Abstract
Puerarin is a natural isoflavone isolated from plants of the genus Pueraria and functions as a protector against cerebral ischemia. We hypothesized that puerarin can be involved in the repair of peripheral nerve injuries. To test this hypothesis, doses of 10, 5, or 2.5 mg/kg per day puerarin (8-(β-D-Glucopyranosyl-7-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) were injected intraperitoneally into mouse models of sciatic nerve injury. Puerarin at the middle and high doses significantly up-regulated the expression of growth-associated protein 43 in the L4–6 segments of the spinal cord from mice at 1, 2, and 4 weeks after modeling, and reduced the atrophy of the triceps surae on the affected side and promoted the regeneration of nerve fibers of the damaged spinal cord at 8 weeks after injury. We conclude that puerarin exerts an ongoing role to activate growth-associated protein 43 in the corresponding segment of the spinal cord after sciatic nerve injury, thus contributing to neural regeneration after sciatic nerve injuries.
Keywords: nerve regeneration, peripheral nerve injury, puerarin, growth-associated protein 43, sciatic nerve, repair, NSFC grant, neural regeneration
Introduction
Medication and surgery are the two most commonly used treatments for sciatic nerve damage, but neither is totally effective. In recent years, natural medicine and its extracts have been shown to stimulate nerve growth factor expression and Schwann cell proliferation after nerve injuries, contributing to neural regeneration and functional recovery (Qiu et al., 1999; Xue et al., 2007; Ye et al., 2007).
Puerarin, known as 8-(β-D-Glucopyranosyl-7-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, is extracted from the root of the kudzu plant, and has been widely used in traditional Chinese herbal medicine for the treatment of various diseases (including brain diseases). Dietary isoflavones have been shown to improve stroke outcome after focal cerebral ischemia/reperfusion injuries in such a way that a higher dietary isoflavone content results in a lower infarct volume and better neurological function (Burguete et al., 2006). Some scholars have found that puerarin can reduce focal cerebral ischemia/reperfusion injuries by scavenging oxygen free radicals, expanding cerebral blood vessels, and increasing cerebral perfusion volume (Han et al., 2007; Xu et al., 2005). The protective effects of puerarin against cerebral ischemia could act by: (1) an antioxidant and anti-free radical scavenger; (2) inhibition of p53 gene expression and anti-neuronal apoptosis; (3) inhibition of inflammatory response caused by focal cerebral ischemia and reperfusion; (4) improvement of cerebral microcirculation (Xu et al., 2005; He 2007; Saida et al., 1978; Schwartz et al., 1982; Ansselin et al., 1990; Pei et al., 1995; Yang, 1999). These findings suggest that puerarin is likely to reduce initial damage and facilitate nerve repair including neural regeneration.
In the present study, we developed a sciatic nerve injury model in BALB/c mice to observe nerve myelin recovery, muscular atrophy and expression of growth-associated protein 43 (GAP43) and its mRNA in the L4–6 spinal segments of the spinal cord following administration with puerarin. This study was designed to explore the effects of puerarin on promoting neural regeneration following peripheral nerve injuries.
Materials and Methods
Animals
In total 160 BALB/c male mice, aged 4–6 weeks, weighing 20 ± 2 g, were provided by the Experimental Animal Center of Jilin University, China, and housed at room temperature and allowed free access to food and water. All the mice were subjected to unilateral sciatic nerve complete transection and microscopic anastomosis. All experimental procedures were carried out in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals (The Ministry of Science and Technology of the People's Republic of China, 2006).
Establishment of a mouse model of sciatic nerve injury
Mice were anesthetized with an intraperitoneal injection of 1% sodium thiopental (100 mg/kg) and fixed in the prone position. After the surgical region was disinfected, a 1.5 cm longitudinal incision was made on the posterior femur of the unilateral lower limb. The sciatic nerve trunk at the lower edge of the piriformis muscle was bluntly stripped from the surrounding tissues (Figure 1). Then, the sciatic nerve was completely transected 0.5 cm below the ischial tuberosity and anastomosed, viewed under a 120-fold microscope. After that, the fascia, subcutaneous tissue and skin were sutured layer by layer.
Figure 1.
Corresponding spinal segments associated with the sciatic nerve during modeling.
A 1.5 cm longitudinal incision was made on the posterior femur of the unilateral lower limb. The sciatic nerve trunk at the lower edge of the piriformis muscle was bluntly separated. The lamina was bitten to ex-pose the spinal segments associated with the sciatic nerve.
Specimen collection and preparation
Ten mice from each group were taken at the corresponding time points and intraperitoneally anesthetized with 1% sodium thiopental (100 mg/kg). The spinal canal was nipped to expose and dissociate the L4–6 spinal cord segment connected with the sciatic nerve. The L4–6 segment on the injury side was harvested, and immersed in liquid nitrogen quickly after being labeled.
Western blot assay of GAP43 protein expression in the injured spinal cord segment
The tissue specimens were taken from liquid nitrogen, and immediately placed in a mortar and pestle to be boiled in loading buffer for 15 minutes followed by centrifugation. The supernatant was taken and treated with radioimmunoprecipitation assay for cell lysis to separate and extract the proteins. The protein samples were electrophoresed in 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. The membrane was incubated with rabbit anti-mouse GAP43 antibodies (dissolved at a ratio of 1:1,000 with PBS containing 1% bovine serum albumin; Sigma, St. Louis, MO, USA) at 4°C overnight, and rinsed with 0.01 mol/L PBS 5 minutes × 4. After additional incubation with goat anti-rabbit IgG (1:10,000; Sigma) at room temperature for 1 hour, the membrane was rinsed with 0.01 mol/L PBS 5 minutes × 4, developed by 3,3′-diaminobenzidine (Sigma) according to instructions, and exposed by X-ray films followed by band scanning and analysis. GAPDH served as an internal reference, and the result was expressed as an absorbance value of GAP43/GAPDH.
Real time-PCR detection of GAP43 mRNA expression in the injured spinal cord segment
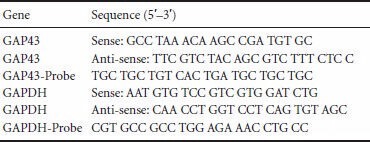
Primer sequence for GAP43 was designed by Beacon Designer 9 software, and GAPDH served as an internal reference. The specificity of the primers was determined using BLAST. The total RNA was extracted from the L4–6 segments on the injury side for real time-PCR detection of GAP43 mRNA. △Ct value was detected, and the experimental results were described as 2-△△Ct.
Primer sequences:
Hematoxylin-eosin and Luxol fast blue staining
Ten mice from each group were selected at the corresponding time points. The neural stem was cut 0.5 cm distal to the sciatic nerve anastomosis (the anastomosis was also resected), and fixed in formalin for over 72 hours. Following dehydration in alcohol and paraffin embedding, the samples were cut into sections for hematoxylin-eosin staining. After initial screening, the damaged site was confirmed and stained with Luxol fast blue staining. Interactive image analysis system (IBAS2000; Kontron, Germany) was used to automatically correct image magnification, and the measured values were the actual values. Under high magnification, four square images per region were randomly selected to measure the diameter of sciatic nerve fibers and count the number of nerve fibers. Measurement by the operator abided by the following rules: (1) the sciatic nerve fibers adjacent to the contact boundaries were not counted; (2) the diameter of the nerve fiber was measured without considering the myelin sheath membrane, and the smallest diameter was selected as the nerve fiber diameter, depending on which, the diameter and area of myelinated nerve fibers of the spinal cord were determined.
Determination of muscular mass index in the soleus muscle
Eight weeks after the sciatic nerve transection, the triceps surae muscle was excised and weighed on an electronic analytical balance. The muscular mass index in the soleus muscle was calculated by the Cuadros method (Cuadros et al., 1987) using the following formula: muscle mass of the soleus muscle/body mass × 100.
Statistical analysis
Measurement data were expressed as mean ± SD, and statistically processed using SPSS 17.0 statistical software (SPSS, Chicago, IL, USA). Multi-group comparison was performed using analysis of variance at each time point, and intergroup comparison was done using the least significant difference test. A value of P < 0.05 was considered significant.
Results
Quantitative analysis of animals
After the initial surgery, 160 mice were equally and randomly divided into low dose, middle dose, high dose, and normal saline groups, and injected with 2.5, 5, 10 mg/kg per day puerarin and an equal volume of saline. Hereafter, 10 mice from each group were selected at 1, 2, 4, 8 weeks after injury, respectively. Finally, all 160 mice were included in result analysis with no dropout.
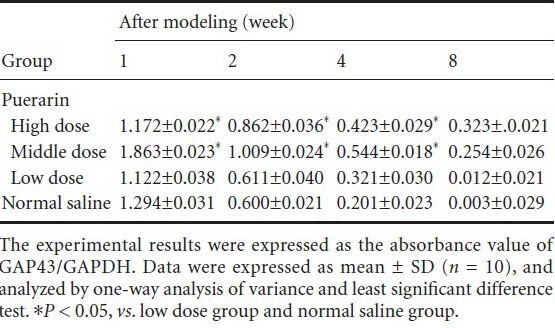
Puerarin effects on mRNA and protein expressions of GAP43 in the L4–6 segment of the injured side of mice
After 1, 2, and 4 weeks of modeling, mRNA and protein expressions of GAP43 were upregulated in the high and middle dose groups as compared with the low dose and normal saline groups (P < 0.05). After 8 weeks, there were no significant differences between the four groups (Figures 2, 3, Table 1).
Figure 2.
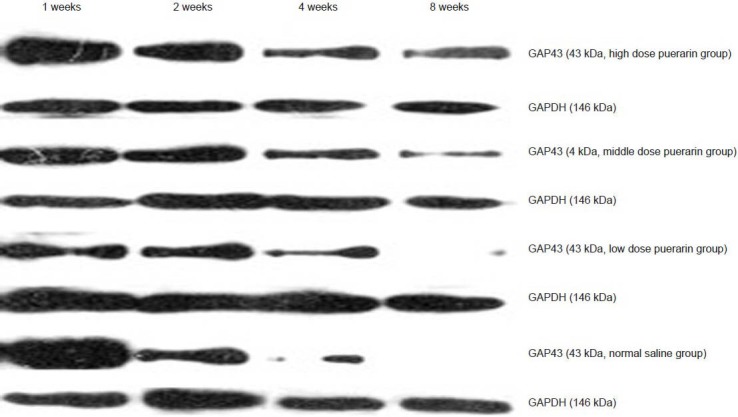
Protein expression of growth-associated protein 43 (GAP43) in the L4–6 segment of the injured side.
After 1, 2, 4, 8 weeks of modeling, the protein expressions of GAP43 were upregulated in the high and middle dose groups as compared with the low dose and normal saline groups.
Figure 3.
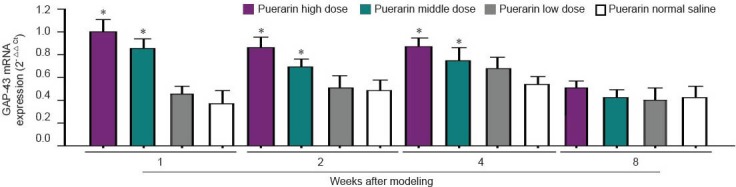
Real time-PCR detection of growth-associated protein 43 (GAP43) mRNA expression (2- △△ Ct).
Data were expressed as mean ± SD (n = 10), and analyzed by one-way analysis of variance and least significant difference test. *P < 0.05, vs. low dose group and normal saline group.
Table 1.
Changes in the protein expression of growth-associated protein 43 (GAP43) in the L4–6 segment of the injured side after administration of puerarin
Puerarin effects on the morphology of the triceps surae and spinal cord tissues on the injured sciatic nerve side of mice
At 8 weeks after injury, the muscle cells in the high and middle dose groups were arranged regularly and tightly, and there was a large cross-sectional area of muscle fibers with small gaps between them. While in the low dose and normal saline groups, varying degrees of muscle atrophy were visible and the muscle fibers arranged loosely with smaller cross-sectional areas (Figure 4).
Figure 4.
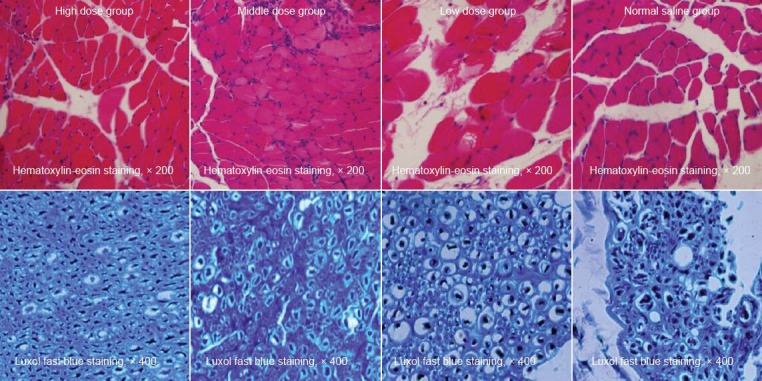
Puerarin effects on the morphology of the triceps surae and nerve fibers of the spinal cord on the injured side at 8 weeks after sciatic nerve surgery.
The hematoxylin-eosin staining showed that muscle cells in the high and middle dose puerarin groups arranged regularly and tightly with a larger cross-sectional area of muscle fibers and smaller gaps. Luxol fast blue staining showed that the myelin sheaths arranged regularly with uniform thickness and clear outline and no proliferated fibrous connective tissues were found in the high and middle dose groups. While in the low dose group, the shape and thickness of the myelin sheaths were both irregular but still with a clear boundary, and fibrous connective tissues had prolifer-ated significantly; the normal saline group displayed disordered myelin sheaths and proliferated fibrous connective tissues.
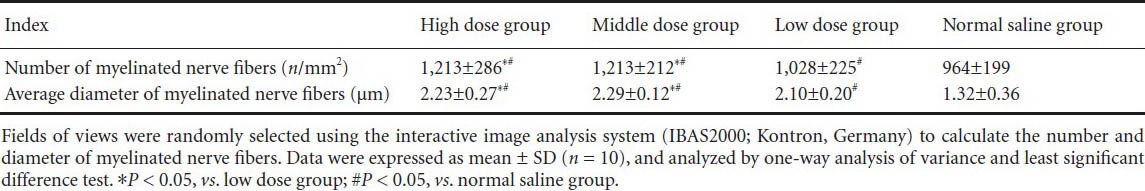
Under the Luxol fast blue staining, the myelin fibers appeared to be blue, and the background was gray in color. At 8 weeks after injury, the high and middle dose groups showed regular arrangement of the myelin sheaths with uniform thickness and clear outline, but there were no proliferated fibrous connective tissues. While in the low dose group, the shape and thickness of the myelin sheaths were both irregular but still with clear boundaries, and the fibrous connective tissues had proliferated significantly; the normal saline group exhibited disordered myelin sheaths and significantly proliferated fibrous connective tissues (Figure 4). Based on randomized fields of view, the number of myelinated nerve fibers and their average diameter were calculated and analyzed statistically. The number and average diameters of myelinated nerve fibers in the high and middle dose groups were significantly higher than in the low dose and normal saline groups. Moreover, the low dose group exhibited less damage than the normal saline group (P < 0.05; Table 2).
Table 2.
Puerarin effects on numbers and average diameters of myelinated nerve fibers at 8 weeks after sciatic nerve injury
Results of muscular mass index in the soleus muscle
At 8 weeks after injury, the muscular mass index in the soleus muscle was significantly higher in the high and middle dose groups (2.32±0.01, 2.09±0.02) than the low dose and normal saline groups (1.82±0.03, 1.36±0.03; P < 0.05). Moreover, there was also a significant difference between the low dose and normal saline groups (P < 0.05).
Discussion
GAP43 is a membrane-associated phosphorylated protein that can be rapidly attached to the membrane. It is closely related to neuronal growth, neurite formation and plasticity (Chen et al., 2012; Zhang et al., 2013). GAP43 can potentially control G proteins, which act as a signal transduction factor and amplifier in membrane signal transduction systems, through its amino terminus domain (Fishman, 1996). Purified GAP43 is a guanine nucleotide release protein that can enhance guanosine diphosphate release, increase the activity of guanosine-5′-triphosphate and stimulate guanine nucleotide binding to guanosine-5′-triphosphate (McIlvain et al., 2003; Mendonça et al., 2010; Zhang et al., 2012; Chen et al., 2013). In this study, we found that after intervention with high- and middle-dose puerarin, the expressions of GAP43 protein and mRNA in the L4–6 segments of the injured side were dramatically increased. Our findings suggest that puerarin can trigger the overexpression of GAP43, thus promoting neural regeneration.
In the present study, Luxol fast blue staining further illustrated that the number, integrity and thickness of the myelin sheaths were increased in the process of neural regeneration following sciatic nerve injury, suggesting that puerarin strongly promotes the growth of damaged nerve myelin. After nerve injury, local muscle atrophy certainly occurs, which can be eased following neural regeneration and functional recovery. Our findings from hematoxylin-eosin staining and muscular mass index in the soleus muscle indicate that puerarin promoted sciatic nerve regeneration better than untreated controls.
Interestingly, after intervention with puerarin for 8 weeks, GAP43 levels showed no differences at any dose, whereas the myelinated nerve and recovery of muscle atrophy were significantly different in the high, middle and low dose groups from the normal saline group. These results indicate that the nerve repair and regeneration were completed at 8 weeks in the high and middle dose groups, followed by decreased GAP43 that was close to the level of the low dose and normal saline groups. Meanwhile, nerves and muscles recovered well.
Taken together, puerarin has an ongoing role in the up-regulation of GAP43 in the corresponding segment after sciatic nerve injury, and thus promotes sciatic nerve repair. During the repair, purerarin could also have antioxidant, anti-apoptotic and anti-inflammatory roles.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81250016.
Conflicts of interest: None declared.
Copyedited by Daws E, Wang J, Wang L, Li CH, Song LP, Zhao M
References
- [1].Ansselin AQ, Pollard JD. lmmunopathological factors in peripheral nerve allograft rejection: quantification of lymphocyte invasion and major histocompatility complex expression. J Neurosci. 1990;96:75–88. doi: 10.1016/0022-510x(90)90058-u. [DOI] [PubMed] [Google Scholar]
- [2].Burguete MC, Torregrosa G, Pérez-Asensio FJ, Castelló-Ruiz M, Salom JB, Gil JV, Alborch E. Dietary phytoestrogens improve stroke outcome after transient focal focal cerebral ischemia in rats. Eur J Neurosci. 2006;23:703–710. doi: 10.1111/j.1460-9568.2006.04599.x. [DOI] [PubMed] [Google Scholar]
- [3].Cuadros CL, Granatir CE. Nerve regeneration through a synthetic microporous tube (expanded polytetrafluoroethylene): experimental study in the sciatic nerve of the rat. Microsurgery. 1987;8:41–47. doi: 10.1002/micr.1920080202. [DOI] [PubMed] [Google Scholar]
- [4].Chen H, Zhang Y, Yang ZJ, Zhang HT. Human umbilical cord Wharton's jelly-derived oligodendrocyte precursor-like cells for axon and myelin sheath regeneration. Neural Regen Res. 2013;8:890–899. doi: 10.3969/j.issn.1673-5374.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen Y, Zhao CL, Zhang CL, Luo LR, Yu G. Influence of chronic intermittent hypoxia on growth associated protein 43 expression in the hippocampus of young rats. Neural Regen Res. 2012;7:1241–1246. doi: 10.3969/j.issn.1673-5374.2012.16.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fishman MC. GAP-43: putting constraints on neuronal plasticity. Perspect Dev Neurobiol. 1996;4:193–198. [PubMed] [Google Scholar]
- [7].Han RM, Tian YX, Becker EM, Andersen ML, Zhang JP, Skibsted LH. Puerarin and conjugate bases as radical scavengers and antioxidants: molecular mechanism and synergism with β-carotene. J Agric Food Chem. 2007;55:2384–2391. doi: 10.1021/jf062796c. [DOI] [PubMed] [Google Scholar]
- [8].He ZG. Protective role of puerarin against cerebral ischemia and its pharmacological mechanism. Zhongguo Linchuang Yiyao Yanjiu Zazhi. 2007;164:46–47. [Google Scholar]
- [9].McIlvain VA, Robertson DR, Maimone MM, McCasland JS. Abnormal thalamocortical pathfinding and terminal arbors lead to enlarged barrels in neonatal GAP-43 heterozygous mice. J Comp Neurol. 2003;462:252–264. doi: 10.1002/cne.10725. [DOI] [PubMed] [Google Scholar]
- [10].Mendonça HR, Araújo SE, Gomes AL, Sholl-Franco A, da Cunha Faria Melibeu A, Serfaty CA, Campello-Costa P. Expression of GAP-43 during development and after monocular enucleation in the rat superior colliculus. Neurosci Lett. 2010;477:23–27. doi: 10.1016/j.neulet.2010.04.027. [DOI] [PubMed] [Google Scholar]
- [11].Pei FX, Yang ZM, Huang FG, Xiang Z, Tan JS, Bu H. Immunoreaction and neural regeneration following peripheral nerve injuries. Zhonghua Xianwei Waike Zazhi. 1995;18:146. [Google Scholar]
- [12].Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, Ng JC, Kao PN. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells andNF-kappaB transcriptional activation. J Biol Chem. 1999;274:13443–13450. doi: 10.1074/jbc.274.19.13443. [DOI] [PubMed] [Google Scholar]
- [13].Saida T, Saida K, Silberdery DH, Brown MJ. Transfer of demyelination by intraneural injection of experimental allergic neuritisserum. Nature. 1978;272:639–641. doi: 10.1038/272639a0. [DOI] [PubMed] [Google Scholar]
- [14].Schwartz M, Sela BA, Eshhar N. Antibodies to ganglioside and myelin autoantigens are produced in mice following sciatic nerve injury. J Neurochem. 1982;38:1192–1195. doi: 10.1111/j.1471-4159.1982.tb07890.x. [DOI] [PubMed] [Google Scholar]
- [15].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [16].Xu X, Zhang S, Zhang L, Yan WM, Zheng XX. The neuroprotection of puerarin against focal cerebral ischemia is associated with the prevention of apoptosis in rats. Planta Med. 2005;71:585–591. doi: 10.1055/s-2005-871261. [DOI] [PubMed] [Google Scholar]
- [17].Xue B, Jiao J, Zhang L, Li KR, Gong YT, Xie JX, Wang XM. Triptolide upregulates NGF synthesis in rat astrocyte cultures. Neurochem Res. 2007;32:1113–1119. doi: 10.1007/s11064-006-9253-1. [DOI] [PubMed] [Google Scholar]
- [18].Yang GZ. Immunology research on traditional Chinese medicine. Zhongguo Zhongxiyi Jiehe Zazhi. 1999;5:259–261. [Google Scholar]
- [19].Ye M, Xie WD, Lei F, Meng Z, Zhao YN, Su H, Du LJ. Brazilein an important immunosuppressive component from Caesalpinia sappan L. Int Immunopharmacol. 2006;6:426–432. doi: 10.1016/j.intimp.2005.09.012. [DOI] [PubMed] [Google Scholar]
- [20].Zhang XH, Chen JJ. The mechanism of astragaloside IV promoting sciatic nerve regeneration. Neural Regen Res. 2013;8:2256–2265. doi: 10.3969/j.issn.1673-5374.2013.24.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang ZY, Zhang H, Du BL, Chen ZQ. Enriched environment upregulates growth-associated protein 43 expression in the hippocampus and enhances cognitive abilities in prenatally stressed rat offspring. Neural Regen Res. 2012;7:1967–1973. doi: 10.3969/j.issn.1673-5374.2012.25.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


