Abstract
Previous studies have shown that baicalin prevented iron accumulation after substantia nigra injury, reduced divalent metal transporter 1 expression, and increased ferroportin 1 expression in the substantia nigra of rotenone-induced Parkinson's disease rats. In the current study, we investigated the relationship between iron accumulation and transferrin expression in C6 cells, to explore the mechanisms of the inhibitory effect of baicalin on iron accumulation observed in Parkinson's disease rats. Iron content was detected using inductively coupled plasma-atomic emission spectroscopy. Results showed that iron content decreased 41% after blocking divalent metal transporter 1 and ferroportin 1 proteins. After treatment with ferric ammonium citrate of differing concentrations (10, 50, 100, 400μg/mL) in C6 glioma cells, cell survival rate and ferroportin 1 expression were negatively correlated with ferric ammonium citrate concentration, but divalent metal transporter 1 expression positively correlated with ferric ammonium citrate concentration. Baicalin or deferoxamine reduced divalent metal transporter 1 expression, but increased ferroportin 1 expression in the 100μg/mL ferric ammonium citrate-loaded C6 cells. These results indicate that baicalin down-regulated iron concentration, which positively regulated divalent metal transporter 1 expression and negatively regulated ferroportin 1 expression, and decreased iron accumulation in the substantia nigra.
Keywords: nerve regeneration, Parkinson's disease, iron, baicalin, divalent metal transporter 1, ferroportin 1, deferoxamine, C6 cells, the Scientific Research Common Program of Beijing Municipal Commission of Education, neural regeneration
Introduction
Parkinson's disease is a disease of the central nervous system. A previous study demonstrated that abnormally increased iron content may be a cause of neurodegenerative diseases[1,2]. A growing number of studies have shown that excessive iron is closely associated with the pathogenesis of Parkinson's disease[3]. In addition, it has been reported that compared with normal persons of the same age, extensive iron deposition is present in the brains of patients with Parkinson's disease[4,5]. MRI studies in patients with Parkinson's disease have shown iron deposition in the substantia nigra[6,7,8], and that iron deposition was associated with disease progression[9,10]. Other researchers have found changes in iron metabolism in patients with Parkinson's disease, abnormally increased iron content, and oxidative stress[11]. Thus, the authors speculated that abnormal iron accumulation-induced oxidative stress and defective anti-oxidation metabolism may be causes of neuronal death[11]. In another study, although iron content was increased in oligodendrocytes, iron content was higher in remaining dopaminergic neurons compared with control rats[12]. Furthermore, progressive death of dopaminergic neurons in the substantia nigra and dopamine depletion in the axon terminals of the striatum has been shown to correlate with abnormally increased iron content[13], but the reason for increased iron content remains unknown. Whether abnormal transferrin expression is associated with increased iron content is currently unknown. Both divalent metal transporter 1 and ferroportin 1 are membrane proteins. Divalent metal transporter 1 is essential for iron uptake, and ferroportin 1 for iron removal[14]. Divalent metal transporter 1 expression increases and iron accumulation appears in the same region of the brain in patients with Parkinson's disease[15]. Iron accumulation in the substantia nigra and divalent metal transporter 1 expression increases were observed in N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 6-hydroxydopamine-induced animal models of Parkinson's disease[1]. Wang[16] observed that ferroportin 1 expression decreased in one injured side of the substantia nigra in a 6-hydroxydopamine-induced rat model of Parkinson's disease. Our previous studies in rats showed tyrosine hydroxylase cell deletion and iron accumulation in the substantia nigra, an increase in divalent metal transporter 1 expression, and a decrease in ferroportin 1 expression, and confirmed that changes in divalent metal transporter 1 and ferroportin 1 expression were associated with the origin and development of iron accumulation in Parkinson's disease rats[17,18].
Baicalin is a major active ingredient of the plant Scutellaria baicalensis Georgi, and has wide pharmacological action. Baicalin has been shown to have protective effects on dopaminergic neurons in the substantia nigra of rotenone-induced Parkinson's disease rats and in C6 cells[17,18,19]. Treatment with baicalin reduced iron accumulation and divalent metal transporter 1 expression, but increased ferroportin 1 expression in the substantia nigra of rotenone-induced Parkinson's disease rats[18,19]. This study sought to investigate the relationship between iron accumulation and transferrin expression in C6 cells, and explore the mechanisms of the inhibitory effect of baicalin on iron accumulation seen in Parkinson's disease rats.
Results
Effects of divalent metal transporter 1 and ferroportin 1 blockage on iron content in C6 cells
C6 cells were incubated for 24 hours and divided into four groups: control, 100 μg/mL ferric ammonium citrate, divalent metal transporter 1 block, and divalent metal transporter 1 + ferroportin 1 block. Iron content was detected using inductively coupled plasma-atomic emission spectroscopy. As shown in Table 1, iron content significantly increased in the 100 μg/mL ferric ammonium citrate group (P < 0.01), but decreased in both the divalent metal transporter 1 block and divalent metal transporter 1 + ferroportin 1 block groups (P < 0.01), but was still higher compared with the control group (P < 0.05). Iron content was significantly higher after blocking divalent metal transporter 1 and ferroportin 1 compared with after blocking divalent metal transporter 1 alone (P < 0.01). Cellular iron content decreased 61% after divalent metal transporter 1 protein block. Moreover, iron content also decreased 41% after blocking of divalent metal transporter 1 and ferroportin 1 proteins. These data indicate that divalent metal transporter 1 played a key role in iron accumulation, and that inhibition of divalent metal transporter 1 function had an impact on iron absorption.
Table 1.
Effects of blocking DMT1 and FP1 on iron content in C6 cells
Effects of different concentrations of iron on C6 cell survival and transferrin divalent metal transporter 1 and ferroportin 1 expression
Cells were incubated for 24 hours and divided into five groups: control, 10 μg/mL ferric ammonium citrate, 50 μg/mL ferric ammonium citrate, 100 μg/mL ferric ammonium citrate, and 400 μg/mL ferric ammonium citrate. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and immunohistochemical staining for divalent metal transporter 1 and ferroportin 1 were conducted. As shown in Table 2, Figure 1, C6 cells were incubated with different concentrations of ferric ammonium citrate. Divalent metal transporter 1 expression positively correlated with ferric ammonium citrate concentration (0–400 μg/mL, r = 0.98). Ferroportin 1 expression negatively correlated with ferric ammonium citrate concentration (0–400 μg/mL, r = −0.95). Divalent metal transporter 1 expression negatively correlated with ferroportin 1 expression (r = −0.98). In addition, the cell survival rate decreased with increasing ferric ammonium citrate concentration. However, no significant difference in ferroportin 1 expression was detected between the 10 μg/mL ferric ammonium citrate group and control group.
Table 2.
Effects of various concentrations of FAC on cell survival rate and expression of DMT1 and FP1 in C6 cells
Figure 1.
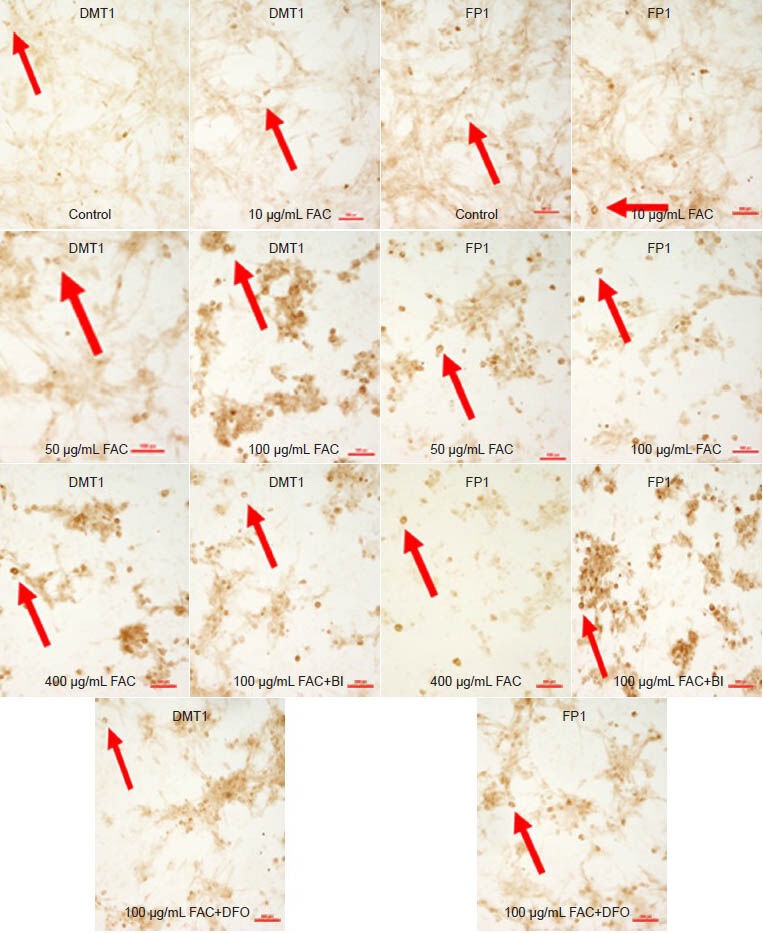
Effects of baicalin on the immunoreactivites of DMT1 and FP1 in C6 cells (immunohistochemical staining, × 200).
C6 cells were incubated with different concentrations of FAC (10, 50, 100, and 400 μg/mL). DMT1 expression increased with a rise in FAC concen-tration, while FP1 expression decreased. DMT1 expression in the FAC + BI and FAC + DFO group was lower compared with the 100 μg/mL FAC group, but higher compared with the FP1 group. DMT1 expression in the FAC + BI group was lower compared with the FAC + DFO group, but FP1 expression was the opposite. Arrows show DMT1 and FP1 expression. DMT1: Divalent metal transporter 1; FP1: ferroportin 1; FAC: ferric ammonium citrate; DFO: deferoxamine; BI: baicalin. Scale bars: 100 μm.
Effects of baicalin and deferoxamine on expression of divalent metal transporter 1 and ferroportin 1 in iron-loaded C6 cells
Cells were incubated for 24 hours and assigned to four groups: control, 100 μg/mL ferric ammonium citrate, 100 μg/mL ferric ammonium citrate + baicalin, 100 μg/mL ferric ammonium citrate + deferoxamine. Immunohistochemical staining for divalent metal transporter 1 and ferroportin 1 was conducted. As shown in Table 3, divalent metal transporter 1 expression in the 100 μg/mL ferric ammonium citrate group was significantly higher compared with the control group (8.56 ± 0.32 vs. 2.86 ± 0.31; n = 6; P < 0.01). Ferroportin 1 expression was lower in the 100 μg/mL ferric ammonium citrate group compared with the control group (2.64 ± 0.48 vs. 4.46 ± 0.40; n = 6; P < 0.01). Divalent metal transporter 1 expression was lower, but ferroportin 1 expression was higher in the 100 μg/mL ferric ammonium citrate + baicalin and 100 μg/mL ferric ammonium citrate + deferoxamine groups compared with the 100 μg/mL ferric ammonium citrate group. These data show that divalent metal transporter 1 expression was lower, but ferroportin 1 expression was higher in the 100 μg/mL ferric ammonium citrate + baicalin group compared with the 100 μg/mL ferric ammonium citrate + deferoxamine group.
Table 3.
Effects of baicalin on expression (integrated absorbance) of DMT1 and FP1 in C6 cells
Discussion
Effects of blocking divalent metal transporter 1 and ferroportin 1 on iron transport in C6 cells
In 1922, Spatz detected iron in brain cells using Perls’ stain, and he verified that iron had unevenly distributed throughout the brain, with the extrapyramidal system the most significantly concentrated region. Moreover, neurons, microglia, oligodendrocytes, and various astrocytes contained high iron content, with iron content highest in oligodendro cytes[20]. Iron-induced oxidative stress has been suggested as a mechanism of nervous degenerative diseases[21]. Chen[22] and Nunez et al.[23] showed that extracellular iron had an anti-proliferative effect on SH-SY5YS cells, and decreased the cell survival rate. In addition, in vitro cerebellar granule cell death was observed in Dulbecco's modified Eagle's medium containing a high-concentration of iron for 7 days. Oxidative stress induced by high iron concentration is a cause of cell death[24].
Expression of divalent metal transporter 1 protein is present in nearly every tissue in the body, and in astrocytes, neurons, and capillary endothelial cells in the brain[25,26,27]. Specifically, divalent metal transporter 1 has been shown to be expressed in the putamen and substantia nigra, suggesting that divalent metal transporter 1 plays a key role in iron metabolism[28,29]. Divalent metal transporter 1 expression is high in the substantia nigra of Parkinson's disease patients and in animal models, indicating that disturbance of iron metabolism may induce selective iron accumulation in the substantia nigra[1,30,31]. Abnormal divalent metal transporter 1 expression is likley an important factor in neurodegenerative diseases, and induces iron metabolism disorder, iron accumulation, and enhances oxidative stress, and eventually leads to neuronal death[32]. Su et al.[33] found increased intracellular iron levels in AdDMT1-infected MES23.5 cells after incubation with iron for 6 hours. Zhang[32] induced increased expression of divalent metal transporter 1 protein and mRNA and found a significant influx of iron in 6-hydroxydopamine-treated MES23.5 cells. A previous study[34] showed high divalent metal transporter 1 expression in 6-hydroxydopamine-treated C6 cells, and increased iron uptake and accumulation in C6 cells.
Ferroportin 1 protein expression has been detected in the small intestine, placenta, spleen, liver, kidney, heart, muscle, lung, and brain[35]. Xenopus oocytes incubated with 55Fe in advance, and then subsequently injected with ferroportin 1 cRNA showed that 80% of iron was released from Xenopus oocytes[36]. Moreover, Donovan et al.[37] showed that iron released from oocytes in the presence of ferroportin 1 protein was 5 × higher compared with the absence of EP1 protein. Wang[16] observed low iron release from C6 cells when ferroportin 1 was blocked; up to 5 × lower compared with controls. Patti et al.[38] observed that upregulated expression of ferroportin 1 protein resulted in increased iron release in C6 cells.
Our previous study suggested that injury to dopamine neurons was accompanied by microglia activation[39], and iron accumulation appeared in the substantia nigra in a rotenone model of Parkinson's disease[17,18]. However, precisely which cells iron accumulation can be detected in has not been completely clarified. Thus, we hypothesized that iron accumulation is mainly detected in neuroglial cells. In this study, C6 cells expressing ferroportin 1 and divalent metal transporter 1 protein showed a relationship between iron transport and divalent metal transporter 1 and ferroportin 1 expression. Cellular iron content decreased 61% after blocking the divalent metal transporter 1 protein, suggesting that divalent metal transporter 1 plays a key role in iron accumulation. Thus, inhibiting its function could impact iron absorption. However, the results show that there may be other iron transport pathways in addition to divalent metal transporter 1. The Ca2+ channel plays an important role in high-concentration iron accumulation in cardiac cells and PC12 cells[40]. For example, nifedipine protected dopaminergic neurons in the substantia nigra of iron-loaded rats[41]. More research is needed to confirm the precise mechanisms involved in iron intake. In this study, iron content also decreased 41% after blocking divalent metal transporter 1 and ferroportin 1 proteins, but iron content was higher than blocking divalent metal transporter 1 protein alone, which indicates that ferroportin 1 is also critical to iron transport. Iron content would increase if ferroportin 1 was blocked. Thus, the two newly discovered proteins can affect iron transporter.
Iron concentration regulates divalent metal transporter 1 and ferroportin 1 expression
Our earlier findings showed that baicalin had a protective effect on dopaminergic nerve cells in Parkinson's disease rats, decreased divalent metal transporter 1 expression, and increased ferroportin 1 expression. We also showed that baicalin protected nerve cells by iron chelation. However, the mechanism of baicalin's effect on protein expression was unknown. We hypothesized that iron chelation diminishes iron concentration and has a secondary action. Thus, we speculated that the concentration of iron adjusted levels of divalent metal transporter 1 and ferroportin 1 protein expression. Our study results revealed that divalent metal transporter 1 expression was positively associated with iron concentration, but ferroportin 1 was negatively associated (data not shown). Thus, iron concentration is important in regulating divalent metal transporter 1 and ferroportin 1 expression. Iron concentration increases in the microenvironment may be induced by iron metabolism disorders. Our previous results showed that iron concentration was increased in the liver, but decreased in the serum of rotenone model Parkinson's disease rats. Thus, it is likley that an iron metabolism disorder occurred in the Parkinson's disease rats.
The above study primarily demonstrated the relationship between iron concentration increases and divalent metal transporter 1 and ferroportin 1 expression associated with nerve cell damage, and showed that the concentration of iron regulates divalent metal transporter 1 and ferroportin 1 expression. Iron concentration plays an important role in the metabolism of iron accumulation in the substantia nigra and progression of Parkinson's disease.
Baicalin and deferoxamine act on divalent metal transporter 1 and ferroportin 1 expression levels via iron chelation in C6 cells
Our previous study showed that iron chelation decreased iron concentration in glial cells, decreased oxidative stress, and played a protective role in nerve cells[42]. This study showed that baicalin and deferoxamine decreased divalent metal transporter 1 expression, but increased ferroportin 1 expression, and subsequently lowered iron concentration in cells and protected nerve cells. Thus, the neuroprotective mechanism of baicalin and deferoxamine appears to be iron chelation. The results of our studies indicate that iron chelation has potential use as a preventive treatment of Parkinson's disease and central nervous system degenerative diseases.
Materials and Methods
Design
A randomized controlled study.
Time and setting
This experiment was performed in the Pharmacology Laboratory of the Traditional Chinese Medicine Academy, Capital Medical University, China, from September 2010 to February 2012.
Materials
Drugs
Drugs included ferric ammonium citrate (Sinopharm Chemical Reagent Co., Ltd., Beijing, China, batch No. F20091029), deferoxamine (Novartis, Basel, Switzerland), and baicalin (the National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China). The molecular formula of baicalin is C21H18O11 and the molecular weight is 446.36.
Methods
Cell culture
C6 cells (Nanjing Institute of Kaiji Biological Engineering, Nanjing, Jiangsu Province, China) were incubated in high-glucose Dulbecco's modified Eagle's medium (Nanjing Institute of Kaiji Biological Engineering) supplemented with 10% fetal bovine serum (Nanjing Institute of Kaiji Biological Engineering), 100 U/mL penicillin and 100 μg/mL streptomycin. The cells were seeded at a density of 104 cells per mL in T-25 culture flasks with 5 mL of complete Dulbecco's modified Eagle's medium and cultured at 37°C with 5% CO2.
Iron content assay
Cells were incubated for 24 hours and assigned to four groups: control, 100 μg/mL ferric ammonium citrate, divalent metal transporter 1 block, and divalent metal transporter 1 + ferroportin 1 block. In the divalent metal transporter 1 block group, 2 μL rabbit anti-rat divalent metal transporter 1 polyclonal antibody (1:2,000; ADI, San Antonio, TX, USA), 100 μL, was added to each well in a wet box at 4°C overnight. In the divalent metal transporter 1 + ferroportin 1 block group, 2 μL divalent metal transporter 1 polyclonal antibody and 2 μL rabbit anti-rat ferroportin 1 polyclonal antibody (1:1,000; Abcam, Hong Kong, China), 100 μL/piece, was added to each well in a wet box at 4°C overnight, for 2 hours. Subsequently, the cells were washed with PBS. The control was incubated in serum-free Dulbecco's modified Eagle's medium. The 100 μg/mL ferric ammonium citrate, divalent metal transporter 1 block, and divalent metal transporter 1+ ferroportin 1 block groups were treated with 100 μg/mL ferric ammonium citrate (Sinopharm Chemical Reagent Co., Ltd., Beijing, China, batch No. F20091029) for 48 hours, 2 mL/well. All cells were incubated in serum-free Dulbecco's modified Eagle's medium (Nanjing Institute of Kaiji Biological Engineering) for an additional 3 hours. After treatment, the cells were collected. Iron content was detected by an inductively coupled plasma emission spectrometer (ICE-APS; Shimadzu, Kyoto, Japan). Each group contained nine parallel wells.
MTT assay
Cells were incubated for 24 hours and divided into five groups: control, 10 μg/mL ferric ammonium citrate, 50 μg/mL ferric ammonium citrate, 100 μg/mL ferric ammonium citrate, and 400 μg/mL ferric ammonium citrate. Cells were washed and treated with different concentrations of ferric ammonium citrate for 48 hours, and then treated with serum-free Dulbecco's modified Eagle's medium for an additional 3 hours. Simultaneously, the control was also incubated in serum-free Dulbecco's modified Eagle's medium. 100 μL of 0.5% (w/v) MTT (Nanjing Institute of Kaiji Biological Engineering) was added to each well. The plates were incubated at 37°C for 4 hours. After incubation, the stain was removed from the plate and 150 μL of dimethyl sulfoxide (Nanjing Institute of Kaiji Biological Engineering) was added and mixed for 20 minutes for visualization. The absorbance was read at 490 nm using an enzyme-linked immunosorbent assay plate reader (Bio-Rad 680, Hercules, CA, USA). The percentage of growth inhibition was calculated and plotted on a graph.
Immunohistochemical staining for divalent metal transporter 1 and ferroportin 1
Cells were cultured on 6-well plates with glass slides for 24 hours and divided into seven groups: control, 10 μg/mL ferric ammonium citrate, 50 μg/mL ferric ammonium citrate, 100 μg/mL ferric ammonium citrate, 400 μg/mL ferric ammonium citrate, 100 μg/mL ferric ammonium citrate + baicalin, 100 μg/mL ferric ammonium citrate + deferoxamine. Subsequently, cells were washed. The control was treated with serum-free Dulbecco's modified Eagle's medium and the others were treated with different concentrations of ferric ammonium citrate for 48 hours. The 100 μg/mL ferric ammonium citrate + baicalin and 100 μg/mL ferric ammonium citrate + deferoxamine groups were treated with 100 μg/mL baicalin and 100 μg/mL deferoxamine, respectively, for an additional 3 hours, other groups were incubated with serum-free Dulbecco's modified Eagle's medium for an additional 3 hours, 2 mL/piece.
The glass slides were removed and washed three times with PBS, 3 minutes each time. Glass slides were fixed in 4% paraformaldehyde for 40 minutes, and maintained at room temperature for 5 minutes, and washed three times with PBS, 3 minutes each time. Glass slides were treated with 3% H2O2 100 μL/piece, for 10 minutes, washed three times with PBS, 3 minutes each time. Specimens were blocked with sheep serum, 100 μL/piece, in a 37°C incubator for 30 minutes, and treated with rabbit anti-rat divalent metal transporter 1 polyclonal antibody (1:2,000; ADI), 100 μL/piece, in a wet box at 4°C overnight or rabbit anti-rat ferroportin 1 polyclonal antibody (1:1,000; Abcam), 100 μL/piece, in a wet box at 4°C overnight. The glass slides were removed and washed three times with PBS, 3 minutes each time. Glass slides were treated with Polymer Helper (Beijing Sequoia Jinqiao Biological Technology Co., Ltd., Beijing, China) 100 μL/piece, in a wet box at 37°C for 1 hour, and washed three times with PBS, 3 minutes each time. Glass slides were treated with poly-horseradish peroxidase anti-rabbit IgG (Beijing Sequoia Jinqiao Biological Technology Co., Ltd.), 100 μL/piece, in a wet box at 37°C for 30 minutes, washed three times with PBS, 3 minutes each time. Glass slides were developed with 3,3′-diaminobenzidine (1:1,000; Beijing Sequoia Jinqiao Biological Technology Co., Ltd.) for 3 minutes.
Samples were dehydrated in a gradient of ethanol and xylene, and mounted with neutral gum. Each experiment contained six parallel wells. Six sections were collected from each group, and observed using a microscope (Nikon, Tokyo, Japan) at 40 × magnification. Four-quadrant images were collected from each section. Integral absorbance values were analyzed using NIS-Elements image analysis software (Ver.2.1; Nikon).
Statistical analysis
Data are expressed as mean ± SD and were evaluated using SPSS 11.5 software (SPSS, Chicago, IL, USA). Measurement data were analyzed using one-way analysis of variance, and groups were compared using the Student-Newman-Keuls test. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This work was supported by the Scientific Research Common Program of Beijing Municipal Commission of Education, No. KM20110025010.
Conflicts of interest: None declared.
Peer review: This study verified that iron concentration regulated the expression of divalent metal transporter 1 and ferroportin 1. Baicalin and deferoxamine reduced divalent metal transporter 1 expression, but increased ferroportin 1 expression, and then protected nerve cells, which provided significant ideas for the prevention and treatment of Parkinson's disease.
Copyedited by Barrett R, Raye W, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- [1].Jiang H, Qian ZM, Xie JX. Increased DMT1 expression and iron content in MPTP-treated C57BL/6 mice. Shengli Xue Bao. 2003;55(5):571–576. [PubMed] [Google Scholar]
- [2].Wang YL, Gao H, Yang XL. Establishment of rat models of Parkinson's disease by unilateral two-point injection with 6-hydroxydopamine. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17(46):8030–8035. [Google Scholar]
- [3].Yang H, Cao XB. 4. Vol. 27. Guowai Yixue: Shenjingbing Xue Shenjing Waike Xue Fence; 2000. Research on iron associated with Parkinson disease; pp. 180–183. [Google Scholar]
- [4].Connot JR, Snyder BS, Arosio P, et al. A quantitative analysis of isoferritins selected regions of aged Parkinsonian and Alzheimer's diseased brains. J Neurochem. 1995;65(2):717–724. doi: 10.1046/j.1471-4159.1995.65020717.x. [DOI] [PubMed] [Google Scholar]
- [5].Berg D, Grote C, Rauseh WD, et al. Iron accumulation in the substantia nigra in rats visualized by ultrasound. Ultrasound Med Biol. 1999;25(6):901–904. doi: 10.1016/s0301-5629(99)00046-0. [DOI] [PubMed] [Google Scholar]
- [6].Gorell JM, Ordidge RJ, Brown GG, et al. Increased iron-related MRI contrast in the substantia nigra in Parkinson's disease. Neurology. 1995;45(6):1138–1143. doi: 10.1212/wnl.45.6.1138. [DOI] [PubMed] [Google Scholar]
- [7].Michaeli S, Oz G, Sorce DJ, et al. Assessment of brain iron and neuronal integrity in patients with Parkinson's disease using novel MRI contrasts. Mov Disord. 2007;22(3):334–340. doi: 10.1002/mds.21227. [DOI] [PubMed] [Google Scholar]
- [8].Martin WR, Wieler M, Gee M. Midbrain iron content in early Parkinson disease-A potential biomarker of disease status. Neurology. 2008;70(6):1411–1417. doi: 10.1212/01.wnl.0000286384.31050.b5. [DOI] [PubMed] [Google Scholar]
- [9].Ryvlin P, Broussolle E, Piollet H, et al. Magnetic-resonance-imaging evidence of decreased putamenal iron content in idiopathic Parkinson's disease. Arch Neurol. 1995;52(6):583–588. doi: 10.1001/archneur.1995.00540300057013. [DOI] [PubMed] [Google Scholar]
- [10].Ye FQ, Allen PS, Martin WR. Basal ganglia iron content in Parkinson's disease measured with magnetic resonance. Mov Disord. 1996;11(3):243–249. doi: 10.1002/mds.870110305. [DOI] [PubMed] [Google Scholar]
- [11].Rao R, Tkac l, Townsend EL, et al. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2000;133(10):3215–3221. doi: 10.1093/jn/133.10.3215. [DOI] [PubMed] [Google Scholar]
- [12].Wang J, Jiang H, Xie JX. Correlation between iron levels and degeneration of dopaminergic neurons in rat nigrostriatal system during the early 6-OHDA lesions in medial forebrain bundle. Acta Physiol Sin. 2003;55(4):422–427. [PubMed] [Google Scholar]
- [13].Jiang H, Xie JX. Intracerebral high-speed rail in the etiology of Parkinson's disease in rats. Zhongguo Shenjing Kexue Zazhi. 2001;17(2):201–204. [Google Scholar]
- [14].Xie JX, Jiang H, Wang J. Guangzhou, Zhongguo: 2004. Involvement of brain iron metabolism of two new proteins-DMT1 and FP1. Advances in neuroscience(III)Proceedings. [Google Scholar]
- [15].Gamek MD, Dolan K, Hothinski C. DMT1: amammalian transporter for multiple metals. Biometals. 2003;16(1):41–54. doi: 10.1023/a:1020702213099. [DOI] [PubMed] [Google Scholar]
- [16].Wang J. Qingdao: Qingdao University; 2005. The roles of iron export proteins in the nigral iron accumulation of Parkinsonian rat models. [Google Scholar]
- [17].Song YW, Chen X, Li C, et al. Divalent metal transporter1 expression and iron deposition in the substantia nigra of a rat model of Parkinson's disease. Neural Regen Res. 2010;5(22):1701–1705. [Google Scholar]
- [18].Song YW, Chen X, Zhang N, et al. Inhibition on iron deposition in SN in PD rats by baicalin and its mechanism. Zhongguo Yaolixue Tongbao. 2011;2(12):1740–1744. [Google Scholar]
- [19].Guo CY, Chen X. Baicalin interferes with iron accumulation in C6 glioma cells. Neural Regen Res. 2011;6(30):2352–2356. [Google Scholar]
- [20].Du Y, Feng YM, Xian ZM. Brain iron, transferrin and transferrin receptor. Sheng Li Ke Xue Jin Zhan. 1999;30(4):337–340. [PubMed] [Google Scholar]
- [21].McKie AT, Barrow D, Latunde DO, et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291(5509):1755–1759. doi: 10.1126/science.1057206. [DOI] [PubMed] [Google Scholar]
- [22].Chen DH. Fuzhou: Fujian Medical University; 2007. Extracellular iron ions on SH_SY5Y cell proliferation and tau protein phosphorylation in vitro. [Google Scholar]
- [23].Nunez MT, Gallardo V, Munoz P, et al. Progressive iron accumulation induces a biphasic change in the glutathione content of neuroblastoma cells. Free Radic Biol Med. 2004;37(7):953–960. doi: 10.1016/j.freeradbiomed.2004.06.005. [DOI] [PubMed] [Google Scholar]
- [24].Pu YM, Wang Q, Qian ZM. Effect of iron and lipid peroxidation on development of cerebellar granule cells in vitro. Neuroscience. 1999;89(3):855–861. doi: 10.1016/s0306-4522(98)00384-4. [DOI] [PubMed] [Google Scholar]
- [25].Burdo JR, Menzies SL, Simpson IA, et al. Distribution of divalent metal transporter l and metal transport protein1 in the normal and Belgrade rat. J Neurosci Res. 2001;66(6):1198–1207. doi: 10.1002/jnr.1256. [DOI] [PubMed] [Google Scholar]
- [26].Siddappa AJ, Rao RB, Wobken JD, et al. Developmental changes in the expression of iron regulatory proteins and iron transport proteins in the perinatal rat brain. J Neurosei Res. 2002;68(6):761–775. doi: 10.1002/jnr.10246. [DOI] [PubMed] [Google Scholar]
- [27].Burdo JR, Simpson IA, Menzies S, et al. Regulation of the profile of iron-management proteins in brain microvaseulature. J Cereb Blood Flow Metab. 2004;24(1):67–74. doi: 10.1097/01.WCB.0000095800.98378.03. [DOI] [PubMed] [Google Scholar]
- [28].Knutson M, Menzies S, Connor J, et al. Developmental, regional, and cellular expression of SFT/UbeH5A and DMT1 mRNA in brain. J Neurosci Res. 2004;76(5):633–641. doi: 10.1002/jnr.20113. [DOI] [PubMed] [Google Scholar]
- [29].Ke Y, Chang YZ, Duan XL, et al. Age-dependent and iron-independent expression of two mRNA isoforms of divalent metal transport 1 in rat brain. Neurobiol Aging. 2005;26(5):739–748. doi: 10.1016/j.neurobiolaging.2004.06.002. [DOI] [PubMed] [Google Scholar]
- [30].Andrews NC. The iron transporter DMT1. Int J Biochem Cell Biol. 1999;31(10):991–994. doi: 10.1016/s1357-2725(99)00065-5. [DOI] [PubMed] [Google Scholar]
- [31].Moos T, Morgan EH. The meltabolism of neuronal iron and its pathogenic role in neurological disease: review. Ann N Y Acad Sci. 2004;1012:14–26. doi: 10.1196/annals.1306.002. [DOI] [PubMed] [Google Scholar]
- [32].Zhang SZ. Qingdao: Qingdao University; 2008. Research on DMT1 for iron accumulation in the 6-OHDA or MPP+ induced MES23.5 cells. [Google Scholar]
- [33].Su MA, Trenor CC, Fleming JC, et al. The G185R mutation disrupts function of the iron transporter Nramp2. Blood. 1998;92(6):2157–2163. [PubMed] [Google Scholar]
- [34].Fleming MD, Romano MA, Garriek LM, et al. Anemia of the Belgrade rat is caused by a mutation in iron transporter protein Nramp2. Proe Natl Acad Sci U S A. 1998;95(3):1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Xu HM. Qingdao: Qingdao University; 2008. The role of over-expressed DMT1 in iron accumulation in MES23.5 cells and the mechanism of the protective effect of ginsenoside Rg1. [Google Scholar]
- [36].Song N, Jiang H, Wang J, et al. Divalent metal transporter 1 up regulation is involved in the 6-hydroxydopamine induced ferrous iron influx. J Neurosci Res. 2007;85(14):3118–3126. doi: 10.1002/jnr.21430. [DOI] [PubMed] [Google Scholar]
- [37].Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40al is essential for iron homeostasis. Cell Metab. 2005;1(3):191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- [38].Patti MC, Persichini T, Mazzone V, et al. Interleukin-β up-regulates iron efflux in rat C6 glioma cells through modulation of ceruloplasmin and ferroportin 1 synthesis. Neurosci Lett. 2004;363(2):182–186. doi: 10.1016/j.neulet.2004.04.005. [DOI] [PubMed] [Google Scholar]
- [39].Chen X, Zhang N, Li C, et al. Parallel relationship between microglial activation and substantia nigra damage in a rotenone-induced Parkinson's disease rat model. Neural Regen Res. 2010;5(4):245–250. [Google Scholar]
- [40].Oudit GY, Sun H, Trivierim G, et al. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med. 2003;9(9):1187–1194. doi: 10.1038/nm920. [DOI] [PubMed] [Google Scholar]
- [41].Wang R, Ma ZG, Xie JX. Neuroprotective effect og Nifdipine on the dopaminer gicneurons in substantia nigra of acute iron-overloaded rats. Qingdao Daxue Yixueyuan Xuebao. 2011;47(5):377–381. [Google Scholar]
- [42].Guo CY, Chen X. Baicalin interferes with iron accumulation in C6 glioma cells. Neural Regen Res. 2011;6(30):2352–2356. [Google Scholar]


