronal regeneration through the use of Rho-associated kinases (ROCKs), a class of serine/threonine kinases, which are important mediators of axonal repair in injuries of the CNS (Zhang et al., 2006). We found that ROCK inhibitors promoted neurite outgrowth via dramatic cytoskeletal reorganization in PC-12 cell lines, in a time- and dose-dependent manner. Furthermore, we discovered that the effect of ROCK inhibitors was mediated via dephosphorylation of cofilin, which when dephosphorylated, binds to actin and enhances depolymerization of actin-associated filaments. Thus, we have shown that ROCK inhibitors could be utilized as a therapeutic technique for treating damaged neurons by promoting their regeneration through axonal repair. However, this technique still needs further investigations to fully understand the mechanisms at hand and to make it as effective as possible in the clinical setting.
ROCK inhibitors have been recently implicated in improving the in vitro and in vivo survivability and neuronal differentiation capacity of transplanted stem cells. Human embryonic stem cells have been hard to culture, propagate and differentiate since they undergo massive apoptosis following dissociation, but this problem was diminished and cloning efficiency increased from 1 to 27% when using the ROCK inhibitor, Y-27632 (Watanabe et al., 2007). Furthermore, Y-27632 allowed stem cells to differentiate into cortical and basal telencephalic progenitors. Moreover, several studies established novel protocols and found that utilizing Y-27632 increased the survivability and enhanced the recovery of cryopreserved human embryonic stem cells (Martin-Ibanez et al., 2008; Li et al., 2009; Martin-Ibanez et al., 2009; Xu et al., 2010). In another study, it was shown that Y-27632 could inhibit the anti-neurogenesis/neurodifferentiation effect of chondroitin sulfate proteoglycan, which is found in glial scar tissue, on mesenchymal stem cells (Lim and Joe, 2013). Thus, this particular study shows that ROCK inhibitors could be employed as an adjuvant to stem cell transplant to counteract the anti-regenerative effects of factors produced by damaged brain tissue. Further research should be done to discover more pharmacological agents such as Y-27632 that are even more efficient in enhancing the survival and neuronal differentiation of transplanted stem cells.
Growth factors used to enhance stem cell therapy
Growth factors have also been extensively studied and have been found to aid the in vitro stem cell culturing and the in vivo transplantation process. In one study, it was found that transplanting human umbilical stem cells, transfected with vascular endothelial growth factor (VEGF) and human fibroblast growth factor 2 (FGF2), into an amyotrophic lateral sclerosis (ALS) rodent model led to the transformation of stem cells into astrocytes (Rizvanov et al., 2011). Without VEGF and FGF2, transplanted stem cells differentiated into endothelial cells and microglia. However, when they received signals from dying motor neurons and autocrine signals from these growth factors, they differentiated into astrocytes and contributed to the survival of damaged neurons. Another study also found a way to enhance the transplantation process, where it was also shown that genetically modifying neural stem cells into expressing brain-derived neurotrophic factor (BDNF) allows a more efficient neuronal differentiation and higher survivability with significantly improved motor functions when transplanted into rats with TBI (Ma et al., 2012). Interestingly, it has also been shown that the proliferation rate of in vitro SVZ-derived cells is increased via transferrin receptor 1 when apotransferrin is added to the culture media (Silvestroff et al., 2013). Such a finding could abolish the fact that we have such a negligible pool of endogenous stem cells, since we can extract some of those cells, proliferate them in culture, and transplant them at a high number to give rapid and effective results. These are just a few of many studies on biological substances and growth factors that may enhance stem cell therapy; it seems only logical that we continue the quest of searching for factors within our bodies that may hold the cure. The cure for neurodegenerative diseases may be inside our bodies after all, but just needs a bit of enhancement. A nice illustration of all of the above neurotherapeutic interventions is depicted in Figure 1.
Figure 1.
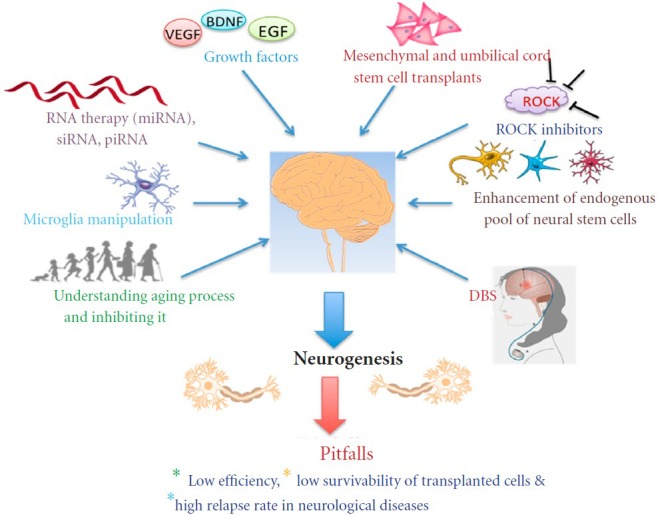
A schematic diagram showing the major methods currently employed for applying and understanding neurogenesis, and the major pitfalls.
VEGF: vascular endothelial growth factor; BDNF: brain-derived neurotrophic factor; EGF: epidermal growth factor; ROCKs: Rho-associated kinas-es; DBS: deep brain stimulation.
Conclusion
The field of neural stem cell research in the area of neurodegenerative disorders is highly promising and still in its infancy. However, we have started to pave our way through and are on our way of uncovering many of the secrets of neurogenesis that are still eluding us. As we have discussed, we currently have many options in which to target neurogenesis as a therapeutic technique for neurodegenerative diseases and injuries, but none of which have actually given a long-term effect. The reason for that lies in the fact that we still have not fully grasped the concept of neurogenesis and how it really works. That is why it is imperative that we further investigate and fully understand this fascinating and complicated mechanism before devising new therapeutic modalities or else we might be wasting a lot of time and money on therapies that may never work.
Acknowledgments:
We acknowledge Prof. Poon WS and Dr. Lam PK of the Chinese University of Hong Kong for their scientific input.
Footnotes
Conflicts of interest: None declared.
References
- 1.Ahlenius H, Visan V, Kokaia M, Lindvall O, Kokaia Z. Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J Neurosci. 2009;29:4408–4419. doi: 10.1523/JNEUROSCI.6003-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antony JM, Paquin A, Nutt SL, Kaplan DR, Miller FD. Endogenous microglia regulate development of embryonic cortical precursor cells. J Neurosci Res. 2011;89:286–298. doi: 10.1002/jnr.22533. [DOI] [PubMed] [Google Scholar]
- 3.Bedard A, Parent A. Evidence of newly generated neurons in the human olfactory bulb. Brain Res Dev Brain Res. 2004;151:159–168. doi: 10.1016/j.devbrainres.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Bruijnzeel AW, Bauzo RM, Munikoti V, Rodrick GB, Yamada H, Fornal CA, Ormerod BK, Jacobs BL. Tobacco smoke diminishes neurogenesis and promotes gliogenesis in the dentate gyrus of adolescent rats. Brain Res. 2011;1413:32–42. doi: 10.1016/j.brainres.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 5.Buzanska L, Jurga M, Stachowiak EK, Stachowiak MK, Domanska-Janik K. Neural stem-like cell line derived from a nonhematopoietic population of human umbilical cord blood. Stem Cells Dev. 2006;15:391–406. doi: 10.1089/scd.2006.15.391. [DOI] [PubMed] [Google Scholar]
- 6.Cramer NP, Chatterjee M, Lischka FW, Juliano SL. Culturing layer-specific neocortical neurons as a cell replacement therapy following traumatic brain injury. Front Neurol. 2014;4:213. doi: 10.3389/fneur.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham CL, Martinez-Cerdeno V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33:4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van Roon-Mom WM, Bjork-Eriksson T, Nordborg C, Frisen J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 9.de Paula S, Greggio S, Marinowic DR, Machado DC, DaCosta JC. The dose-response effect of acute intravenous transplantation of human umbilical cord blood cells on brain damage and spatial memory deficits in neonatal hypoxia-ischemia. Neuroscience. 2012;210:431–441. doi: 10.1016/j.neuroscience.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Encinas JM, Hamani C, Lozano AM, Enikolopov G. Neurogenic hippocampal targets of deep brain stimulation. J Comp Neurol. 2011;519:6–20. doi: 10.1002/cne.22503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farioli-Vecchioli S, Mattera A, Micheli L, Ceccarelli M, Leonardi L, Saraulli D, Costanzi M, Cestari V, Rouault JP, Tirone F. Running rescues defective adult neurogenesis by shortening the length of the cell cycle of neural stem and progenitor cells. Stem Cells. 2014 doi: 10.1002/stem.1679. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Faul M, Xu L, Wald M, Coronado V. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. [Google Scholar]
- 13.Frinchi M, Bonomo A, Trovato-Salinaro A, Condorelli DF, Fuxe K, Spampinato MG, Mudo G. Fibroblast growth factor-2 and its receptor expression in proliferating precursor cells of the subventricular zone in the adult rat brain. Neurosci Lett. 2008;447:20–25. doi: 10.1016/j.neulet.2008.09.059. [DOI] [PubMed] [Google Scholar]
- 14.Geissler M, Dinse HR, Neuhoff S, Kreikemeier K, Meier C. Human umbilical cord blood cells restore brain damage induced changes in rat somatosensory cortex. PLoS One. 2011;6:e20194. doi: 10.1371/journal.pone.0020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldmann T, Wieghofer P, Muller PF, Wolf Y, Varol D, Yona S, Brendecke SM, Kierdorf K, Staszewski O, Datta M, Luedde T, Heikenwalder M, Jung S, Prinz M. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat Neurosci. 2013;16:1618–1626. doi: 10.1038/nn.3531. [DOI] [PubMed] [Google Scholar]
- 16.Harting MT, Jimenez F, Xue H, Fischer UM, Baumgartner J, Dash PK, Cox CS. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg. 2009;110:1189–1197. doi: 10.3171/2008.9.JNS08158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010;30:3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kempermann G. New neurons for ‘survival of the fittest’. Nat Rev Neurosci. 2012;13:727–736. doi: 10.1038/nrn3319. [DOI] [PubMed] [Google Scholar]
- 20.Kim SS, Ye C, Kumar P, Chiu I, Subramanya S, Wu H, Shankar P, Manjunath N. Targeted delivery of siRNA to macrophages for anti-inflammatory treatment. Mol Ther. 2010;18:993–1001. doi: 10.1038/mt.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirschenbaum B, Nedergaard M, Preuss A, Barami K, Fraser RA, Goldman SA. In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cereb Cortex. 1994;4:576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- 22.Kohman RA, Rhodes JS. Neurogenesis, inflammation and behavior. Brain Behav Immun. 2013;27:22–32. doi: 10.1016/j.bbi.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kole AJ, Swahari V, Hammond SM, Deshmukh M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev. 2011;25:125–130. doi: 10.1101/gad.1975411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam PK, Lo AW, Wang KK, Lau HC, Leung KK, Li KT, Lai PB, Poon WS. Transplantation of mesenchymal stem cells to the brain by topical application in an experimental traumatic brain injury model. J Clin Neurosci. 2013;20:306–309. doi: 10.1016/j.jocn.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Lee EJ, Banerjee S, Zhou H, Jammalamadaka A, Arcila M, Manjunath BS, Kosik KS. Identification of piRNAs in the central nervous system. RNA. 2011;17:1090–1099. doi: 10.1261/rna.2565011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Krawetz R, Liu S, Meng G, Rancourt DE. ROCK inhibitor improves survival of cryopreserved serum/feeder-free single human embryonic stem cells. Hum Reprod. 2009;24:580–589. doi: 10.1093/humrep/den404. [DOI] [PubMed] [Google Scholar]
- 28.Lim HS, Joe YA. A ROCK inhibitor blocks the inhibitory effect of chondroitin sulfate proteoglycan on morphological changes of mesenchymal stromal/stem cells into neuron-like cells. Biomol Ther (Seoul) 2013;21:447–453. doi: 10.4062/biomolther.2013.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loi M, Koricka S, Lucassen PJ, Joels M. Age- and sex-dependent effects of early life stress on hippocampal neurogenesis. Front Endocrinol. 2014;5:13. doi: 10.3389/fendo.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma H, Yu B, Kong L, Zhang Y, Shi Y. Neural stem cells over-expressing brain-derived neurotrophic factor (BDNF) stimulate synaptic protein expression and promote functional recovery following transplantation in rat model of traumatic brain injury. Neurochem Res. 2012;37:69–83. doi: 10.1007/s11064-011-0584-1. [DOI] [PubMed] [Google Scholar]
- 31.Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maldonado-Soto AR, Oakley DH, Wichterle H, Stein J, Doetsch FK, Henderson CE. Stem cells in the nervous system. Am J Phys Med Rehabil. 2014 doi: 10.1097/PHM.0000000000000111. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandal PK, Blanpain C, Rossi DJ. DNA damage response in adult stem cells: pathways and consequences. Nat Rev Mol Cell Biol. 2011;12:198–202. doi: 10.1038/nrm3060. [DOI] [PubMed] [Google Scholar]
- 34.Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- 35.Martin-Ibanez R, Stromberg AM, Hovatta O, Canals JM. Cryopreservation of dissociated human embryonic stem cells in the presence of ROCK inhibitor. Curr Protoc Stem Cell Biol. 2009;(Chapter 1: Unit 1C 8) doi: 10.1002/9780470151808.sc01c08s10. [DOI] [PubMed] [Google Scholar]
- 36.Martin-Ibanez R, Unger C, Stromberg A, Baker D, Canals JM, Hovatta O. Novel cryopreservation method for dissociated human embryonic stem cells in the presence of a ROCK inhibitor. Hum Reprod. 2008;23:2744–2754. doi: 10.1093/humrep/den316. [DOI] [PubMed] [Google Scholar]
- 37.Monier A, Evrard P, Gressens P, Verney C. Distribution and differentiation of microglia in the human encephalon during the first two trimesters of gestation. J Comp Neurol. 2006;499:565–582. doi: 10.1002/cne.21123. [DOI] [PubMed] [Google Scholar]
- 38.Nakajima K, Kohsaka S. Microglia: neuroprotective and neurotrophic cells in the central nervous system. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:65–84. doi: 10.2174/1568006043481284. [DOI] [PubMed] [Google Scholar]
- 39.Ng SY, Johnson R, Stanton LW. Human long non-coding RNAs promote pluripotency and neuronal differentiation by association with chromatin modifiers and transcription factors. EMBO J. 2012;31:522–533. doi: 10.1038/emboj.2011.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Institute of Neurological Disorders and Stroke. Parkinson's Disease: Hope Through Research. 2014a doi: 10.1002/ana.24261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Institute of Neurological Disorders and Stroke. Multiple Sclerosis: Hope Through Research. 2014b [Google Scholar]
- 42.Pastrana E, Cheng LC, Doetsch F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc Natl Acad Sci U S A. 2009;106:6387–6392. doi: 10.1073/pnas.0810407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pincus DW, Keyoung HM, Harrison-Restelli C, Goodman RR, Fraser RA, Edgar M, Sakakibara S, Okano H, Nedergaard M, Goldman SA. Fibroblast growth factor-2/brain-derived neurotrophic factor-associated maturation of new neurons generated from adult human subependymal cells. Ann Neurol. 1998;43:576–585. doi: 10.1002/ana.410430505. [DOI] [PubMed] [Google Scholar]
- 44.Rajasethupathy P, Antonov I, Sheridan R, Frey S, Sander C, Tuschl T, Kandel ER. A role for neuronal piRNAs in the epigenetic control of memory-related synaptic plasticity. Cell. 2012;149:693–707. doi: 10.1016/j.cell.2012.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos-Rodriguez JJ, Molina-Gil S, Ortiz-Barajas O, Jimenez-Palomares M, Perdomo G, Cozar-Castellano I, Lechuga-Sancho AM, Garcia-Alloza M. Central proliferation and neurogenesis is impaired in type 2 diabetes and prediabetes animal models. PLoS One. 2014;9:e89229. doi: 10.1371/journal.pone.0089229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rizvanov AA, Guseva DS, Salafutdinov, Kudryashova NV, Bashirov FV, Kiyasov AP, Yalvac ME, Gazizov IM, Kaligin MS, Sahin F, Mukhamedyarov MA, Palotas A, Islamov RR. Genetically modified human umbilical cord blood cells expressing vascular endothelial growth factor and fibroblast growth factor 2 differentiate into glial cells after transplantation into amyotrophic lateral sclerosis transgenic mice. Exp Biol Med (Maywood) 2011;236:91–98. doi: 10.1258/ebm.2010.010172. [DOI] [PubMed] [Google Scholar]
- 47.Salthouse TA. When does age-related cognitive decline begin? Neurobiol Aging. 2009;30:507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawada M, Sawamoto K. Mechanisms of neurogenesis in the normal and injured adult brain. Keio J Med. 2013;62:13–28. doi: 10.2302/kjm.2012-0005-re. [DOI] [PubMed] [Google Scholar]
- 49.Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silvestroff L, Franco PG, Pasquini JM. Neural and oligodendrocyte progenitor cells: transferrin effects on cell proliferation. ASN Neuro. 2013;5:e00107. doi: 10.1042/AN20120075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor SR, Smith C, Harris BT, Costine BA, Duhaime AC. Maturation-dependent response of neurogenesis after traumatic brain injury in children. J Neurosurg Pediatr. 2013;12:545–554. doi: 10.3171/2013.8.PEDS13154. [DOI] [PubMed] [Google Scholar]
- 52.Tropepe V, Craig CG, Morshead CM, van der Kooy D. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16:543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- 54.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verney C, Monier A, Fallet-Bianco C, Gressens P. Early microglial colonization of the human forebrain and possible involvement in periventricular white-matter injury of preterm infants. J Anat. 2010;217:436–448. doi: 10.1111/j.1469-7580.2010.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Visvanathan J, Lee S, Lee B, Lee JW, Lee SK. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007;21:744–749. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker PA, Shah SK, Harting MT, Cox CS., Jr Progenitor cell therapies for traumatic brain injury: barriers and opportunities in translation. Dis Model Mech. 2009;2:23–38. doi: 10.1242/dmm.001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang XL, Zhao YS, Hu MY, Sun YQ, Chen YX, Bi XH. Umbilical cord blood cells regulate endogenous neural stem cell proliferation via hedgehog signaling in hypoxic ischemic neonatal rats. Brain Res. 2013;1518:26–35. doi: 10.1016/j.brainres.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 60.Wolf Y, Yona S, Kim KW, Jung S. Microglia seen from the CX-3CR1 angle. Front Cell Neurosci. 2013;7:26. doi: 10.3389/fncel.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu X, Cowley S, Flaim CJ, James W, Seymour LW, Cui Z. Enhancement of cell recovery for dissociated human embryonic stem cells after cryopreservation. Biotechnol Prog. 2010;26:781–788. doi: 10.1002/btpr.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Z, Ottens AK, Larner SF, Kobeissy FH, Williams ML, Hayes RL, Wang KK. Direct Rho-associated kinase inhibition [correction of inhibiton] induces cofilin dephosphorylation and neurite outgrowth in PC-12 cells. Cell Mol Biol Lett. 2006;11:12–29. doi: 10.2478/s11658-006-0002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


