The functional loss that occurs after retinal/optic nerve injury is permanent and can arise through trauma or neurodegenerative conditions such as glaucoma. Neurotrophic factors (NTFs) promote survival of injured retinal ganglion cells (RGCs) and regeneration of their axons, suggesting their clinical utility to prevent further damage and restore lost function. Delivery of optimal concentrations of NTFs to RGCs is difficult to achieve by injection but single implants of stem cells which naturally secrete multiple NTFs for sustained periods better addresses this problem. This review discusses a relatively new source of adult stem cells, the dental pulp stem cells, and compares their efficacy and feasibility with other stem cells, such as the well-studied bone marrow-derived mesenchymal stem cells (BMSCs), in the context of cellular therapy for the retina.
Retinal and/or optic nerve damage after trauma or degenerative diseases leads to partial or complete blindness. Like other parts of the central nervous system (CNS), RGC axons in the optic nerve fail to regenerate after injury and RGCs are not replaced after death (reviewed in Berry et al., 2008). Unlike the rest of the CNS, death of RGCs is rapid after optic nerve injury with over 90% lost after 21 days (Berkelaar et al., 1994), as there are no collateral axons to maintain the retrograde delivery of neuroprotective NTFs to RGC somata. Thus, a major therapeutic goal is to develop new strategies to promote both RGC neuroprotection and regeneration of their axons after trauma and in degenerative retinal disease.
A requirement for neuroprotection and axon regeneration is the delivery of an effective titre of NTFs to neuronal cell bodies. However, effective delivery of exogenous NTFs to the eye is limited by disadvantages such as the need for repeated intravitrealed injections (Ko et al., 2000, 2001), the down-regulation of TrK receptors after bolus administration (Sommerfeld et al., 2000; Chen and Weber, 2004) and the lack of efficacy when single NTFs are used (Logan et al., 2006).
We have previously explored the application of cellular therapy to address these problems by intravitreal transplantation of cells which, due to their placement at close proximity to the retina, will continuously supply multiple NTFs directly to the RGC somata, promoting both RGC survival and the regeneration of their axons after optic nerve injury. Intravitreal rather than intranerve transplantation of NTF secreting cells avoids the formation of a sink of high NTF concentration that traps axons within transplant sites, preventing their forward growth along the optic nerve. In our first study (Logan et al., 2006), we achieved this by intravitreal grafting of fibroblasts engineered to express fibroblast growth factor-2 (FGF-2), neurotrophin-3 (NT-3) and brain-derived neurotrophic factor (BDNF) which led to survival and axon regeneration of approximately 1.25% of RGCs (compared to intact retina) with axons extending 2 mm distal to the crush site.
Genetic modification of autologously transplanted cells however adds costs and time required to prepare the therapy, making translation to the clinic more challenging.
An emerging new therapeutic theme is the use of stem cells as a sustained source of multiple NTFs for CNS injury. For example, various studies have attributed functional recovery after transplantation in models of spinal cord injury to stem cell-derived NTFs (reviewed in Li and Lepski, 2013). These studies have demonstrated improvements in locomotion, but few stem cell-derived neurons are formed, causing us and others to speculate that a paracrine mechanism, rather than neuronal differentiation is responsible for the restoration of function (Burdon et al., 2011). Studies from other laboratories in ocular models have explored this phenomenon by transplanting BMSCs into the rat vitreous body after either optic nerve transection (Levkovitch-Verbin et al., 2010) or episcleral vein ligation/trabecular meshwork laser photocoagulation induced-glaucoma (Yu et al., 2006; Johnson et al., 2010). Following BMSC transplantation, these authors demonstrated significant RGC neuroprotection with the number of surviving RGCs increased by 10–20% in animal models of glaucoma (Yu et al., 2006; Johnson et al., 2010) and by 20% at 8 days after optic nerve transection (Levkovitch-Verbin et al., 2010) compared to survival of RGCs in untreated animals. Notably, GFP+ BMSCs survived in the eye for at least 4 weeks post-transplantation (Yu et al., 2006; Johnson et al., 2010, Levkovitch-Verbin et al., 2010). The failure of BMSCs to differentiate into neurons and/or migrate and integrate into the retina, along with the positive expression of NTFs by the transplanted BMSCs (Yu et al., 2006; Levkovitch-Verbin et al., 2010) strongly suggests that their neuroprotective effects are paracrine-mediated.
We have recently explored the alternative use of dental pulp stem cells (DPSCs) for neural protection and regeneration in the eye. The use of DPSCs is a relatively recent development in the field of neuroregenerative medicine and is of particular interest since they are neural crest-derived cells that can be isolated from exfoliated or extracted adult teeth, making them an easily accessible stem cell from patients of all ages (Gronthos et al., 2000). We hypothesized that their neural crest origin and neural characteristics make DPSCs more suited than other mesenchymal stem cell sources, such as BMSCs, in the treatment of CNS injuries. Conflicting evidence exists for the successful differentiation of DPSCs into neurons in vitro with evidence for (Arthur et al., 2008; Kiraly et al., 2009) and against, both from us (our unpublished data) and others (Aanismaa et al., 2012). The evidence for a paracrine mechanism of DPSC action in neural support (Nosrat et al., 2001) has recently been strengthened by the results of a study using a rodent model of spinal cord injury (Sakai et al., 2012) in which, compared to BMSCs, DPSCs significantly restored locomotory function. Recovery was attributed to paracrine mechanisms, with the gene expression of many NTFs, such as nerve growth factor (NGF), BDNF and NT-3, being greater in DPSCs than BMSCs. However, other mechanisms of action such as their neuronal and oligodendrocyte differentiation could not be ruled out in this spinal cord injury study.
We addressed this possibility in our latest study in which we studied the paracrine-mediated neuroprotective and pro-regenerative properties of DPSCs compared to BMSCs for axotomised RGCs both in vitro and in an optic nerve crush model after intravitreal transplantation (Mead et al., 2013). In vitro, we showed not only that DPSCs are more neuroprotective and neuritogenic than BMSCs, but that these effects were abolished after Fc-TrK blockade. This paracrine-mediated effect was corroborated by ELISA showing greater titres of NGF, BDNF and NT-3 in the DPSC's secretome compared to that of BMSCs. Finally, we transplanted DPSCs into the rat vitreous body and observed a 27% increase in the number of surviving RGCs 21 days after optic nerve crush compared to the survival of RGCs in untreated/dead cell transplanted animals. This survival was significantly greater than was seen after intravitreal BMSC transplants which yielded an 11% increase in the number of surviving RGCs 21 days after optic nerve crush compared to the survival of RGCs in untreated/dead cell transplanted animals. Compared to BMSCs, DPSC transplantation promoted over twice the number of regenerating RGC axons which grew through the optic nerve and lesion scar and over 1.2 mm into the distal optic nerve segment. Duplication of the in vitro experiments using human-derived stem cells have now yielded similar results (Figure 1; unpublished data).
Figure 1.
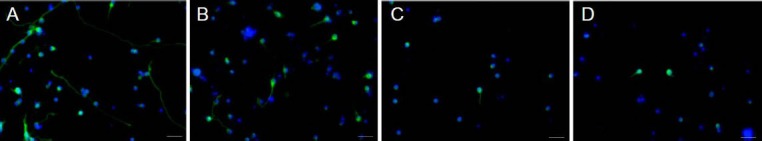
Neuroprotection and neuritogenesis of axotomized/injured adult βIII-tubulin-positive (green) retinal neurons after culture with human-derived dental pulp stem cells (DPSCs; A) and human-derived BMSCs (B). DPSCs more significantly promoted neuroprotection of βIII-tubulin-positive retinal neurons and regeneration of their neurites compared to untreated βIII-tubulin-positive retinal neurons (C) or those treated with bone marrow-derived mesenchymal stem cells (BMSCs). These effects are neurotrophin-dependent as emphasized by the response abolition after addition of TrKA/B/C inhibitors (D), which block nerve growth factor, brain-derived neurotrophic factor and NT-3 receptor binding. Representative images shown with DAPI (blue) used as a nuclear counterstain, scale bars represent 50 μm.
The paracrine basis of neuroprotection and axogenesis is the subject of our current study and, although DPSC-derived NTFs have been clearly implicated, the large and diverse secretomes of BMSCs and DPSCs suggest other secreted molecules may also be involved in the stem cell-mediated neuroprotection/axogenesis, such as platelet-derived growth factor (Johnson et al., 2013). One candidate neurotrophic signalling cascade is the mTOR pathway which, when activated, promotes pronounced regeneration of axons in the optic nerve (Park et al., 2008), although it is currently unknown if stem cells significantly activate the mTOR pathway. It is also unknown if secreted growth factors directly interact with their cognate receptors on the injured neurons or activate glia to signal RGC protection and axon regeneration indirectly (Muller et al., 2009). Glia are activated after DPSC transplantation (Mead et al., 2013) and this juxtacrine mechanism could supplement the local NTF supply.
Although we have shown DPSCs perform better as a cell therapy than BMSCs in our models of retinal/optic nerve injury, comparisons with other stem cells are necessary to ensure the correct stem cell type is taken forward into clinical trials. Adipose-derived stem cells have proven efficacy in promoting neuritogenesis (Kalbermatten et al., 2011), yet have not been tested in the eye. Similarly neural stem cells (NSCs), isolated from foetal spinal cord and transplanted with growth factors have promoted some of the most significant axon regeneration seen to date after transplantation in spinal cord injury sites (Lu et al., 2012), probably explained by NSC differentiation into neurons which directly integrate into the host circuitry, and not by paracrine mechanisms. However, intravitreal transplantation of undifferentiated NSCs has not yet been explored and would determine any paracrine properties. Despite their efficacy, their foetal and cadaveric source poses both ethical issues and a significant challenge in obtaining adequate numbers of cells for transplantation, particular if the therapy was to translate to the clinic. Secondly, unlike DPSCs, for which autologous transplantation is feasible, patients receiving NSCs would require lifelong immunosuppressive treatment.
With BMSCs already being used in clinical trials for retinal and optic nerve damage (www.clinicaltrials.gov/show/NCT01920867), the future for stem cell therapy in treating traumatic and degenerative ocular conditions is fast becoming a reality. We have evidence that DPSCs may be a more appropriate cell type than BMSCs for retinal therapy (although NSCs may also be concluded as a strong alternative candidate)(Mead et al., 2013) and we are engaged in further work to substantiate this claim. Thus, an in depth comparison with other available stem cells is necessary as well as research into the exact mechanism behind DPSC-mediated RGC neuroprotection and axon regeneration to support the preclinical and translational development of this cellular therapy.
Footnotes
Funding: The studentship of Ben Mead was funded by the BBSRC (grant number BB/F017553/1) and the Rosetrees Trust.
Conflicts of interest: None declared.
References
- [1].Aanismaa R, Hautala J, Vuorinen A, Miettinen S, Narkilahti S. Human dental pulp stem cells differentiate into neural precursors but not into mature functional neurons. Stem Cell Discovery. 2012;2:85–91. [Google Scholar]
- [2].Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787–1795. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- [3].Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994;14:4368–4374. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Berry M, Ahmed Z, Lorber B, Douglas M, Logan A. Regeneration of axons in the visual system. Restor Neurol Neurosci. 2008;26:147–174. [PubMed] [Google Scholar]
- [5].Burdon TJ, Paul A, Noiseux N, Prakash S, Shum-Tim D. Bone marrow stem cell derived paracrine factors for regenerative medicine: current perspectives and therapeutic potential. Bone Marrow Res 2011. 2011 doi: 10.1155/2011/207326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen H, Weber AJ. Brain-derived neurotrophic factor reduces TrkB protein and mRNA in the normal retina and following optic nerve crush in adult rats. Brain Res. 2004;1011:99–106. doi: 10.1016/j.brainres.2004.03.024. [DOI] [PubMed] [Google Scholar]
- [7].Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51:2051–2059. doi: 10.1167/iovs.09-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Johnson TV, Dekorver NW, Levasseur VA, Osborne A, Tassoni A, Lorber B, Heller JP, Villasmil R, Bull ND, Martin KR, Tomarev SI. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain. 2014;137(Pt 2):503–519. doi: 10.1093/brain/awt292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kalbermatten DF, Schaakxs D, Kingham PJ, Wiberg M. Neurotrophic activity of human adipose stem cells isolated from deep and superficial layers of abdominal fat. Cell Tissue Res. 2011;344:251–260. doi: 10.1007/s00441-011-1142-5. [DOI] [PubMed] [Google Scholar]
- [11].Kiraly M, Porcsalmy B, Pataki A, Kadar K, Jelitai M, Molnar B, Hermann P, Gera I, Grimm WD, Ganss B, Zsembery A, Varga G. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem Int. 2009;55:323–332. doi: 10.1016/j.neuint.2009.03.017. [DOI] [PubMed] [Google Scholar]
- [12].Ko ML, Hu DN, Ritch R, Sharma SC. The combined effect of brain-derived neurotrophic factor and a free radical scavenger in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000;41:2967–2971. [PubMed] [Google Scholar]
- [13].Ko ML, Hu DN, Ritch R, Sharma SC, Chen CF. Patterns of retinal ganglion cell survival after brain-derived neurotrophic factor administration in hypertensive eyes of rats. Neurosci Lett. 2001;305:139–142. doi: 10.1016/s0304-3940(01)01830-4. [DOI] [PubMed] [Google Scholar]
- [14].Levkovitch-Verbin H, Sadan O, Vander S, Rosner M, Barhum Y, Melamed E, Offen D, Melamed S. Intravitreal injections of neurotrophic factors secreting mesenchymal stem cells are neuroprotective in rat eyes following optic nerve transection. Invest Ophthalmol Vis Sci. 2010;51:6394–6400. doi: 10.1167/iovs.09-4310. [DOI] [PubMed] [Google Scholar]
- [15].Li J, Lepski G. Cell transplantation for spinal cord injury: a systematic review. Biomed Res Int 2013. 2013 doi: 10.1155/2013/786475. 786475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Logan A, Ahmed Z, Baird A, Gonzalez AM, Berry M. Neurotrophic factor synergy is required for neuronal survival and disinhibited axon regeneration after CNS injury. Brain. 2006;129:490–502. doi: 10.1093/brain/awh706. [DOI] [PubMed] [Google Scholar]
- [17].Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci. 2013;54:7544–7556. doi: 10.1167/iovs.13-13045. [DOI] [PubMed] [Google Scholar]
- [19].Muller A, Hauk TG, Leibinger M, Marienfeld R, Fischer D. Exogenous CNTF stimulates axon regeneration of retinal ganglion cells partially via endogenous CNTF. Mol Cell Neurosci. 2009;41:233–246. doi: 10.1016/j.mcn.2009.03.002. [DOI] [PubMed] [Google Scholar]
- [20].Nosrat IV, Widenfalk J, Olson L, Nosrat CA. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol. 2001;238:120–132. doi: 10.1006/dbio.2001.0400. [DOI] [PubMed] [Google Scholar]
- [21].Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting Axon Regeneration in the Adult CNS by Modulation of the PTEN/mTOR Pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S, Hibi H, Kadomatsu K, Ishiguro N, Ueda M. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122:80–90. doi: 10.1172/JCI59251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sommerfeld MT, Schweigreiter R, Barde YA, Hoppe E. Down-regulation of the neurotrophin receptor TrkB following ligand binding. Evidence for an involvement of the proteasome and differential regulation of TrkA and TrkB. J Biol Chem. 2000;275:8982–8990. doi: 10.1074/jbc.275.12.8982. [DOI] [PubMed] [Google Scholar]
- [24].Yu S, Tanabe T, Dezawa M, Ishikawa H, Yoshimura N. Effects of bone marrow stromal cell injection in an experimental glaucoma model. Biochem Biophys Res Commun. 2006;344:1071–1079. doi: 10.1016/j.bbrc.2006.03.231. [DOI] [PubMed] [Google Scholar]


