The P2X7 receptor is a member of the family of purinoceptors, which are ligand-gated membrane ion channels activated by extracellular adenosine 5′-triphosphate. A unique feature of the P2X7 receptor is that its activation can result in the formation of large plasma membrane pores that allow the flux of ions as well as hydrophilic molecules of up to 900 Da. Recent studies indicate that P2X7-mediated signaling can trigger apoptotic cell death after ischemia and during the course of certain neurodegenerative disorders. Expression of the P2X7 receptor has been demonstrated in most cell types in the retina and is related to retinal neurotransmission, and may modulate both photoreceptor and rod bipolar cell responses. In addition to its physiological roles, receptor stimulation has been reported to be involved in neuronal death in the retina. Stimulation of P2X7 receptors can kill retinal ganglion cells (RGCs) by a mechanism dependent on increased intracellular Ca2+. This mechanism might play a role in ischemia-induced neuronal damage and optic nerve injury. Given that extracellular ATP levels and P2X7 receptor expression in the retina increase with elevated intraocular pressure, stimulation of P2X7 receptors may exert a deleterious effect on RGCs in glaucomatous eyes. P2X7 receptor activation may also be associated with the up-regulation of inflammatory cytokine expression, e.g., interleukin-1β and tumor necrosis factor-α. Based on its reported effects, the P2X7 receptor is a potential therapeutic target of pharmacological strategies designed to prevent neuronal death in ocular diseases including glaucoma.
What P2X7 receptors are?
Extracellular adenosine 5′-triphosphate (ATP) is an excitatory transmitter in both the peripheral and central nervous systems. P2X receptors are a family of ligand-gated membrane ion channels activated by extracellular ATP. P2X receptors consist of seven isoforms designated P2X1 to P2X7 (North, 2002; Kaczmarek-Hájek et al., 2012) and are widely distributed in most types of cells of nearly every origin. These receptors have many functions such as synaptic transmission in the peripheral and central nervous systems, contraction of smooth muscle, platelet aggregation, macrophage activation, cell death and immunomodulation (Burnstock et al., 2010, 2011).
In contrast to other ligand-gated channels in the purinoceptor family, the P2X7 receptor possesses unique features that are likely to be of both physiological and pathophysiological significance. Most importantly, not only does the initial activation of these receptors result in the opening of a non-selective plasma membrane channel, but in many types of cells, sustained activation causes the formation of trans-membrane pores that are permeable to hydrophilic molecules of up to 900 Da (Valera et al., 1994; Falzoni et al., 1995).
Indicative of the P2X7 receptor having a role in cell pathology, this receptor has been found to be highly up-regulated in neurons and glial cells located in the ischemic cerebral cortex (Franke et al., 2004). P2X7-mediated signaling is also implicated in neurodegenerative diseases, such as Parkinson's disease, Alzheimer's disease and multiple sclerosis (Romagnoli et al., 2008).
P2X7 receptors in the retina
Expression of the P2X7 receptor has been demonstrated in most cell types in the retina; these include neurons such as the retinal ganglion cells (RGCs) (Brändle et al., 1998; Ishii et al., 2003; Wheeler-Schlling et al., 2001), as well as glia (Morigiwa et al., 2000; Pannicke et al., 2000) and vascular cells (Kawamura et al., 2003). In the adult rat retina, immunolabeling for the P2X7 receptor is detected in a number of cells in the inner nuclear layer and ganglion cell layer, suggestive of amacrine cells and RGCs (Brändle et al., 1998). This research group later confirmed that P2X7 receptors are expressed in identified RGCs using reverse transcription polymerase chain reaction (Wheeler-Schilling et al., 2001). These receptors were also found in presynaptic processes of rod bipolar cells, as well as other conventional synapses, suggesting that purines play a role in neurotransmission within the retina, and may modulate both photoreceptor and rod bipolar cell responses (Puthussery and Fletcher, 2004). Moreover, based on experiments using the P2X7 receptor knockout mouse, it was suggested that these receptors provide excitatory input to photoreceptor terminals or to inhibitory cells that regulate both the rod and cone pathway response (Vessey and Fletcher, 2012). Another group suggested that activation of this receptor may affect uptake of neurotransmitters from the extracellular space by Müller cells in the retina (Pannicke et al., 2000).
P2X7 receptors and neuronal death in the retina
In addition to the putative physiological roles of P2X7 receptors, stimulation of these receptors has been reported to be involved in neuronal death in the retina. It was reported that ATP induces the death of developing avian retinal neurons in culture via activation of P2X7 receptors (Anccasi et al., 2013). The potential neuroprotective effect of a P2X7 receptor antagonist on photoreceptor cell death was reported using primary retinal cell cultures (Notomi et al., 2011). This antagonist has also been reported to inhibit apoptosis in cultured human retinal pigment epithelium (Yang et al., 2011). Moreover, stimulation of P2X7 receptors can kill RGCs in vitro and in vivo through a mechanism that is likely dependent on a rise in intracellular Ca2+ (Zhang et al., 2005; Hu et al., 2010). It was also suggested that the balance between extracellular ATP and its protective metabolite adenosine can influence RGC survival in the eye (Hu et al, 2010). Another study demonstrated that early up-regulation of neuronal P2X7 receptors may cause injury to retinal neurons which may contribute to retinal damage (Franke et al., 2005). Furthermore, data from our laboratory indicate that the activation of P2X7 receptors is involved in hypoxia-induced death of retinal neurons (Sugiyama et al, 2010). Using human organotypic retinal cultures, it was demonstrated that stimulation of P2X7 receptors can mediate RGC death and that this mechanism plays a role in ischemia-induced neurodegeneration (Niyadurupola et al., 2013). Moreover, it was also reported that ischemic damage was attenuated by P2X7 receptor antagonists in isolated optic nerves as well as in cultured oligodendrocytes (Domercq et al., 2010). Consistent with this, our recent study revealed that P2X7 antagonists prevent loss of RGCs after optic nerve crush (Figure 1), and that this protective effect is possibly mediated through suppression of P2X7 receptor over-expression in the retina (Kakurai et al., 2013). In addition, multiple combinations of three Ca2+ channel inhibitors including a P2X7 receptor antagonist have been indicated in the preservation of visual function in a rat model of partial optic nerve transection (Savigni et al., 2011).
Figure 1.
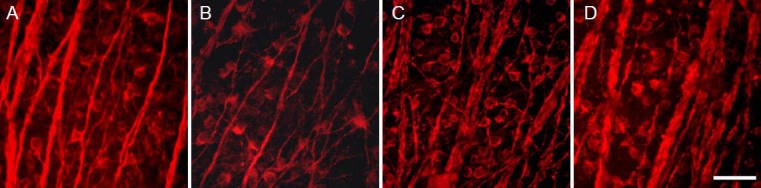
Representative photomicrographs of TUJ1-positive cells on day 7 after optic nerve crush (ONC) injury, with and without P2X7 antagonists (OxATP and BBG) in corresponding regions (1 mm from the optic nerve head).
(A) Normal eye; (B) an eye with ONC + phosphate buffered saline; (C) an eye with ONC + 30 μmol/L OxATP; (D) an eye with ONC + 0.3 μmol/L BBG. Scale bar: 50 μm. TUJ1: Neuron-specific β-tubulin; OxATP: oxidized adenosine triphosphate; BBG: brilliant blue G. From Kakurai et al. (2013).
P2X7 receptors and glaucoma
A group of researchers have demonstrated that ATP is released in response to an acute rise in ocular pressure both in vitro (Reigada et al., 2008), and in vivo (Zhang et al., 2007) and have recently shown that ATP release accompanies chronic elevation in pressure (Li et al., 2011). It has also been suggested that mechanical strain triggers ATP release directly from retinal ganglion cells and that this released ATP autostimulates P2X7 receptors (Xia et al., 2012). Recently, our group has also demonstrated that acute elevation of intraocular pressure can induce the up-regulation of P2X7 receptor expression (Figure 2) (Sugiyama et al., 2013). Given that extracellular ATP levels and P2X7 receptor expression in the retina increase with elevated intraocular pressure, and stimulation of P2X7 receptors in RGCs can be lethal, this autocrine response may exert a deleterious effect on RGCs in glaucomatous eyes.
Figure 2.
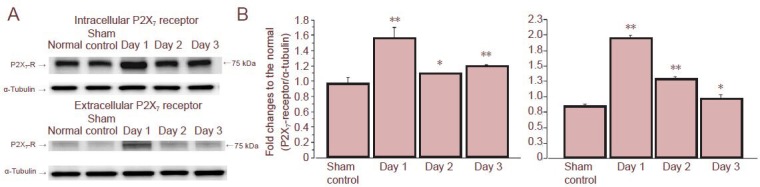
Western blot analysis of P2X7 receptor (P2X7-R) protein levels in whole retina after intraocular pressure (IOP) elevation.
(A) Representative immunoreactive bands of intracellular and extracellular P2X7 receptors in normal, sham control, and treated retinas on days 1, 2, and 3 after IOP elevation. (B) Densitometric quantification of immunoreactive bands of intracellular (left) and extracellular (right) P2X7 receptor in sham control and treated retinas on days 1, 2, and 3 compared to normal retina. Data are expressed as mean ± SD (n = 3). The asterisks indicate significant differences from the sham control eyes (unpaired t-test, *P < 0.05, **P < 0.01). From Sugiyama et al. (2013).
There are a number of reports linking P2X7 receptor activation in the retina with the expression of inflammatory cytokines (Skaper et al., 2010; Weisman et al., 2012). For example, P2X7 agonists enhance the release of interleukin (IL)-1β and tumor necrosis factor (TNF)-α from hypoxia-activated retinal microglia (Morigiwa et al., 2000). In addition, our data suggest that the up-regulation of TNF-α, IL-1β, and IL-6 may be involved in RGC death that occurs when P2X7 receptors are activated after an increase in intraocular pressure (Figure 3) (Sugiyama et al., 2013).
Figure 3.
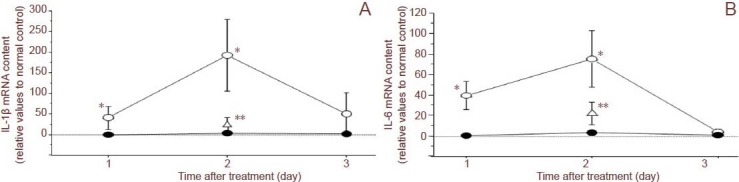
Effects of intraocular pressure (IOP) elevation on the expression of interleukin (IL)-1β and IL-6 mRNA in the retina.
(A) Alterations in IL-1β mRNA content in the retina after IOP elevation (open circles) or after sham treatment (closed circles). (B) Alterations in IL-6 mRNA content in the retina after IOP elevation (open circles) or sham treatment (closed circles). Open triangles represent samples from eyes treated with10 μmol/L OxATP just after IOP elevation. Data are expressed as mean ± SEM (n = 4–5). The asterisks indicate significant differences from the sham control eyes (*) or from the eyes after IOP elevation (**; Mann-Whitney U-test, P < 0.05). From Sugiyama et al. (2013).
In conclusion, a variety of recent experimental studies are providing evidence that the P2X7 purinoceptor is a potential therapeutic target of pharmacological strategies designed to diminish or prevent neuronal death in ocular diseases including glaucoma.
Acknowledgments:
I would like to express my appreciation to Professor Puro DG for leading me to this research topic during my stay as a research fellow in his laboratory at the University of Michigan in 2001, and also to Professor Ikeda T for giving me the opportunity to study abroad and then to continue to investigate this topic in the Department of Ophthalmology at Osaka Medical College, Japan.
References
- [1].Anccasi RM, Ornelas IM, Cossenza M, Persechini PM, Ventura AL. ATP induces the death of developing avian retinal neurons in culture via activation of P2X7 and glutamate receptors. Purinergic Signal. 2013;9:15–29. doi: 10.1007/s11302-012-9324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brändle U, Kohler K, Wheeler-Schilling TH. Expression of the P2X7 -receptor subunit in neurons of the rat retina. Brain Res Mol Brain Res. 1998;62:106–109. doi: 10.1016/s0169-328x(98)00254-x. [DOI] [PubMed] [Google Scholar]
- [3].Burnstock G, Fredholm BB, North RA, Verkhratsky A. The birth and postnatal development of purinergic signalling. Acta Physiol (Oxf) 2010;199:93–147. doi: 10.1111/j.1748-1716.2010.02114.x. [DOI] [PubMed] [Google Scholar]
- [4].Burnstock G, Kennedy C. P2X receptors in health and disease. Adv Pharmacol. 2011;61:333–372. doi: 10.1016/B978-0-12-385526-8.00011-4. [DOI] [PubMed] [Google Scholar]
- [5].Domercq M, Perez-Samartin A, Aparicio D, Alberdi E, Pampliega O, Matute C. P2X7 receptors mediate ischemic damage to oligodendrocytes. Glia. 2010;58:730–740. doi: 10.1002/glia.20958. [DOI] [PubMed] [Google Scholar]
- [6].Falzoni S, Munerati M, Ferrari D, Spisani S, Moretti S, Di Virgilio F. The purinergic P2Z receptor of human macrophage cells. Characterization and possible physiological role. J Clin Invest. 1995;95:1207–1216. doi: 10.1172/JCI117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Franke H, Günther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004;63:686–699. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- [8].Franke H, Klimke K, Brinckmann U, Grosche J, Francke M, Sperlagh B, Reichenbach A, Liebert UG, Illes P. P2X7 receptor-mRNA and -protein in the mouse retina; changes during retinal degeneration in BALBCrds mice. Neurochem Int. 2005;47:235–242. doi: 10.1016/j.neuint.2005.04.022. [DOI] [PubMed] [Google Scholar]
- [9].Hu H, Lu W, Zhang M, Zhang X, Argall AJ, Patel S, Lee GE, Kim YC, Jacobson KA, Laties AM, Mitchell CH. Stimulation of the P2X7 receptor kills rat retinal ganglion cells in vivo. Exp Eye Res. 2010;91:425–432. doi: 10.1016/j.exer.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ishii K, Kaneda M, Li H, Rockland KS, Hashikawa T. Neuron-specific distribution of P2X7 purinergic receptors in the monkey retina. J Comp Neurol. 2003;459:267–277. doi: 10.1002/cne.10608. [DOI] [PubMed] [Google Scholar]
- [11].Kaczmarek-Hájek K, Lörinczi E, Hausmann R, Nicke A. Molecular and functional properties of P2X receptors-recent progress and persisting challenges. Purinergic Signal. 2012;8:375–417. doi: 10.1007/s11302-012-9314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kakurai K, Sugiyama T, Kurimoto T, Oku H, Ikeda T. Involvement of P2X7 receptors in retinal ganglion cell death after optic nerve crush injury in rats. Neurosci Lett. 2013;534:237–241. doi: 10.1016/j.neulet.2012.11.060. [DOI] [PubMed] [Google Scholar]
- [13].Kawamura H, Sugiyama T, Wu DM, Kobayashi M, Yamanishi S, Katsumura K, Puro DG. ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol. 2003;551(pt 3):787–799. doi: 10.1113/jphysiol.2003.047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li A, Zhang X, Zheng D, Ge J, Laties AM, Mitchell CH. Sustained elevation of extracellular ATP in aqueous humor from humans with primary chronic angle-closure glaucoma. Exp Eye Res. 2011;93:528–533. doi: 10.1016/j.exer.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Morigiwa K, Quan M, Murakami M, Yamashita M, Fukuda Y. P2 purinoceptor expression and functional changes of hypoxia-activated cultured rat retinal microglia. Neurosci Lett. 2000;282:153–156. doi: 10.1016/s0304-3940(00)00887-9. [DOI] [PubMed] [Google Scholar]
- [16].Niyadurupola N, Sidaway P, Ma N, Rhodes JD, Broadway DC, Sanderson J. P2X7 receptor activation mediates retinal ganglion cell death in a human retina model of ischemic neurodegeneration. Invest Ophthalmol Vis Sci. 2013;54:2163–2170. doi: 10.1167/iovs.12-10968. [DOI] [PubMed] [Google Scholar]
- [17].North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- [18].Notomi S, Hisatomi T, Kanemaru T, Takeda A, Ikeda Y, Enaida H, Kroemer G, Ishibashi T. Critical involvement of extracellular ATP acting on P2RX 7 purinergic receptors in photoreceptor cell death. Am J Pathol. 2011;179:2798–2809. doi: 10.1016/j.ajpath.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pannicke T, Fischer W, Biedermann B, Schädlich H, Grosche J, Faude F, Wiedemann P, Allgaier C, Illes P, Burnstock G, Reichenbach A. P2X7 receptors in Müller glial cells from the human retina. J Neurosci. 2000;20:5965–5972. doi: 10.1523/JNEUROSCI.20-16-05965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Puthussery T, Fletcher EL. Synaptic localization of P2X7 receptors in the rat retina. J Comp Neurol. 2004;472:13–23. doi: 10.1002/cne.20045. [DOI] [PubMed] [Google Scholar]
- [21].Reigada D, Lu W, Zhang M, Mitchell CH. Elevated pressure triggers a physiological release of ATP from the retina: possible role for pannexin hemichannels. Neuroscience. 2008;157:396–404. doi: 10.1016/j.neuroscience.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Romagnoli R, Baraldi PG, Cruz-Lopez O, Lopez-Cara C, Preti D, Borea PA, Gessi S. The P2X7 receptor as a therapeutic target. Expert Opin Ther Targets. 2008;12:647–661. doi: 10.1517/14728222.12.5.647. [DOI] [PubMed] [Google Scholar]
- [23].Savigni DL, O’Hare Doig RL, Szymanski CR, Bartlett CA, Loziæ I, Smith NM, Fitzgerald M. Three Ca2+ channel inhibitors in combination limit chronic secondary degeneration following neurotrauma. Neuropharmacology. 2013;75:380–390. doi: 10.1016/j.neuropharm.2013.07.034. [DOI] [PubMed] [Google Scholar]
- [24].Skaper SD, Debetto P, Giusti P. The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J. 2010;24:337–345. doi: 10.1096/fj.09-138883. [DOI] [PubMed] [Google Scholar]
- [25].Sugiyama T, Oku H, Shibata M, Fukuhara M, Yoshida H, Ikeda T. Involvement of P2X7 receptors in the hypoxia-induced death of rat retinal neurons. Invest Ophthalmol Vis Sci. 2010;51:3236–3243. doi: 10.1167/iovs.09-4192. [DOI] [PubMed] [Google Scholar]
- [26].Sugiyama T, Lee SY, Horie T, Oku H, Takai S, Tanioka H, Kuriki Y, Kojima S, Ikeda T. P2X7 receptor activation may be involved in neuronal loss in the retinal ganglion cell layer after acute elevation of intraocular pressure in rats. Mol Vis. 2013;19:2080–2091. [PMC free article] [PubMed] [Google Scholar]
- [27].Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- [28].Vessey KA, Fletcher EL. Rod and cone pathway signalling is altered in the P2X7 receptor knock out mouse. PLoS One. 2012;7:e29990. doi: 10.1371/journal.pone.0029990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Weisman GA, Camden JM, Peterson TS, Ajit D, Woods LT, Erb L. P2 receptors for extracellular nucleotides in the central nervous system: role of P2X7 and P2Y2 receptor interactions in neuroinflammation. Mol Neurobiol. 2012;46:96–113. doi: 10.1007/s12035-012-8263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wheeler-Schilling TH, Marquordt K, Kohler K, Guenther E, Jabs R. Identification of purinergic receptors in retinal ganglion cells. Brain Res Mol Brain Res. 2001;92:177–180. doi: 10.1016/s0169-328x(01)00160-7. [DOI] [PubMed] [Google Scholar]
- [31].Xia J, Lim JC, Lu W, Beckel JM, Macarak EJ, Laties AM, Mitchell CH. Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7 receptors. J Physiol. 2012;590(Pt 10):2285–2304. doi: 10.1113/jphysiol.2012.227983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yang D, Elner SG, Clark AJ, Hughes BA, Petty HR, Elner VM. Activation of P2X receptors induces apoptosis in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011;52:1522–1530. doi: 10.1167/iovs.10-6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang X, Zhang M, Laties AM, Mitchell CH. Stimulation of P2X7 receptors elevates Ca2+ and kills retinal ganglion cells. Invest Ophthalmol Vis Sci. 2005;46:2183–2191. doi: 10.1167/iovs.05-0052. [DOI] [PubMed] [Google Scholar]
- [34].Zhang X, Li A, Ge J, Reigada D, Laties AM, Mitchell CH. Acute increase of intraocular pressure releases ATP into the anterior chamber. Exp Eye Res. 2007;85:637–643. doi: 10.1016/j.exer.2007.07.016. [DOI] [PubMed] [Google Scholar]


