Abstract
When repairing nerves with adhesives, most researchers place glue directly on the nerve stumps, but this method does not fix the nerve ends well and allows glue to easily invade the nerve ends. In this study, we established a rat model of completely transected sciatic nerve injury and repaired it using a modified 1 cm-length conduit with inner diameter of 1.5 mm. Each end of the cylindrical conduit contains a short linear channel, while the enclosed central tube protects the nerve ends well. Nerves were repaired with 2-octyl-cyanoacrylate and suture, which complement the function of the modified conduit. The results demonstrated that for the same conduit, the average operation time using the adhesive method was much shorter than with the suture method. No significant differences were found between the two groups in sciatic function index, motor evoked potential latency, motor evoked potential amplitude, muscular recovery rate, number of medullated nerve fibers, axon diameter, or medullary sheath thickness. Thus, the adhesive method for repairing nerves using a modified conduit is feasible and effective, and reduces the operation time while providing an equivalent repair effect.
Keywords: nerve regeneration, nerve repair, adhesive anastomosis, cyanoacrylate, nerve conduits, sciatic nerve, electrophysiology, muscle recovery, the International Technology Cooperation Program, neural regeneration
Introduction
Nerve anastomosis is an important mechanism in the fields of neurosurgery and microsurgery that is vital to reconstruct nerve function and to increase the success rate of reconstructions. Currently, suture remains the gold standard for peripheral nerve anastomosis. However, both epineurial and perineurial sutures unavoidably injure the nerve fiber. The suture material itself can also possibly induce an inflammatory reaction (Harris and Tindall, 1991; Diao and Vannuyen, 2000; Choi et al., 2004).
Because of the shortcomings of suturing, many researchers have developed different instruments and methods for better restoration of neurologic motion and sensory functions (Braun, 1966; de Medinaceli et al., 1983; Strauch, 2000; Wieken et al., 2003). In recent years, sutureless methods have been gaining traction. One method using lasers could offer certain advantages over sutures such as less tissue injury, a smaller inflammatory reaction, and a shorter procedure (Beggs et al., 1986; Almquist, 1988; Bailes et al., 1989; Korff et al., 1992; Dubuisson and Kline, 1993; Menovsky et al., 1995). However, the clinical application of this method is still limited because of the high risk of dehiscence after the operation (Korff et al., 1992; Dubuisson and Kline, 1993; Menovsky et al., 1994; Menovsky et al., 1996). Fibrin glue has also been suggested instead of the laser. This glue is less expensive and simpler than the laser setup, though the main disadvantages of glue are similar to those of laser methods: a low bonding strength and the occurrence of allergic reactions (Matras et al., 1972; Cruz et al., 1986; Smahel et al., 1987; Narakas, 1988; Maragh et al., 1990). Cyanoacrylate has gradually caught the interest of researchers not only because it is cheap and simple to use, but also because it offers sufficient tensile strength. Many researchers have studied the repair of peripheral nerves using cyanoacrylate in animal models and obtained encouraging results (Awe et al., 1963; Choi et al., 2004; Lee et al., 2006; Merolli et al., 2009; Landegren et al., 2010). Pineros-Fernandez et al. (2005) and Elgazzar et al. (2007) achieved end-to-end nerve anastomosis using octyl 2-cyanoacrylate and n-butyl-2-cyanoacrylate and concluded that this method was effective and safe. Nevertheless, both groups glued the cyanoacrylate directly onto the nerve. Nerve is very soft, making it difficult to maintain the correct position of the nerve ends when gluing them. To improve sutureless anastomosis, a conduit may be a useful assistant instrument because it can contribute to better fixing of the nerve ends and to avoiding the invasion of glue into the nerve ends. The concept of nerve conduits was introduced in neurosurgery several decades ago, and has gradually become widely accepted (Doolabh et al., 1996; Bertleff et al., 2005; Sinis et al., 2005; Taras et al., 2005; Lee et al., 2006; Schlosshauer et al., 2006; Dornseifer et al., 2011). With the help of a conduit, a tiny gap can be created between the two nerve stumps, and it is widely accepted that if the gap width is 10 mm or less, the autograft nerve and conduit can achieve a good repair (Stang et al., 2005; Schlosshauer et al., 2006; Bozkurt et al., 2007; Pfister et al., 2007; Madaghiele et al., 2008). Nevertheless, few papers have addressed the design of the special nerve conduit needed for the adhesive technique, including definition of the conduit structural parameters. Ordinarily, nerve conduits are cylindrical. For nerve repair, the two ends are inserted into the tube to fix their positions, and then glue is placed onto the ends of the tube to bond the nerve stumps. However, it is difficult to insert the nerve ends into the conduit because the nerve is soft and there is frictional resistance. The length of nerve that should be inserted into the conduit is also unclear. Merolli et al. (2009) successfully anastomosed rat sciatic nerve with cyanoacrylate and a homemade nerve conduit. In that experiment, the authors ingeniously reshaped the cylindrical conduit into a double-halved cuboid. This conduit is a novel instrument, and nerve stumps can be easily inserted into the conduit and fixed well. However, the procedures with this conduit become a little more complicated, reducing the time savings, which is an important feature of the adhesive method. Taken together, these reports suggest that the adhesive technique is a promising method, and is better suited for nerve repair when a nerve conduit is used, but the conventional conduit shape may not be ideal for the adhesive bonding technique.
To improve this sutureless method, we designed a special conduit for the adhesive technique and defined the best parameters for its use through in vitro testing, and then repaired nerves with cyanoacrylate and the modified conduit in an in vivo rat model.
Materials and Methods
Animals
A total of 60 male Sprague-Dawley rats, weighing 280–320 g, were housed in special cages with free access to food and water. They received humane care in compliance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Science, National Research Council. Washington, DC: National Academies Press, 1996). The animal experimental protocol was approved by the local Ethics Committee of the Chinese PLA General Hospital and the Committee of the Animal Experiment Center of the Chinese PLA Postgraduate Medical School.
Study design and experimental groups
The 60 rats were randomly divided into three groups. One in vitro experiment was performed to determine the appropriate length of the nerve ends that should be inserted into the conduit. A small amount of glue was siphoned into the conduit if not enough nerve was placed inside it, and that glue could invade the nerve end and affect the reconstruction. The length required depends on the length of the conduit. Forty sciatic nerves taken from twenty rats in this experiment were randomly divided into four groups, each with a different nerve insertion length into the conduit: 2, 3, 4, or 5 mm to mimic the process of repair (n = 10).
Another in vitro experiment was performed to determine the appropriate conduit inner diameter. To use as little glue as possible to reduce the biological toxicity caused by cyanoacrylate, we only placed a single drop of glue on each conduit end. Thus, the different inner conduit diameters led to different tensile strengths that the specimens could support. However, the larger the inner diameter of the conduit, the easier it was to put the nerve end into the conduit. Because the average diameter of normal rat sciatic nerve was 1.3 mm (based on our measurements), we designed three conduits with inner diameters of 1.5, 1.8, and 2.0 mm. Forty sciatic nerves taken from twenty rats for this experiment were randomly divided into four groups (n = 10): a suture control group that used 9-0 suture, and the three different conduit inner diameter groups (1.5, 1.8, and 2.0 mm) to mimic the process of repair.
An in vivo animal experiment was performed in 20 Sprague-Dawley rats to determine the feasibility and effectiveness of this method. The rats were randomly divided into the experimental or control group as follows. In the experimental group (n = 10), the right sciatic nerve was sharply transected and anastomosed with 2-octyl-cyanoacrylate and a redesigned conduit. In the control group (n = 10), anastomosis of the right sciatic nerve was performed using 9-0 microsurgery sutures and a nerve conduit.
Nerve conduit
Two small linear channels were made on the conduit surface in a straight line, but not connected, from each conduit end. The beginning of the channel was at the notch, and its length was slightly shorter than the length of the inserted nerve, which makes nerve insertion along the channel, and avoiding curves, simple. Moreover, the intact portion covered the ends completely, better protecting them (Figure 1). The conduit material was silicone, because the protective effect of silicone is reliable (Lundborg et al., 1982; Merle et al., 1989; Lundborg et al., 1997; Braga-Silva, 1999), and the aim of this study was to evaluate the feasibility and effectiveness of this new method. The conduit inner diameter was first set as 1.5 mm, and the length was first set as 10 mm, with the final parameters defined through the in vitro testing.
Figure 1.
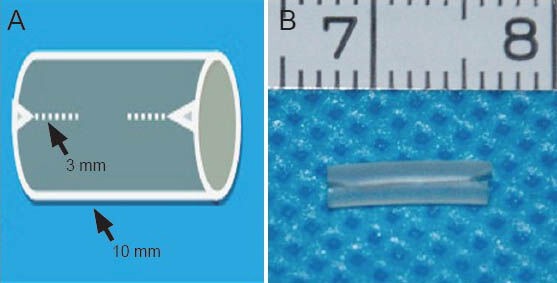
A sketch of the modified nerve conduit.
There are two linear, unconnected channels. (A) A sketch of the con-duit with the two small channels. The length of the channels is 3 mm (arrow) and the length of the conduit is 10 mm (arrow). (B) An image of the conduit (7, 8: 7, 8 cm).
Adhesive and container
The adhesive used in this study was 2-octyl-cyanoacrylate (Dermabond, Ethicon Inc., Somerville, NJ, USA). The advantages of this glue include low biotoxicity and strong tensile resistance. For convenience during the operation, the glue was placed in a sterile and soft plastic container with a cone-shaped head and cylindrical base. A 1 mL syringe needle was installed on the container head, allowing us to easily control the quantity of glue applied.
Surgical procedure for the in vitro experiments
All rats used for the in vitro tests were sacrificed with a lethal intraperitoneal dose of ketamine hydrochloride. The skin and subcutaneous fascia of the right leg were dissected, and the sciatic nerve was exposed by dissecting the hamstring muscles. Approximately 2 cm of the nerve was cut out and soaked in normal saline, and then the same procedure was repeated on the left leg. All sciatic nerves were harvested following this method.
For the first in vitro experiment, 40 nerve specimens were randomly divided into the four groups to compare insertion lengths, as defined above. In the 2 mm group, the specimen was transected in the middle, and the stumps were inserted 2 mm into the conduit, and then one drop of glue was placed on each conduit end where it connected with the nerve. After about 15 seconds, the glue was solidified, and the condition of the nerve end was observed. The same procedure was done for the 3, 4, and 5 mm insertion groups.
For the second in vitro experiment, an additional 40 nerve specimens were harveste and randomly divided into four groups to compare conduit inner diameters, as defined above. In the suture group, the specimen was transected in the middle, each nerve end was connected using the 9-0 perineurial suture method with six stitches, and then the tension strength of the samples was tested. In the other three adhesive groups, all of the specimens were inserted 4 mm into the conduit. The specimens were transected in the middle, and the stumps were placed into the 1.5, 1.8, or 2.0 mm inner diameter conduits. One drop of glue was placed on each conduit end at the connection to the nerve. After the glue solidified, the tensile strength of the samples was tested.
Surgical procedure of animal experiment
The animals were anesthetized by intraperitoneal injection of a combination of ketamine hydrochloride (40–50 mg/kg; Wanhe Pharmaceutical Co., Ltd., Beijing, China) and xylazine (10 mg/kg, Wanhe Pharmaceutical Co., Ltd.). The right sciatic nerve was exposed, and the left was left intact. Immediately, the nerve was sharply transected 1 cm away from the distal sciatic trifurcation using microscissors. In the experimental group, the two nerve ends were inserted 4 mm into the conduit using microscope forceps along the linear. One drop of glue was placed on each conduit end by gently pinching it, and the glue took 5–10 seconds to solidify. Then, a drop of glue was similarly placed on the other conduit end, finishing the repair procedure (Figure 2). In the control group, the suture needle was first inserted through the conduit outside wall at 11 o’clock more than 4 mm from the end of the conduit. Next, the needle was pushed through the epineurium of one nerve end, and then through the conduit from the inside wall at 1 o’clock. This procedure was repeated for the symmetric part of the same conduit and nerve end. These procedures were then repeated on the other nerve end. Finally, the stumps were inserted into the conduit by pulling the eight 9-0 silk suture ends (Figure 3). For both groups, a gap of up to 2 mm between the two nerve ends remained. When the anastomosis was complete, the muscle and skin were sutured closed by layer, and the rats were returned to their cages.
Figure 2.
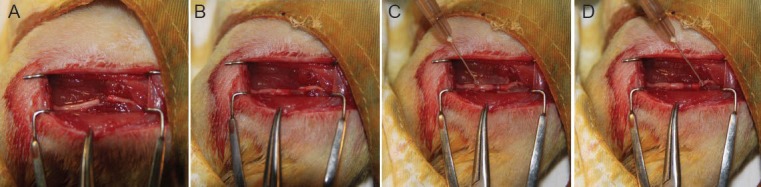
The procedure for repairing the nerve with the adhesive and conduit.
(A) The nerve was transected using microscissors. (B) One nerve end was placed into the conduit using microforceps along the linear channel, and the same thing was done on the other side. (C) One drop of glue was placed on one conduit end and allowed to solidify for 5–10 seconds. (D) The other side was then similarly glued. These procedures took approximately 1.5 minutes on average in total.
Figure 3.
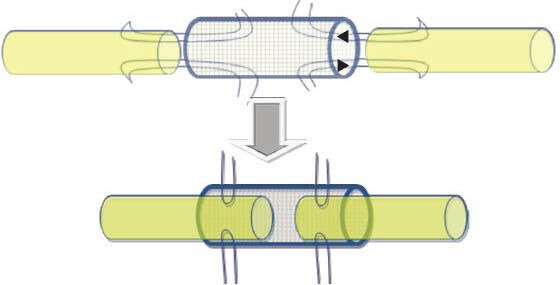
Schematic showing the nerve repair using sutures and the conduit.
The arrows show the direction of fitting.
Defining the conduit parameters
For the first in vitro experiment, after the glue solidified, the conduit was split, and the axon of the nerve end was observed. Whether glue spilled out or not was also observed. The results were recorded to define the best insertion length. For the second in vitro experiment, after the glue solidified, every specimen was fixed on the DTG-1 tension meter (Digital Inc., Tokyo, Japan) and the maximum tension they could withstand was recorded. The tensile results from each group were compared to define the best conduit inner diameter.
Operation time and functional assessment
The operation timer was started upon transection of the nerve, and was stopped when the nerve ends were connected. The functional recovery of the rats was evaluated using the sciatic function index (Bain et al., 1989) prior to the surgery and 2, 4, 8, and 12 weeks after surgery. The sciatic function index was calculated using Bain's formula: sciatic function index = 109.5×(ETS–NTS)/NTS − 38.3×(EPL–NPL)/NPL + 13.3×(EIT–NIT)/NIT − 8.8, where E is the injury side, Ns the normal side, TS the distance from the first to the fifth digit, Pl the distance from the third digit to the end of the footmark, and IT the distance from the second to the forth digits. A sciatic function index of 0 implies normal function, whereas an index of −100 indicates complete injury.
Electromyography and muscle wet weight ratio
After 3 months, all rats were again anesthetized, their sciatic nerves were exposed, and somatosensory evoked potentials were recorded in both the operated and intact hind limbs of the adhesive and suture groups rats using a portable electromyography instrument (Keypoint, Medtronic Danmark A/S, Copenhagen, Denmark). The active electrode was inserted into the proximal end of the anastomotic stoma and the recording electrode was inserted into the gastrocnemius muscle. Five hundred stimulation pulses of 0.2 ms each were generated at a rate of 1.5 Hz. The stimulus intensity was 6.0 mA, and a slight twitching of the limb was observed in all rats. Motor evoked potential latency and motor evoked potential amplitude (positive wave peaks) were measured. Then, all rats were killed while under deep anesthesia in an atmosphere saturated with CO2. The triceps surae (gastrocnemius and soleus) muscles were then harvested and the same procedure was performed on the other leg. Blood was cleaned from the muscles using filter papers, they were weighed on an electrical scale, and then the wet weight ratio of the operated to non-operated side was calculated. Muscle atrophies if nerve control is lost, and the weight will recover with nerve regeneration. Thus, higher mass ratios indicate better recovery.
Histological analysis
Approximately 1 cm of all the operated nerves on the right side was retrieved after general observation, including the distal and proximal 5 mm next to the anastomosis. The retrieved nerves were fixed in glutaraldehyde for at most 24 hours, dehydrated in serial passages of acetone, and then embedded in araldite. Semi- and super-thin traverse sections were cut in the distal 3 mm of the anastomosis, and then a vertical section was cut. The longitudinal sections were stained with Luxol fast blue and counterstained with hematoxylin and eosin, and the semi-thin traverse sections were stained with toluidine blue. The samples were observed by light microscopy (Olympus, Tokyo, Japan). The super-thin sections were stained with uranium-lead dyeing and observed by transmission electron microscopy (Phenom Inc., Amsterdam, Netherlands). The number of medullated fibers, axonal diameter, and the thickness of the medullary sheet were measured by image analysis software (Image Pro Plus 6.0, Media Cybernetics Inc., Rockville, MD, USA).
Statistical analysis
All quantitative experimental data were presented as mean ± SD. Qualitative results were presented as percentages. The data were statistically analyzed using independent-sample t-test and Pearson Chi-square test, with Kruskal-Wallis H test when appropriate, using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA) with P-values less than 0.05 considered significant.
Results
Conduit structural parameters
For the first in vitro experiment, glue spilled into the ends of seven specimens in the 2-mm insertion group, and into the ends of five specimens in the 3-mm group. No glue spillage occurred in the 4- or 5-mm groups. Thus, insertion of at least 4 mm of the nerve end into the conduit was effective for avoiding glue spillage. Based on this, we recommend a conduit length of 10 mm. The 2-mm gap permitted between the two ends was for reducing the tensile strength caused by neural retraction. The length of the channels on the conduit surface was 3 mm.
The average tensile strength was 2.48 ± 0.32 N in the 1.5-mm inner diameter conduit group, 1.93 ± 0.26 N in the 1.8-mm group, 1.26 ± 0.41 N in the 2.0-mm group, and 2.56 ± 0.28 N in the suture group. There was no significant difference between the 1.5-mm and suture groups, but the differences between the 1.8- and 2.0-mm groups and the suture group was significant. Therefore, the 1.5 mm inner diameter conduit was used for the in vivo study.
Operation time
The time required to complete nerve anastomosis was significantly decreased using the adhesive method. With the use of the adhesive, anastomosis required 1.47 ± 0.42 minutes. With the suture method, the time was 12.24 ± 2.85 minutes, a significantly longer operation time (P < 0.05). All procedures were performed by the first author alone.
Assessment of sciatic nerve function after repair
Two weeks after the operation, the sciatic function index scores tended towards −100 in both groups, showing a distinct sign of nearly complete loss of function. After 2 weeks, both groups underwent progressive recovery through 12 weeks (Figure 4). There were no significant differences in the sciatic function index between the two groups at 2, 4, 8, or 12 weeks.
Figure 4.
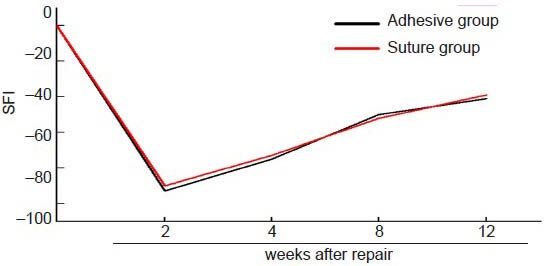
The change in sciatic function index (SFI) of the two groups at different time points after sciatic nerve repair with two methods.
The nerve function progressively increased after reaching the lowest level at 2 weeks. There were no significant differences in SFI between the two groups at any time point.
Changes in motor evoked potential latency, motor evoked potential amplitude and wet weight of triceps surae muscle after sciatic nerve repair
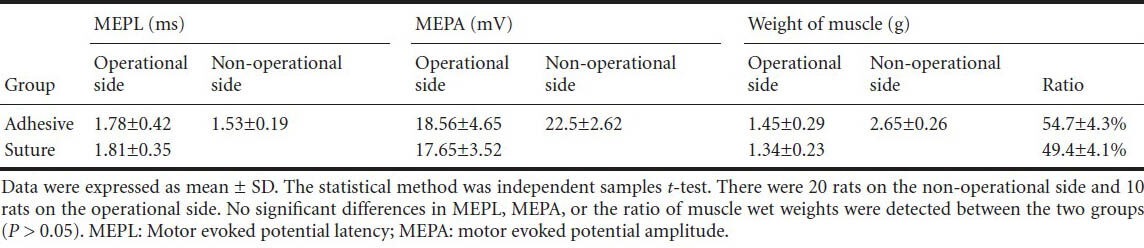
The motor evoked potential latency of the treated limbs in both groups were longer than their corresponding non-operated sides (P < 0.05), and the motor evoked potential amplitudes of the treated limbs in both groups were lower than their corresponding non-operated sides (P < 0.05) at 12 weeks. There was no difference in motor evoked potential latency or motor evoked potential amplitude between the two groups. Atrophy of the triceps surae muscle was visible in all rats at 12 weeks. However, no significant difference in the ratio of muscle wet weight was detected between the two groups (P > 0.05, Table 1).
Table 1.
MEPL, MEPA, and wet weight of the triceps surae muscle at 12 weeks
Sciatic nerve morphology at 12 weeks after repair
Macroscopic examination revealed no signs of nerve dehiscence. The gross appearance of the repaired nerves showed good cooptation and all conduits were normally shaped in all rats. The degree of the inflammatory reaction was mild, but slightly more obvious in the adhesive group. No neuroma formed (Figure 5).
Figure 5.
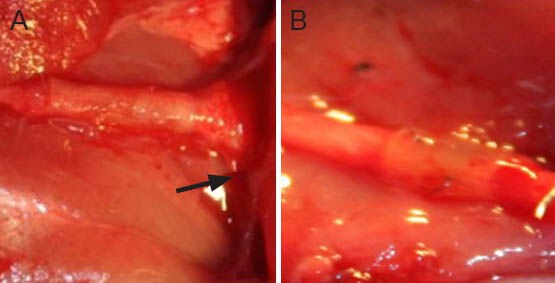
Macroscale images for general observation of the sciatic nerve in the two groups at 12 weeks after repair.
(A) Adhesive group, (B) suture group. The repaired nerves showed good cooptation. The degree of tissue adhesion was mild, but slightly more clear in the distal end of the adhesive group (arrow). No neuro-ma formed in either group.
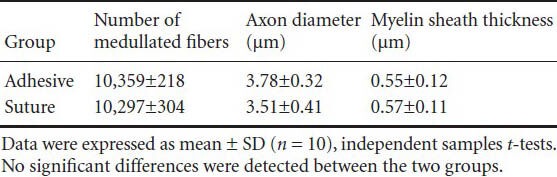
Light microscopic examination revealed anatomical continuity and various degrees of axonal regeneration. The two repair methods showed several common morphological features, and the differences were mainly found in original data. In both groups, the longitudinal sections of the repair site showed fascicular pattern loss and neatly arranged nerve fibers running continuously through the anastomotic stoma (Figure 6). In the distal zone, no significant difference in the number of medullated nerve fibers was detected between the adhesive and suture groups. Tiny myelinated and nonmyelinated fibers were visible in both groups. This result suggested that regenerated axons entered the distal stump and that myelinization gradually occurred (Figure 6). No significant differences in axon diameter or myelin sheath thickness were found between the two groups (Table 2).
Figure 6.
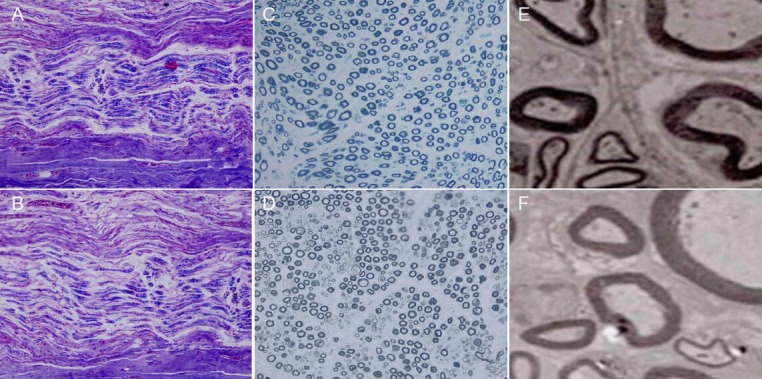
Histology of the sciatic nerve in the two groups after repair by the two methods at 12 weeks.
(A, C, E) Adhesive group; (B, D, F) suture group. In both (A) and (B), relatively neatly arranged nerve fibers, multiple axons, and Schwann cells were present, as well as slight edema that occurred in (B; × 40), but did not appear in (A; × 40). In (C) and (D), tiny myelinated and unmyelinated fibers were observed by light microscopy (× 100). In (E) and (F), the myelin sheath was observed by transmission electron microscopy (× 1,000).
Table 2.
The number of medullated nerve fibers, axon diameter, and myelin sheath thickness in the distal zone.
Assessment of adhesive integrity
No rats died during surgery or recovery. In the first 2 weeks, sciatic nerve paralysis was clearly observed, and skin ulcers were found on the feet of all rats in both groups. Subsequently, nerve regeneration occurred in all rats. The integrity of nerve adhesion in the adhesive group was as reliable as in the suture group. There was little tissue inflammatory reaction in either group. The shape of the conduit was the same in both groups.
Discussion
The repair of peripheral nerves using sutures, no matter epineurial or perineurial, is the gold standard of care. Many researchers have recognized the shortcomings of conventional sutures (Norris et al., 1988; Maragh et al., 1990; Myles et al., 1992; Sierra, 1993). Several sutureless methods have been developed that, no matter what method they use, must fulfill certain criteria, including having enough tensile strength, cause less neural trauma than sutures, and be minimally toxic to tissues. Our goal is to find a high quality technique for nerve repair to replace sutures.
Recently, adhesive bonding techniques have been gaining more attention. The main advantage of adhesive techniques is their simplicity, which greatly shortens the operation time while maintaining satisfactory repair quality. This contributes to a decreased time of wound exposure, and reduces the relative risk of infection. Another advantage of this sutureless technique is that it avoids injuring the axon with needles, and the lack of foreign bodies minimizes the inflammatory reaction. These advantages contribute to better nerve recovery. Moreover, this adhesive technique is easily to master than microscopic suturing. Based on these advantages, researchers have used fibrin glue instead of sutures for almost 30 years (Steube et al., 1988; Maragh et al., 1990; Palazzi et al., 1995). Although they reported reasonable results, but other researchers found that this glue did not provide enough bonding strength (Narakas, 1988; Cruz et al., 1986).
Cyanoacrylates demonstrate better adhesive strength, lower biotoxicity, biodegradability, and bacteriostatic features than fibrin glue (Elgazzar et al., 2007). Two research groups have even reported the successful repair of neural transection injury with cyanoacrylate, such as Pineros-Fernandez (Pineros-Fernandez et al., 2005) and Elgazzar (Elgazzar et al., 2007), and both of them achieved satisfying results. Cyanoacrylate polymers produce formaldehyde as a by-product of hydrolytic degradation, which can cause biological toxicity. However, the production rate of formaldehyde decreased with an increasing length of alkyl groups and the molecular weight of the resulting cyanoacrylate polymers, such as n-decyl, n-octyl, n-heptyl, n- or isobutyl, and methyl, which lowers the toxicity (Tseng et al., 1990; Toriumi et al., 1991; Locatelli et al., 2009). The glue 2-octyl-cyanoacrylate is the first approved for use in the clinic in the United Stated. The tensile strength of 2-octyl-cyanoacrylate has been reported to be three times larger than butyl cyanoacrylate (Penoff, 1999; Hall et al., 2000; Ang et al., 2001; Souza et al., 2008; Pope and Knowles, 2013). Thus, we used 2-octyl-cyanoacrylate as the adhesive, which is a key point of the study.
Many researchers place glue around the anastomotic stoma directly without using any instruments. Owing to the properties of neural tissue, it is very difficult to keep the nerve stumps in the ideal position during the operation, and if the nerve ends are misdirected, the neural functional recovery will be affected. On the other side, the glue could easily flow into the gap between the two ends and then solidify, which could delay nerve regeneration. In addition, when the nerve is transected, retraction can occur. If the nerves are repaired end to end by placing glue directly around the anastomotic stoma, the adhesive strength of the repair would be increased to better resist tensile stress.
The nerve conduit, another important aspect of our technique, could be a useful instrument to solve these problems. The concept of the conduit was introduced into neurosurgery several years ago and has been supported by positive results in animal experiments and in clinical treatments (Doolabh et al., 1996; Bertleff et al., 2005; Schlosshauer et al., 2006). The conduit provides an advantageous environment for nerve regeneration by protecting and fixing nerve stumps, preventing neuroma formation, decreasing neurotrophic factor-loss, and allowing a small gap to exist between the two stumps (Doolabh et al., 1996; Fine et al., 2002; Keilhoff et al., 2003; Sundback et al., 2003; Sinis et al., 2005; Yang et al., 2010). Most researchers sutured the conduit to the nerve in their experiments, and repaired the nerve successfully, which was replicated in the present study with the suture conduit control group. With the aim of developing a better nerve conduit, researchers have explored different conduit materials, injecting different factors into the conduit, and changing its structure. The nerve conduit could be a suitable alternative to autologous grafts, and may be widely used clinically for the repair of defective nerves in the future. Based on our literature survey, few studies have combined a conduit with an adhesive technique to reconstruct nerves, and only a few papers have reported the development of special conduits for adhesive techniques by modifying the structure for easier operations. Merolli et al. (2009) successfully repaired rat sciatic nerve with glue and a Chinese-made conduit. The authors presented an ingenious design for the conduit to enhance the repair effect, but this original conduit was not particularly suited for adhesive techniques. The aims of that paper were to use the conduit instead of an autologous graft and to modify the cylindrical conduit that was designed for the adhesive technique. Using this design, the nipping site can approximate the nerve end, which helped to overcome friction inside the conduit and to avoid nerve curvature. Silicone was chosen as the conduit material because it is histocompatible, stiff enough to avoid collapse (de Ruiter et al., 2009), and demonstrates appropriate elasticity. Also, the channel on the conduit surface automatically closes after the forceps are placed into the nerve. For these reasons, we choose this material for the present experiment. As expected, none of the conduits experienced deformation during the experiments. However, this material is not degradable, so it requires another operation for retrieval. A more suitable material for conduits should be explored in the future.
With these two key techniques, the use of an adhesive and conduit, this sutureless method makes nerve repair simpler and more efficient, shortening operation time by about 10 minutes compared with the suture technique. Using the conduit, a 2-mm gap can be left between the two nerve ends, and it has been shown to work during nerve repair by several papers (Doolabh et al., 1996; Sinis et al., 2005; Merolli et al., 2009). This gap can help to avoid tensional anastomosis, which does not benefit nerve recovery. Furthermore, with the help of this modified conduit, the glue can be prevented from contacting the nerve ends directly, which decrease the influence of adhesive toxicity. The present study showed that sutureless adhesive bonding anastomosis is feasible and efficient, while achieving similar results as the suture method, based on the timing record, general observation, electrophysiology, muscle recovery, and histological analysis.
In summary, both cyanoacrylate and the Chinese-made conduit played an important role in nerve repair. The custom conduit with specific parameters can improve the adhesive technique. This method of repairing nerves with these two techniques is feasible, convenient, and effective. This technique is promising for clinical treatment, and future studies should be performed to further improve it.
Acknowledgments:
We are very grateful to Professor Guo from Beijing University of Aeronautics and Astronautics in China for critical revision of this article.
Footnotes
Funding: This study was supported by the International Technology Cooperation Program, No. S2014ZR0393.
Conflicts of interest: None declared.
Copyedited by Pack M, Robens J, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- [1].Almquist EE. Nerve repair by laser. Orthop Clin North Am. 1988;19:201–208. [PubMed] [Google Scholar]
- [2].Ang ES, Tan KC, Tan LH, Ng RT, Song IC. 2-octylcyanoacrylate-assisted microvascular anastomosis: comparison with a conventional suture technique in rat femoral arteries. J Reconstr Microsurg. 2001;17:193–201. doi: 10.1055/s-2001-14351. [DOI] [PubMed] [Google Scholar]
- [3].Awe WC, Roberts W, Braunwald NS. Rapidly polymerizing adhesive as a hemostatic agent: study of tissue responce and bacteriological properties. Surgery. 1963;54:322–328. [PubMed] [Google Scholar]
- [4].Bailes JE, Cozzens JW, Hudson AR, Kline DG, Ciric I, Gianaris P, Bernstein LP, Hunter D. Laser-assisted nerve repair in primates. J Neurosurg. 1989;71:266–272. doi: 10.3171/jns.1989.71.2.0266. [DOI] [PubMed] [Google Scholar]
- [5].Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129–138. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- [6].Beggs JL, Fischer DW, Shetter AG. Comparative study of rat sciatic nerve microepineurial anastomoses made with carbon dioxide laser and suture techniques: Part 2. A morphometric analysis of myelinated nerve fibers. Neurosurgery. 1986;18:266–269. doi: 10.1227/00006123-198603000-00002. [DOI] [PubMed] [Google Scholar]
- [7].Bertleff MJ, Meek MF, Nicolai JP. A prospective clinical evaluation of biodegradable neurolac nerve guides for sensory nerve repair in the hand. J Hand Surg Am. 2005;30:513–518. doi: 10.1016/j.jhsa.2004.12.009. [DOI] [PubMed] [Google Scholar]
- [8].Bozkurt A, Brook GA, Moellers S, Lassner F, Sellhaus B, Weis J, Woeltje M, Tank J, Beckmann C, Fuchs P, Damink LO, Schugner F, Heschel I, Pallua N. In vitro assessment of axonal growth using dorsal root ganglia explants in a novel three-dimensional collagen matrix. Tissue Eng. 2007;13:2971–2979. doi: 10.1089/ten.2007.0116. [DOI] [PubMed] [Google Scholar]
- [9].Braga-Silva J. J Hand Surg Br. Vol. 24. Edinburgh, Scotland: 1999. The use of silicone tubing in the late repair of the median and ulnar nerves in the forearm; pp. 703–706. [DOI] [PubMed] [Google Scholar]
- [10].Braun RM. Comparative studies of neurorrhaphy and sutureless peripheral nerve repair. Surg Gynecol Obstet. 1966;122:15–18. [PubMed] [Google Scholar]
- [11].Choi BH, Kim BY, Huh JY, Lee SH, Zhu SJ, Jung JH, Cho BP. Microneural anastomosis using cyanoacrylate adhesives. Int J Oral Maxillofac Surg. 2004;33:777–780. doi: 10.1016/j.ijom.2004.03.006. [DOI] [PubMed] [Google Scholar]
- [12].Cruz NI, Debs N, Fiol RE. Evaluation of fibrin glue in rat sciatic nerve repairs. Plast Reconstr Surg. 1986;78:369–373. doi: 10.1097/00006534-198609000-00015. [DOI] [PubMed] [Google Scholar]
- [13].de Medinaceli L, Wyatt RJ, Freed WJ. Peripheral nerve reconnection: mechanical, thermal, and ionic conditions that promote the return of function. Exp Neurol. 1983;81:469–487. doi: 10.1016/0014-4886(83)90276-5. [DOI] [PubMed] [Google Scholar]
- [14].de Ruiter GC, Malessy MJ, Yaszemski MJ, Windebank AJ, Spinner RJ. Designing ideal conduits for peripheral nerve repair. Neurosurg Focus. 2009;26:E5. doi: 10.3171/FOC.2009.26.2.E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Diao E, Vannuyen T. Techniques for primary nerve repair. Hand Clin. 2000;16:53–66. viii. [PubMed] [Google Scholar]
- [16].Doolabh VB, Hertl MC, Mackinnon SE. The role of conduits in nerve repair: a review. Rev Neurosci. 1996;7:47–84. doi: 10.1515/revneuro.1996.7.1.47. [DOI] [PubMed] [Google Scholar]
- [17].Dornseifer U, Fichter AM, Leichtle S, Wilson A, Rupp A, Rodenacker K, Ninkovic M, Biemer E, Machens HG, Matiasek K, Papadopulos NA. Peripheral nerve reconstruction with collagen tubes filled with denatured autologous muscle tissue in the rat model. Microsurgery. 2011;31:632–641. doi: 10.1002/micr.20926. [DOI] [PubMed] [Google Scholar]
- [18].Dubuisson AS, Kline DG. Is laser repair effective for secondary repair of a focal lesion in continuity? Microsurgery. 1993;14:398–401. doi: 10.1002/micr.1920140609. discussion 402-393. [DOI] [PubMed] [Google Scholar]
- [19].Elgazzar RF, Abdulmajeed I, Mutabbakani M. Cyanoacrylate glue versus suture in peripheral nerve reanastomosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:465–472. doi: 10.1016/j.tripleo.2007.01.019. [DOI] [PubMed] [Google Scholar]
- [20].Fine EG, Decosterd I, Papaloizos M, Zurn AD, Aebischer P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur J Neurosc. 2002;15:589–601. doi: 10.1046/j.1460-9568.2002.01892.x. [DOI] [PubMed] [Google Scholar]
- [21].Hall WW, Wrye SW, Banducci DR, Ehrlich P. Microvascular anastomosis using 2-octyl cyanoacrylate in the rat femoral artery. Ann Plast Surg. 2000;44:508–511. doi: 10.1097/00000637-200044050-00008. [DOI] [PubMed] [Google Scholar]
- [22].Harris ME, Tindall SC. Techniques of peripheral nerve repair. Neurosurg Clin N Am. 1991;2:93–104. [PubMed] [Google Scholar]
- [23].Keilhoff G, Stang F, Wolf G, Fansa H. Bio-compatibility of type I/III collagen matrix for peripheral nerve reconstruction. Biomaterials. 2003;24:2779–2787. doi: 10.1016/s0142-9612(03)00084-x. [DOI] [PubMed] [Google Scholar]
- [24].Korff M, Bent SW, Havig MT, Schwaber MK, Ossoff RH, Zealear DL. An investigation of the potential for laser nerve welding. Otolaryngol Head Neck Surg. 1992;106:345–350. doi: 10.1177/019459989210600405. [DOI] [PubMed] [Google Scholar]
- [25].Landegren T, Risling M, Persson JK, Sonden A. Cyanoacrylate in nerve repair: transient cytotoxic effect. Int J Oral Maxillofac Surg. 2010;39:705–712. doi: 10.1016/j.ijom.2010.03.008. [DOI] [PubMed] [Google Scholar]
- [26].Lee DY, Choi BH, Park JH, Zhu SJ, Kim BY, Huh JY, Lee SH, Jung JH, Kim SH. Nerve regeneration with the use of a poly(l-lactide-co-glycolic acid)-coated collagen tube filled with collagen gel. J Craniomaxillofac Surg. 2006;34:50–56. doi: 10.1016/j.jcms.2005.07.011. [DOI] [PubMed] [Google Scholar]
- [27].Locatelli D, Vitali M, Custodi VM, Scagnelli P, Castelnuovo P, Canevari FR. Endonasal approaches to the sellar and parasellar regions: closure techniques using biomaterials. Acta Neurochir. 2009;151:1431–1437. doi: 10.1007/s00701-009-0428-9. [DOI] [PubMed] [Google Scholar]
- [28].Lundborg G, Dahlin L, Dohi D, Kanje M, Terada N. A new type of “bioartificial” nerve graft for bridging extended defects in nerves. J Hand Surg Br. 1997;22:299–303. doi: 10.1016/s0266-7681(97)80390-7. [DOI] [PubMed] [Google Scholar]
- [29].Lundborg G, Dahlin LB, Danielsen N, Hansson HA, Johannesson A, Longo FM, Varon S. Nerve regeneration across an extended gap: a neurobiological view of nerve repair and the possible involvement of neuronotrophic factors. J Hand Surg Am. 1982;7:580–587. doi: 10.1016/s0363-5023(82)80107-x. [DOI] [PubMed] [Google Scholar]
- [30].Madaghiele M, Sannino A, Yannas IV, Spector M. Collagen-based matrices with axially oriented pores. J Biomed Mater Res A. 2008;85:757–767. doi: 10.1002/jbm.a.31517. [DOI] [PubMed] [Google Scholar]
- [31].Maragh H, Meyer BS, Davenport D, Gould JD, Terzis JK. Morphofunctional evaluation of fibrin glue versus microsuture nerve repairs. J Reconstr Microsurg. 1990;6:331–337. doi: 10.1055/s-2007-1006838. [DOI] [PubMed] [Google Scholar]
- [32].Matras H, Dinges HP, Lassmann H, Mamoli B. Suture-free interfascicular nerve transplantation in animal experiments. Wien Med Wochenschr. 1972;122:517–523. [PubMed] [Google Scholar]
- [33].Menovsky T, Beek JF, van Gemert MJ. CO2 laser nerve welding: optimal laser parameters and the use of solders in vitro. Microsurgery. 1994;15:44–51. doi: 10.1002/micr.1920150112. [DOI] [PubMed] [Google Scholar]
- [34].Menovsky T, Beek JF, Thomsen SL. Laser(-assisted) nerve repair. A review. Neurosurg Rev. 1995;18:225–235. doi: 10.1007/BF00383873. [DOI] [PubMed] [Google Scholar]
- [35].Menovsky T, Beek JF, van Gemert MJ, Roux FX, Bown SG. Interstitial laser thermotherapy in neurosurgery: a review. Acta Neurochi. 1996;138:1019–1026. doi: 10.1007/BF01412303. [DOI] [PubMed] [Google Scholar]
- [36].Merle M, Dellon AL, Campbell JN, Chang PS. Complications from silicon-polymer intubulation of nerves. Microsurgery. 1989;10:130–133. doi: 10.1002/micr.1920100213. [DOI] [PubMed] [Google Scholar]
- [37].Merolli A, Rocchi L, Catalano F, Planell J, Engel E, Martinez E, Sbernardori MC, Marceddu S, Leali PT. In vivo regeneration of rat sciatic nerve in a double-halved stitch-less guide: a pilot-study. Microsurgery. 2009;29:310–318. doi: 10.1002/micr.20622. [DOI] [PubMed] [Google Scholar]
- [38].Myles LM, Gilmour JA, Glasby MA. Effects of different methods of peripheral nerve repair on the number and distribution of muscle afferent neurons in rat dorsal root ganglion. J Neurosurg. 1992;77:457–462. doi: 10.3171/jns.1992.77.3.0457. [DOI] [PubMed] [Google Scholar]
- [39].Narakas A. The use of fibrin glue in repair of peripheral nerves. Orthop Clin North Am. 1988;19:187–199. [PubMed] [Google Scholar]
- [40].Norris RW, Glasby MA, Gattuso JM, Bowden RE. Peripheral nerve repair in humans using muscle autografts. A new technique. J Bone Joint Surg Br. 1988;70:530–533. doi: 10.1302/0301-620X.70B4.3403592. [DOI] [PubMed] [Google Scholar]
- [41].Palazzi S, Vila-Torres J, Lorenzo JC. Fibrin glue is a sealant and not a nerve barrier. J Reconstr Microsurg. 1995;11:135–139. doi: 10.1055/s-2007-1006521. [DOI] [PubMed] [Google Scholar]
- [42].Penoff J. Skin closures using cyanoacrylate tissue adhesives. Plastic Surgery Educational Foundation DATA Committee. Device and Technique Assessment. Plast Reconstr Surg. 1999;103:730–731. doi: 10.1097/00006534-199902000-00061. [DOI] [PubMed] [Google Scholar]
- [43].Pfister LA, Papaloizos M, Merkle HP, Gander B. Nerve conduits and growth factor delivery in peripheral nerve repair. J Peripher Nerv Syst. 2007;12:65–82. doi: 10.1111/j.1529-8027.2007.00125.x. [DOI] [PubMed] [Google Scholar]
- [44].Pineros-Fernandez A, Rodeheaver PF, Rodeheaver GT. Octyl 2-cyanoacrylate for repair of peripheral nerve. Ann Plast Surg. 2005;55:188–195. doi: 10.1097/01.sap.0000164386.72523.3c. [DOI] [PubMed] [Google Scholar]
- [45].Pope JF, Knowles T. The efficacy of n-butyl-cyanoacrylate tissue adhesive for closure of canine laparoscopic ovariectomy port site incisions. J Small Anim Pract. 2013;54:190–194. doi: 10.1111/jsap.12047. [DOI] [PubMed] [Google Scholar]
- [46].Schlosshauer B, Dreesmann L, Schaller HE, Sinis N. Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery. 2006;59:740–747. doi: 10.1227/01.NEU.0000235197.36789.42. discussion 747-748. [DOI] [PubMed] [Google Scholar]
- [47].Sierra DH. Fibrin sealant adhesive systems: a review of their chemistry, material properties and clinical applications. J Biomater Appl. 1993;7:309–352. doi: 10.1177/088532829300700402. [DOI] [PubMed] [Google Scholar]
- [48].Sinis N, Schaller HE, Schulte-Eversum C, Schlosshauer B, Doser M, Dietz K, Rosner H, Muller HW, Haerle M. Nerve regeneration across a 2-cm gap in the rat median nerve using a resorbable nerve conduit filled with Schwann cells. J Neurosurg. 2005;103:1067–1076. doi: 10.3171/jns.2005.103.6.1067. [DOI] [PubMed] [Google Scholar]
- [49].Smahel J, Meyer VE, Bachem U. Glueing of peripheral nerves with fibrin: experimental studies. J Reconstr Microsurg. 1987;3:211–220. doi: 10.1055/s-2007-1006987. [DOI] [PubMed] [Google Scholar]
- [51].Souza EC, Fitaroni RB, Januzelli DM, Macruz HM, Camacho JC, Souza MR. Use of 2-octyl cyanoacrylate for skin closure of sternal incisions in cardiac surgery: observations of microbial barrier effects. Curr Med Res Opin. 2008;24:151–155. doi: 10.1185/030079908x253807. [DOI] [PubMed] [Google Scholar]
- [52].Stang F, Fansa H, Wolf G, Reppin M, Keilhoff G. Structural parameters of collagen nerve grafts influence peripheral nerve regeneration. Biomaterials. 2005;26:3083–3091. doi: 10.1016/j.biomaterials.2004.07.060. [DOI] [PubMed] [Google Scholar]
- [53].Steube D, Hamm KD, Pothe H, Schreiber D, Beer R. Fibrin glue on the Cohn I fraction basis in repairing cerebral and dura defects--an experimental study on rats. Folia Haematol Int Mag Klin Morphol Blutforsch. 1988;115:213–217. [PubMed] [Google Scholar]
- [54].Strauch B. Use of nerve conduits in peripheral nerve repair. Hand Clin. 2000;16:123–130. [PubMed] [Google Scholar]
- [55].Sundback C, Hadlock T, Cheney M, Vacanti J. Manufacture of porous polymer nerve conduits by a novel low-pressure injection molding process. Biomaterials. 2003;24:819–830. doi: 10.1016/s0142-9612(02)00409-x. [DOI] [PubMed] [Google Scholar]
- [56].Taras JS, Nanavati V, Steelman P. Nerve conduits. J Hand Ther. 2005;18:191–197. doi: 10.1197/j.jht.2005.02.012. [DOI] [PubMed] [Google Scholar]
- [57].Toriumi DM, Raslan WF, Friedman M, Tardy ME., Jr Variable histotoxicity of histoacryl when used in a subcutaneous site: an experimental study. Laryngoscope. 1991;101:339–343. doi: 10.1002/lary.1991.101.4.339. [DOI] [PubMed] [Google Scholar]
- [58].Tseng YC, Tabata Y, Hyon SH, Ikada Y. In vitro toxicity test of 2-cyanoacrylate polymers by cell culture method. J Biomed Mater Res. 1990;24:1355–1367. doi: 10.1002/jbm.820241007. [DOI] [PubMed] [Google Scholar]
- [59].Wieken K, Angioi-Duprez K, Lim A, Marchal L, Merle M. Nerve anastomosis with glue: comparative histologic study of fibrin and cyanoacrylate glue. J Reconstr Microsurg. 2003;19:17–20. doi: 10.1055/s-2003-37186. [DOI] [PubMed] [Google Scholar]
- [60].Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, Chang SY, Lee OK, Wu KJ. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12:982–992. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]


