Introduction
Aging is the accumulation of multidimensional deterioration of processing of biological, psychological, and social changes with expansion over time (Bowen and Atwood, 2004; Grady, 2012). Aging-related changes are typically accompanied by decline in cognitive function, urinary control, sensory-motor function, and gait ability (Bradley et al., 1991; Bowen and Atwood, 2004; Hedden and Gabrieli, 2004; Grady, 2012; Moran et al., 2012). In addition, a number of studies have suggested changes in brain structure with normal aging, such as decrease in cortical thickness or increase in ventricular width (Blatter et al., 1995; Tang et al., 1997; Uylings and de Brabander, 2002; Preul et al., 2006; Apostolova et al., 2012). In particular, ventricular enlargement has been suggested as a structural biomarker for normal aging and progression of some illnesses, such as Alzheimer's disease (Blatter et al., 1995; Tang et al., 1997; Uylings and de Brabander, 2002; Preul et al., 2006; Apostolova et al., 2012). However, the question of how this structural change in the brain in normal elderly affects change of white matters remains a topic of interest and concern.
Diffusion tensor imaging allows for evaluation of white matter due to its ability to capture and quantify water diffusion characteristics (Mori et al., 1999; Assaf and Pasternak, 2008; Neil, 2008). In normal white matter, water molecules move relatively freely in a direction parallel to nerve fiber tracts, however, their movements are restricted across tracts, which cause diffusion anisotropy in white matter. This selective restriction of water molecule movement allows for exploration of physical changes in white matter caused by normal aging using diffusion anisotropy. Some studies using DTI have reported on changes in periventricular white matter in patients with stroke or other brain injuries (Yeo et al., 2011; Hattori et al., 2012; Jang et al., 2013). In the current study, using DTI, we investigated ventricular enlargement with normal aging, and its effect on periventricular white matter.
Subjects and Methods
Subjects
Sixty subjects (31 males, 29 females; mean age, 49.1 ± 17.6 years; range, 20–78 years) with no history of neurologic or psychiatric disease and head trauma were recruited by advertisements in a regional daily newspaper for the study. All subjects provided written informed consent prior to the study. This study was approved by the Institutional Review Board of Yeungnam University Medical Center in South Korea.
Diffusion tensor image
Diffusion tensor imaging data were acquired using a 1.5T Philips Gyroscan Intera system (Hoffman-LaRoche, Mijdrecht, the Netherlands) equipped with a Synergy-L Sensitivity Encoding head coil using a single-shot spin echo-planar imaging sequence. For each of the 32 noncollinear and noncoplanar diffusion-sensitizing gradients, we acquired 60 contiguous slices parallel to the anterior commissure-posterior commissure line. The imaging parameters used were: matrix = 128 × 128; field of view = 221 × 221 mm2; echo time = 76 ms; repetition time = 10,726 ms; sensitivity encoding factor = 2; echo planar imaging factor = 67; b = 1,000 s/mm2; number of excitations = 1; and a slice thickness of 2.3 mm.
Eddy current-induced image distortions and motion artifacts were reduced using affine multi scale two-dimensional registration (Smith et al., 2004). Diffusion tensor imaging datasets were preprocessed using the Oxford Centre for Functional Magnetic Resonance Imaging of Brain Software Library. White matter was evaluated using DTI-Studio software (CMRM, Johns Hopkins Medical Institute, Baltimore, MD, USA).
Diffusion tensor imaging parameters were estimated in the following four regions of interest in periventricular white matter, which is concerned with cognitive function, urinary control, sensory-motor function, and gait ability (Newton et al., 2006; Jang, 2009; Han et al., 2010; Hong et al., 2010; Klein et al., 2010; Tadic et al., 2010; Yeo et al., 2012); the anterior corona radiata, the posterior corona radiata, genu of the corpus callosum, and splenium of the corpus callosum (Figure 1). In the anterior corona radiata, we placed a region of interest in the area defined by the medial half of the line drawn from the most lateral point of the anterior horn of the lateral ventricle to the most lateral point of the white matter horizontally and the middle third of the line drawn from the most anterior point of the anterior horn of the lateral ventricle to the middle point of the lateral ventricle vertically. In the posterior corona radiata, we placed a region of interest in an area defined by the medial half of the line drawn from the most lateral point of the posterior horn of the lateral ventricle to the most lateral point of the white matter horizontally and the middle third of the line drawn from the middle point of the lateral ventricle to the most posterior point of the posterior horn of the lateral ventricle. In the corpus callosum, we placed a region of interest on the middle third of the genu of the corpus callosum, the splenium of the corpus callosum (Yeo et al., 2011; Jang et al., 2013). We measured fractional anisotropy and apparent diffusion coefficient in each region of interest.
Figure 1.
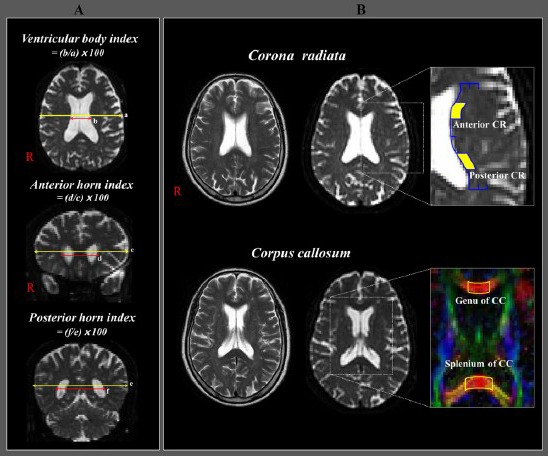
Measurement of ventricular width and regions of interest in the four regions of periventricular white matter in normal subjects.
(A) Measurement of ventricular width and calculations of relative width ratios for the ventricular body, anterior horn, and posterior horn. (B) Regions of interest in the four regions of periventricular white matter. Anterior corona radiata (CR), posterior CR, genu of the corpus callosum (CC), and splenium of the CC. Yellow blocks mean the loca-tion of region of interest for the anterior and posterior corona radiata, and blue lines mean the outline of region of interest for the definition of anterior and posterior corona radiata.
Measurement of ventricular width
We used the ventricular body index, anterior horn index, and posterior horn index, which could estimate the effects of hydrocephalus on the lateral ventricle (Jang et al., 2013) (Figure 1). The relative width ratios for the ventricular body index, anterior horn index, and posterior horn index were defined as follows: the ratio between the maximum distance of the ventricular walls at the body of the ventricle level and the maximum width of the brain on the axial image, the ratio between the maximum widths of tips on both anterior horns and maximum width of the brain at the same level on the coronal image, and the ratio between the maximum widths of tips on both posterior horns and the maximum width of the brain at the same level on the coronal image were defined as the ventricular body index, anterior horn index, and posterior horn index, respectively.
Statistical analysis
Spearman's correlation test was used for determination of correlation between age, diffusion tensor imaging parameters of each region of interest, and each index of ventricular width. Bonferroni correction was used to correct for multiple comparisons of correlation test. Software (version 15.0; SPSS, Chicago, IL, USA) was used in performance of statistical analyses, and statistical significance was set at P < 0.001.
Results
Correlation between ventricular parameters and age
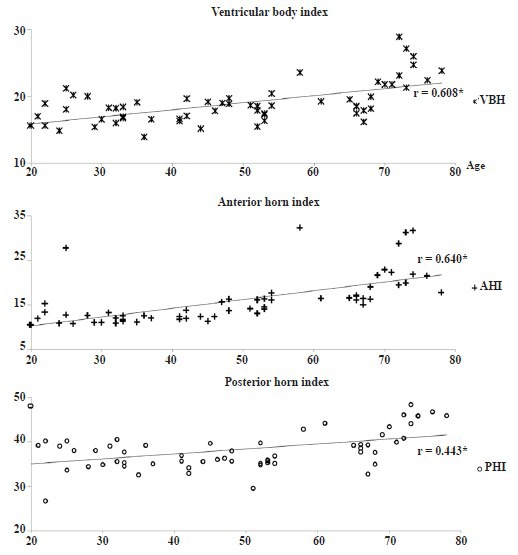
Regarding the ventricular parameters, we found significant correlation with age in all indexes for ventricular width. In particular, ventricular body index (P < 0.001) and anterior horn index (P < 0.001) showed strong positive correlation with age. In addition, the posterior horn index showed moderate positive correlation with age (P < 0.001; Table 1 and Figure 2).
Table 1.
Correlation between each ventricular index and age
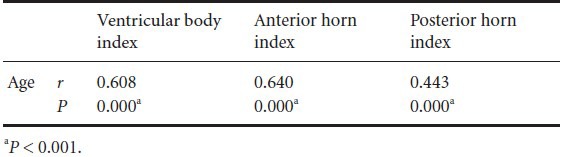
Figure 2.
Scatter diagram for correlation test between ventricular index and age.
Spearman's correlation test was used for determination of correlation between ventricular index and age. *P < 0.001. CR: Corona radiate.
Correlation between diffusion tensor imaging parameters and age
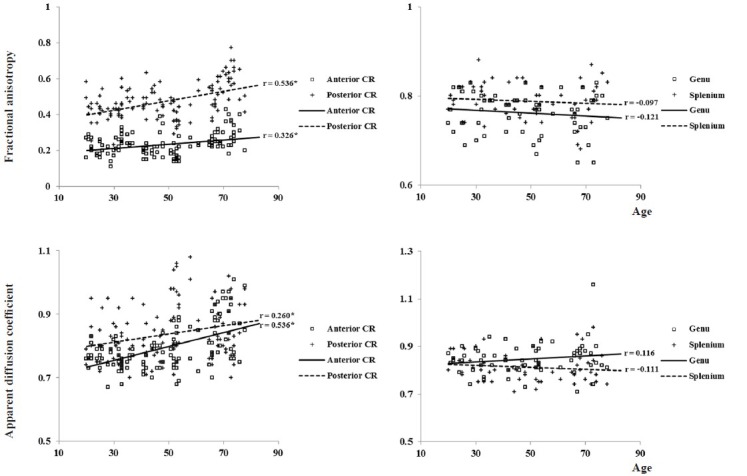
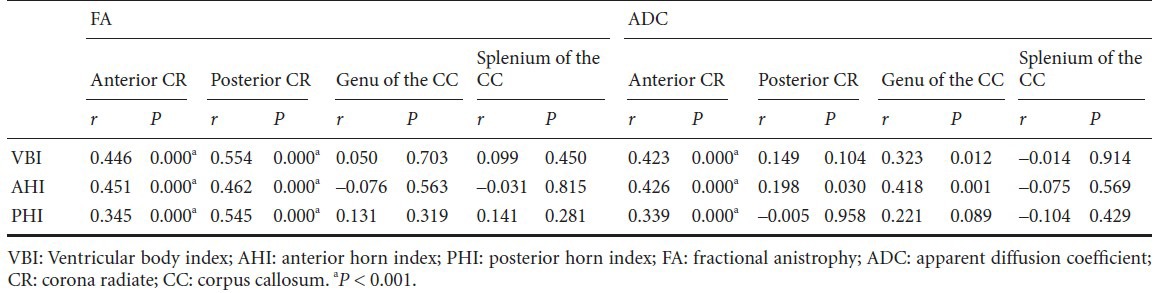
In the anterior corona radiata, we observed weak to moderate positive correlation between fractional anisotropy and age (P < 0.001), and apparent diffusion coefficient and age (P < 0.001). In addition, posterior corona radiata also showed moderate positive correlation between fractional anisotropy and age (P < 0.001). In contrast, apparent diffusion coefficient value of posterior corona radiata and diffusion tensor imaging parameters of the genu and splenium of the corpus callosum did not show significant correlation with age (P > 0.001) (Table 2; Figure 3).
Table 2.
Correlation between diffusion tensor imaging parameters in each region of white matter and age
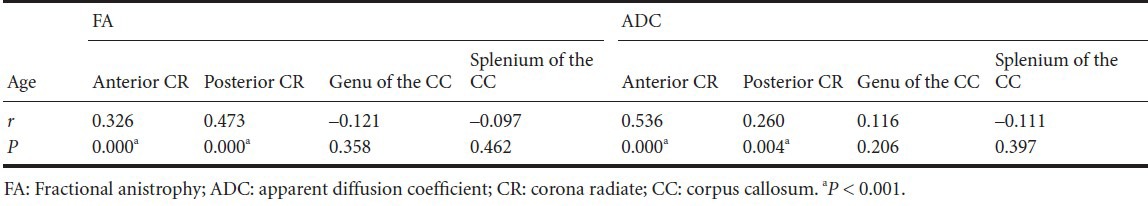
Figure 3.
Scatter diagram for correlation test between diffusion tensor imaging parameters and age.
Spearman's correlation test was used for determination of correlation between diffusion tensor imaging parameters of each region of interest and age. *P < 0.001. VBI: Ventricular body index; AHI: anterior horn index; PHI: posterior horn index.
Correlation between diffusion tensor imaging parameters in white matter and ventricular parameters
In terms of correlation between ventricular parameters and regions of white matter, we found mild to moderate positive correlation between ventricular parameters and fractional anisotropy value of anterior corona radiata (P < 0.001), and fractional anisotropy value of posterior corona radiata (P < 0.001). In addition, apparent diffusion coefficient value of anterior corona radiata also showed mild to moderate positive correlation with each ventricular parameter (P < 0.001). On the other hand, apparent diffusion coefficient value of posterior corona radiata, and fractional anisotropy and apparent diffusion coefficient value of the genu and splenium of the corpus callosum did not show significant correlation with ventricular parameters (P > 0.001; (Table 3).
Table 3.
Correlation between diffusion tensor imaging parameters in each region of white matter and ventricular index
Discussion
In the current study, we investigated the effects of ventricular enlargement in normal aging on periventricular white matter. We found that the fractional anisotropy and apparent diffusion coefficient values of the anterior corona radiata and posterior corona radiata showed positive correlation with age and its related ventricular enlargement. Fractional anisotropy value, which is the most widely used diffusion tensor imaging parameter, represents the degree of directionality of the microstructures (e.g., axons, myelin, microtubules), and the apparent diffusion coefficient value indicates the magnitude of water diffusion (Mori et al., 1999; Assaf and Pasternak, 2008; Neil, 2008). Therefore, mechanical pressure by ventricular enlargement appeared to cause higher packing of fibers and increased fiber density per unit area, resulting in increased fractional anisotropy and apparent diffusion coefficient values of anterior and posterior corona radiata. According to our result, ventricular enlargement with normal aging appears to have the greatest effect on anterior and posterior periventricular white matter. Consequently, this characteristic might be vulnerable to pressure by ventricular enlargement.
Decline of cognitive function, urinary control, sensory-motor function, and gait ability are well-known as aging related changes of normal elderly (Bradley et al., 1991; Bowen and Atwood, 2004; Hedden and Gabrieli, 2004; Grady, 2012; Moran et al., 2012). Many neural tracts from the frontal lobe, such as thalamocortical tracts between the thalamus and the prefrontal cortex, corticoreticulospinal tract, and the neural tract for urinary control are known to pass through the anterior corona radiata (Newton et al., 2006; Klein et al., 2010; Tadic et al., 2010; Yeo et al., 2012). By contrast, somatosensory and motor neural tracts, such as the corticospinal tract, spinothalamic tract, and medial lemniscus are known to ascend or descend through the posterior corona radiata (Jang, 2009; Han et al., 2010; Hong et al., 2010). In the current study, although the discrimination of neural tract on the anterior and posterior corona radiata was not available using region of interest based measure technique, we think that our results, which showed compression of the anterior corona radiata by ventricular enlargement, might be related to the above clinical manifestations of frontal lobe function. In addition, decline of fine motor function and sensory perception might be related to compression in the posterior corona radiata by ventricular enlargement. These results appear to be compatible with the results of the previous studies, which showed changing of white matter with cognitive impairments in aging (Vernooij et al., 2009; Ziegler et al., 2010; Bennett et al., 2011; Borghesani et al., 2013). By contrast, genu and splenium of the corpus callosum, which facilitate inter-hemispheric communication, showed correlation with some cognitive and sensory function, respectively, however, were not responsible for these functions (Bogousslavsky, 2005; Jea et al., 2008; Jang et al., 2012). In addition, the corpus callosum is a colossal commissure between cerebral hemispheres, consequently (Bogousslavsky, 2005; Jea et al., 2008), genu and splenium of the corpus callosum might be less affected by age-related ventricular enlargement.
Several previous studies have reported ventricular enlargement with Alzheimer's disease and hydrocephalus due to stroke or other brain injury (Uylings and de Brabander, 2002; Yeo et al., 2011; Hattori et al., 2012; Jang et al., 2013). On the other hand, even without exact estimation of change of periventricular white matter, only a few studies have demonstrated ventricular enlargement with normal aging (Preul et al., 2006; Apostolova et al., 2012). Using MRI and 3D multi slice modified driven equilibrium Fourier transform, Preul et al. (2006) evaluated cortical thickness and ventricular width throughout normal aging. They demonstrated that an increase in ventricular width occurs in normal aging, accompanied by a decrease in cortical thickness. Recently, Apostolova et al. (2012) reported an association of normal aging with hippocampal atrophy and ventricular enlargement. However, they suggested that progression of ventricular enlargement over time was relatively slow compared to patients with Alzheimer's disease.
In conclusion, we observed ventricular enlargement with normal aging and its related changes in periventricular white matter. We believe that our results are likely to be useful for diagnosis and management of normal aging. However, some limitations to this study should be considered in the interpretation of the results. First, current region of interest approach technique is operator-dependent and it is difficult to full reflect the underlying neural fiber. Therefore, region of interest approach may underestimate the fiber tracts. Second, we could not evaluate declination of cognitive functions and motor functions concerned with aging. Additional studies are needed to report clinical correlation between changes of periventricular white matter and normal aging.
Acknowledgments
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, No. 2012R1A1B4003477.
Footnotes
Conflicts of interest: None declared.
Copyedited by Wang J, Li CH, Song LP, Zhao M
References
- 1.Apostolova LG, Green AE, Babakchanian S, Hwang KS, Chou YY, Toga AW, Thompson PM. Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment (MCI), and Alzheimer disease. Alzheimer Dis Assoc Disord. 2012;26:17–27. doi: 10.1097/WAD.0b013e3182163b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- 3.Bennett IJ, Madden DJ, Vaidya CJ, Howard JH, Jr, Howard DV. White matter integrity correlates of implicit sequence learning in healthy aging. Neurobiol Aging. 2011;32:2317. doi: 10.1016/j.neurobiolaging.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM, Parker N, Kurth S, Horn SD. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. AJNR Am J Neuroradiol. 1995;16:241–251. [PMC free article] [PubMed] [Google Scholar]
- 5.Bogousslavsky J. Stroke syndromes: relevance for therapy. J Neurol Sci. 2005;238:S35–35. [Google Scholar]
- 6.Borghesani PR, Madhyastha TM, Aylward EH, Reiter MA, Swarny BR, Schaie KW, Willis SL. The association between higher order abilities, processing speed, and age are variably mediated by white matter integrity during typical aging. Neuropsychologia. 2013;51:1435–1444. doi: 10.1016/j.neuropsychologia.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowen RL, Atwood CS. Living and dying for sex. A theory of aging based on the modulation of cell cycle signaling by reproductive hormones. Gerontology. 2004;50:265–290. doi: 10.1159/000079125. [DOI] [PubMed] [Google Scholar]
- 8.Bradley WG, Daroff RB, Fenichel GM, Marsden CD. Stoneham, MA: Butterworth-Heinemann; 1991. Neurology in Clinical Practice. [Google Scholar]
- 9.Grady C. The cognitive neuroscience of ageing. Nat Rev Neurosci. 2012;13:491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han BS, Hong JH, Hong C, Yeo SS, Lee D, Cho HK, Jang SH. Location of the corticospinal tract at the corona radiata in human brain. Brain Res. 2010;1326:75–80. doi: 10.1016/j.brainres.2010.02.050. [DOI] [PubMed] [Google Scholar]
- 11.Hattori T, Ito K, Aoki S, Yuasa T, Sato R, Ishikawa M, Sawaura H, Hori M, Mizusawa H. White matter alteration in idiopathic normal pressure hydrocephalus: tract-based spatial statistics study. AJNR Am J Neuroradiol. 2012;33:97–103. doi: 10.3174/ajnr.A2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 13.Hong JH, Son SM, Jang SH. Identification of spinothalamic tract and its related thalamocortical fibers in human brain. Neurosci Lett. 2010;468:102–105. doi: 10.1016/j.neulet.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 14.Jang SH. A review of corticospinal tract location at corona radiata and posterior limb of the internal capsule in human brain. Neurorehabilitation. 2009;24:279–283. doi: 10.3233/NRE-2009-0479. [DOI] [PubMed] [Google Scholar]
- 15.Jang SH, Lee J, Yeo SS, Chang MC. Callosal disconnection syndrome after corpus callosum infarct: a diffusion tensor tractography study. J Stroke Cerebrovasc Dis. 2013;22:e240–244. doi: 10.1016/j.jstrokecerebrovasdis.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Jang SH, Choi BY, Chang CH, Jung YJ, Byun WM, Kim SH, Yeo SS. The effects of hydrocephalus on the periventricular white matter in intracerebral hemorrhage: a diffuser tensor imaging study. Int J Neurosci. 2013;123:420–424. doi: 10.3109/00207454.2012.763164. [DOI] [PubMed] [Google Scholar]
- 17.Jea A, Vachhrajani S, Widjaja E, Nilsson D, Raybaud C, Shroff M, Rutka JT. Corpus callosotomy in children and the disconnection syndromes: a review. Childs Nerv Syst. 2008;24:685–692. doi: 10.1007/s00381-008-0626-4. [DOI] [PubMed] [Google Scholar]
- 18.Klein JC, Rushworth MF, Behrens TE, Mackay CE, de Crespigny AJ, D’Arceuil H, Johansen-Berg H. Topography of connections between human prefrontal cortex and mediodorsal thalamus studied with diffusion tractography. Neuroimage. 2010;51:555–564. doi: 10.1016/j.neuroimage.2010.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran JM, Jolly E, Mitchell JP. Social-cognitive deficits in normal aging. J Neurosci. 2012;32:5553–5561. doi: 10.1523/JNEUROSCI.5511-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Neil JJ. Diffusion imaging concepts for clinicians. J Magn Reson Imaging. 2008;27:1–7. doi: 10.1002/jmri.21087. [DOI] [PubMed] [Google Scholar]
- 22.Newton JM, Ward NS, Parker GJ, Deichmann R, Alexander DC, Friston KJ, Frackowiak RS. Non-invasive mapping of corticofugal fibres from multiple motor areas--relevance to stroke recovery. Brain. 2006;129:1844–1858. doi: 10.1093/brain/awl106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preul C, Hund-Georgiadis M, Forstmann BU, Lohmann G. Characterization of cortical thickness and ventricular width in normal aging: a morphometric study at 3 Tesla. J Magn Reson Imaging. 2006;24:513–519. doi: 10.1002/jmri.20665. [DOI] [PubMed] [Google Scholar]
- 24.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 25.Tadic SD, Griffiths D, Murrin A, Schaefer W, Aizenstein HJ, Resnick NM. Brain activity during bladder filling is related to white matter structural changes in older women with urinary incontinence. Neuroimage. 2010;51:1294–1302. doi: 10.1016/j.neuroimage.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ. Age-induced white matter changes in the human brain: a stereological investigation. Neurobiol Aging. 1997;18:609–615. doi: 10.1016/s0197-4580(97)00155-3. [DOI] [PubMed] [Google Scholar]
- 27.Uylings HB, de Brabander JM. Neuronal changes in normal human aging and Alzheimer's disease. Brain Cogn. 2002;49:268–276. doi: 10.1006/brcg.2001.1500. [DOI] [PubMed] [Google Scholar]
- 28.Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, Niessen WJ, Van der Lugt A, Breteler MM. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry. 2009;66:545–553. doi: 10.1001/archgenpsychiatry.2009.5. [DOI] [PubMed] [Google Scholar]
- 29.Yeo SS, Chang MC, Kwon YH, Jung YJ, Jang SH. Corticoreticular pathway in the human brain: diffusion tensor tractography study. Neurosci Lett. 2012;508:9–12. doi: 10.1016/j.neulet.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 30.Yeo SS, Choi BY, Chang CH, Jung YJ, Ahn SH, Son SM, Byun WM, Jang SH. Periventricular white matter injury by primary intraventricular hemorrhage: a diffusion tensor imaging study. Eur Neurol. 2011;66:235–241. doi: 10.1159/000330942. [DOI] [PubMed] [Google Scholar]
- 31.Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiol Aging. 2010;31:1912–1926. doi: 10.1016/j.neurobiolaging.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


