Abstract
The American Heart Association and the European Resuscitation Council recently recommended that vasopressin can be used for cardiopulmonary resuscitation, instead of epinephrine. However, the guidelines do not discuss the effects of vasopressin during cerebral resuscitation. In this study, we intraperitoneally injected epinephrine and/or vasopressin during cardiopulmonary resuscitation in a rat model of asphyxial cardiac arrest. The results demonstrated that, compared with epinephrine alone, the pathological damage to nerve cells was lessened, and the levels of c-Jun N-terminal kinase and p38 expression were significantly decreased in the hippocampus after treatment with vasopressin alone or the vasopressin and epinephrine combination. No significant difference in resuscitation effects was detected between vasopressin alone and the vasopressin and epinephrine combination. These results suggest that vasopressin alone or the vasopressin and epinephrine combination suppress the activation of mitogen-activated protein kinase and c-Jun N-terminal kinase signaling pathways and reduce neuronal apoptosis during cardiopulmonary resuscitation.
Keywords: nerve regeneration, brain injury, cardiopulmonary resuscitation, epinephrine, vasopressin, c-Jun N-terminal kinase, p38 mitogen activated protein kinase, cardiac arrest, neural regeneration
Introduction
During cardiac compression and artificial ventilation, it is important to find one or several kinds of drugs with good therapeutic effects so as to elevate the cardiac, pulmonary and cerebral resuscitation rates and improve prognosis in the clinical treatment of cardiopulmonary resuscitation. Epinephrine has been shown to be a first-choice drug for cardiopulmonary resuscitation[1]. Nevertheless, its β-adrenergic effect probably increases myocardial oxygen consumption and leads to severe cardiac and cerebral injuries; moreover, epinephrine does not elevate long-term survival rates[2,3,4,5,6]. The 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care recommended the application of vasopressin, instead of the first or second dose of epinephrine, to elevate the success rate of cardiopulmonary resuscitation[7]. Moreover, the European Resuscitation Council makes the same recommendation[8]. A clinical study showed that vasopressin increased blood flow to important organs, expanded cerebral vessels, improved cerebral oxygen supply, elevated coronary perfusion pressure, and enhanced the recovery of neural function after cardiopulmonary resuscitation[9]. Compared with epinephrine, vasopressin could not excite β receptors, or induce an increased heart rate or heart failure[9]. Few studies have addressed the protective effects of vasopressin on the brain during cerebral resuscitation. Cerebral ischemia/reperfusion-induced injuries are key risk factors for cardiopulmonary resuscitation, but the underlying mechanisms remain poorly understood.
Mitogen-activated protein kinases are key regulators of cell stress and injury reactions, and can transmit signals from the extracellular space to nuclei. Numerous studies have confirmed that mitogen-activated protein kinase is activated during ischemia and reperfusion of heart, kidney and nerve[10,11]. Mitogen-activated protein kinase participates in cell proliferation, differentiation, transformation, apoptosis, inflammatory reaction and tumorigenesis. c-Jun N-terminal kinase is involved in the apoptosis of various cells, and plays a crucial role in ischemia/reperfusion injury. The c-Jun N-terminal kinase pathway is a major pathway involved in cell stress-induced cell apoptosis[12,13,14,15]. p38 can be activated by ultraviolet light, osmotic pressure changes, physical stress and cytokines, and has an essential effect on inflammatory reactions and stress[16]. A previous study verified that the p38 mitogen-activated protein kinase pathway was activated in rats after cardiopulmonary resuscitation, resulting in the injuries to the heart and brain and release of a large amount of inflammatory factors[17].
Whether vasopressin has a more effective effect on cerebral resuscitation than epinephrine is unknown. In this study, we compared the effects of epinephrine, vasopressin and their combination on the c-Jun N-terminal kinase and p38 pathways after cardiopulmonary and cerebral resuscitation in a rat model of asphyxial cardiac arrest.
Results
Quantitative analysis of experimental animals
Two-hundred and fifty rats were equally and randomly assigned to a sham surgery group, a model group, an epinephrine group, a vasopressin group and a combination group (vasopressin + epinephrine). After 10 minutes of asphyxia, cardiopulmonary resuscitation was performed in rats. At 1, 4, and 7 minutes after resuscitation, the rat models in the model, epinephrine, vasopressin and combination groups were respectively administered physiological saline, epinephrine, vasopressin and epinephrine + vasopressin via intraperitoneal injection. The rats were observed at 1, 3, 6 and 12 hours after resuscitation. The main reason for rat deaths was the failure of cardiopulmonary resuscitation. Four rats in the model group were successfully resuscitated. Behavioral observations were conducted in one rat at each time point. Twenty-four, 39 and 42 rats were successfully resuscitated in the epinephrine, vasopressin and combination groups, respectively. Thus, six rats were selected for analysis at each time point. Ten rats from the sham surgery group were selected at each time point.
Vasopressin restored spontaneous circulation in rats after cardiopulmonary resuscitation
Pulseless electrical activity, blood pressure < 10 mmHg, and respiratory arrest were observed after asphyxia. The number of rats in the combination group showing restored spontaneous circulation (success rate of resuscitation) was significantly greater than that in the epinephrine group (P < 0.05; Table 1).
Table 1.
Vasopressin effects on the return of spontaneous circulation in rats undergoing cardiopulmonary resuscitation
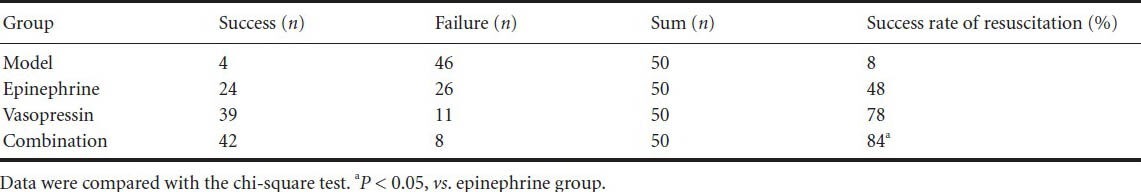
Vasopressin lessened histopathological injury in the hippocampi of rats undergoing cardiopulmonary resuscitation
Nerve cells were normal and no edema was visible in the sham surgery group. Cellular edema occurred, nuclei were vague or invisible, and cytoplasmic vacuolization was aggravated over time in the epinephrine and vasopressin groups. Cellular edema was not apparent and nuclei were unclear in the combination group. The pathological injury was aggravated with increased time in the epinephrine, vasopressin, and combination groups (Figure 1).
Figure 1.
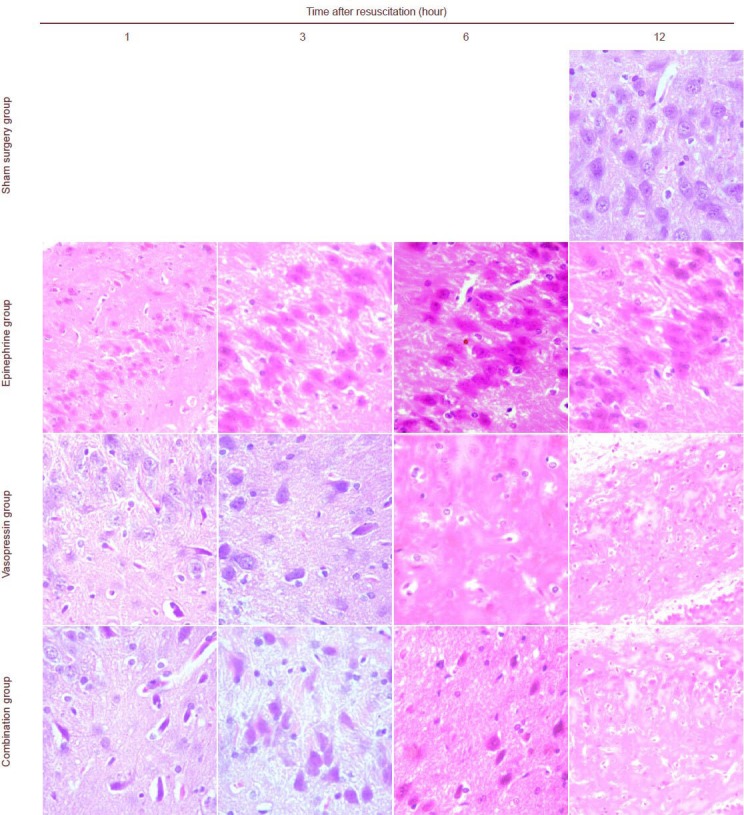
Vasopressin effects on histopathological injury in the hippocampus of rats undergoing cardiopulmonary resuscitation (hematoxylin-eosin staining).
In hematoxylin-eosin staining, cell nuclei are stained blue, the cytoplasm is stained red, and erythrocytes are orange. Other components are dark or light red. Cellular edema occurred, and nuclei were vague or invisible in the epinephrine and vasopressin groups. Cellular edema was not apparent and nuclei were unclear in the combination group. The pathological injury was aggravated with increasing time in the epinephrine, vasopressin, and combination groups, which did not show significant differences. Vasopressin and epinephrine groups at 12 hours, combination group at 1 hour: × 200; others: × 400.
Vasopressin reduced the levels of c-Jun N-terminal kinase and p38 expression in the hippocampi of rats undergoing cardiopulmonary resuscitation
Immunohistochemical staining revealed that some c-Jun N-terminal kinase and p38 expression could be detected in the sham surgery group. c-Jun N-terminal kinase and p38 expression was mainly detectable in the cell membrane, cytoplasm and nuclei in the hippocampi of rats in the epinephrine, vasopressin and combination groups. Moreover, with prolonged time after cardiopulmonary resuscitation, the c-Jun N-terminal kinase and p38 expression levels gradually increased, and peaked at 6 hours. c-Jun N-terminal kinase and p38 expressions were lower in the vasopressin and combination groups than in the epinephrine group (P < 0.05). No significant difference in c-Jun N-terminal kinase and p38 expression levels was observed in the vasopressin and combination groups (P > 0.05; Figures 2, 3, Tables 2, 3).
Figure 2.
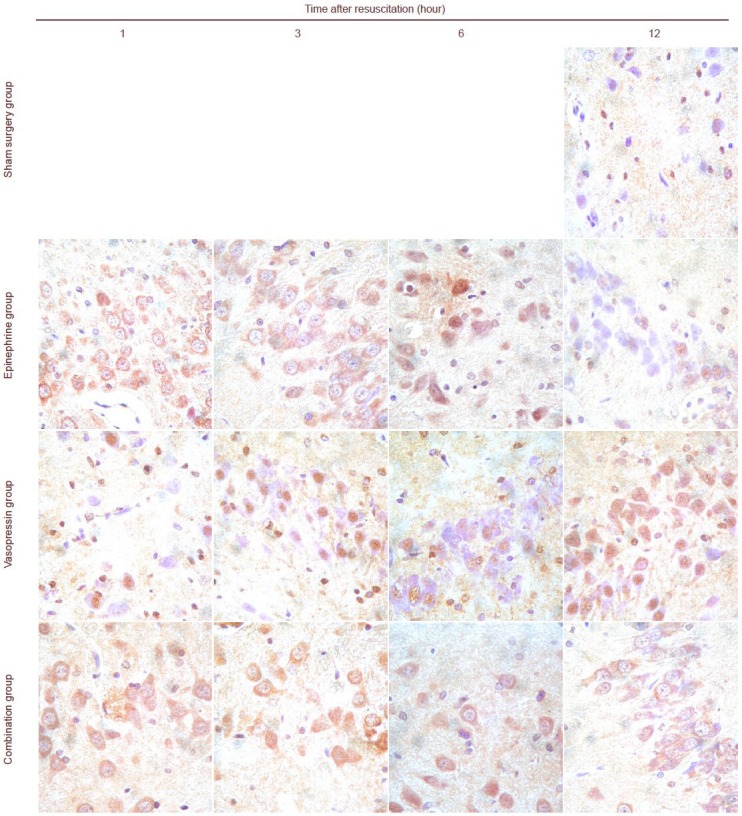
Vasopressin effects on c-Jun N-terminal kinase expression in the hippocampi of rats undergoing cardiopulmonary resuscitation (immunohistochemistry staining, × 400).
c-Jun N-terminal kinase expression in the cytoplasm and nuclei is represented by brown staining. c-Jun N-terminal kinase expression could not be seen or was only sometimes visible in the rat hippocampus in the sham surgery group. c-Jun N-terminal kinase expression was observed in the epinephrine, vasopressin and combination groups. Expression intensity: epinephrine group > vasopressin group = combination group.
Figure 3.
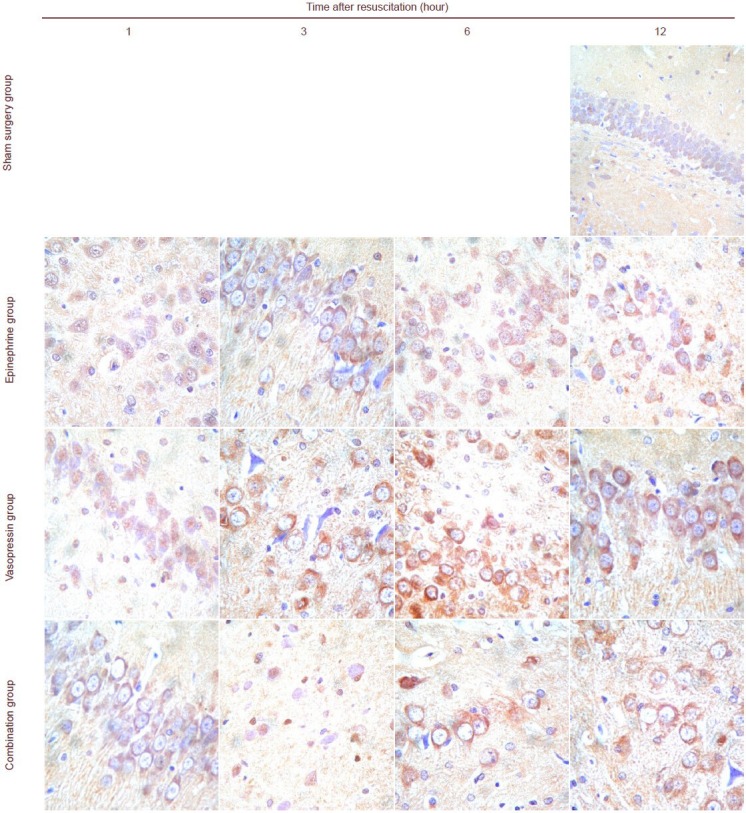
Vasopressin effects on p38 expression in the hippocampi of rats undergoing cardiopulmonary resuscitation (immunohistochemistry, × 400).
P38 expression in the cytoplasm and nuclei is represented by brown staining. p38 expression could not be seen or was only sometimes visible in the rat hippocampus in the sham surgery group. p38 expression was observed in the epinephrine, vasopressin and combination groups. Expression intensity: epinephrine group > vasopressin group = combination group.
Table 2.
Vasopressin effects on immunohistochemical score for c-Jun N-terminal kinase in the hippocampi of rats after cardiopulmonary resuscitation
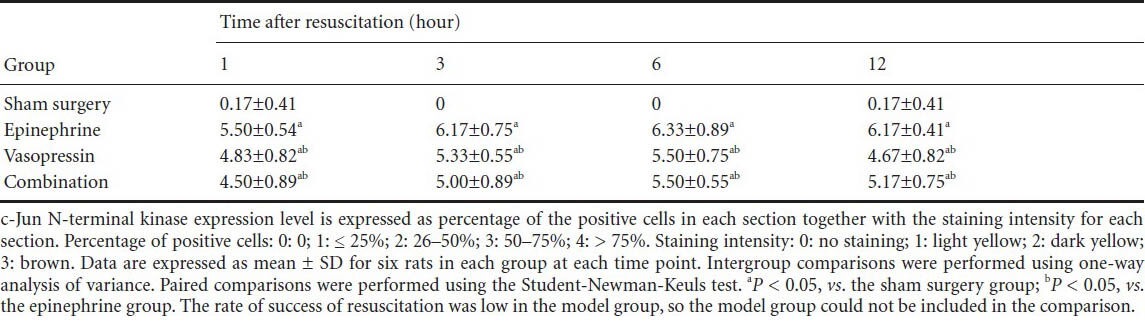
Table 3.
Vasopressin effects on immunohistochemical score for p38 in the hippocampus of rats after cardiopulmonary resuscitation
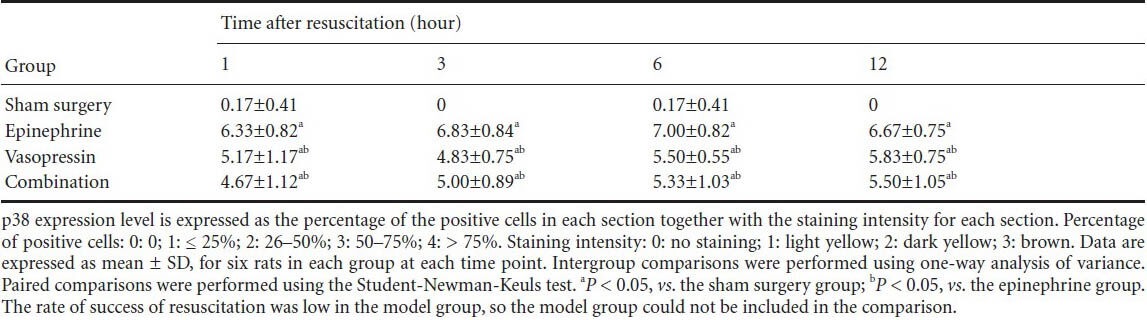
Discussion
Wenzel et al.[18] confirmed that the survival rate associated with using vasopressin was three times that associated with using epinephrine. Stiell et al.[19] suggested that the therapeutic effects of vasopressin were not better than those of epinephrine in asystole patients. However, a recent animal study demonstrated that vasopressin elevated coronary perfusion pressure, contracted peripheral blood vessels, maintained the blood supply to vital organs, did not increase oxygen consumption in the myocardium, and was helpful for the recovery of neurological function. Wenzel et al.[20,21] found that vasopressin significantly diminished after treatment of pigsthat underwent resuscitation with vasopressin. Moreover, myocardial blood flow, oxygen supply, mean arterial blood pressure, and the perfusion volume of vital organs were noticeably greater in pigs treated with vasopressin compared with pigs treated with epinephrine. Lindner et al.[22] showed that vasopressin produced evidently higher perfusion pressure and cerebral blood flow in a pig model of ventricular fibrillation compared with epinephrine. No significant difference in the rate of success of resuscitation was observed in a rat model of asphyxia between treatment with vasopressin and epinephrine[23]. However, diastolic pressure and systolic pressure were higher, while heart rate was lower in the vasopressin group compared with the epinephrine group[23]. Moreover, no significant difference in the results of blood gas analysis was detectable[23]. High-dose vasopressin remarkably relieved myocardial injury, elevated the rate of cardiopulmonary resuscitation, and maintained mean arterial blood pressure for a long period compared with epinephrine in cats after asphyxia during cardiopulmonary resuscitation[24]. Nevertheless, the effects of vasopressin and epinephrine on blood gas analysis were similar[24]. Ou et al.[25] verified that coronary perfusion pressure was significantly higher after combined application of epinephrine and vasopressin compared with the application of vasopressin or epinephrine alone in a rabbit model of asphyxia. Numerous studies have shown that the effects of vasopressin combined with other drugs were better than those of vasopressin alone. Mayr et al.[26] confirmed that the combined application of vasopressin and epinephrine rapidly elevated coronary perfusion pressure and the return of spontaneous circulation in pigs during cardiopulmonary resuscitation. Stadlbauer et al.[27] observed that the combined application of vasopressin and epinephrine obviously increased coronary perfusion pressure, and returned spontaneous circulationin patients. Another study suggested that cerebral blood flow was reduced in patients treated with both vasopressin and epinephrine compared with those treated with vasopressin or epinephrinealone[28]. Nevertheless, the effects of vasopressin alone or combined with epinephrine on cerebral blood flow perfusion were better than those of epinephrine alone[29]. This study verified that vasopressin had better effects on the return of spontaneous circulation and improvement of histopathological injury in the hippocampi of rats during cardiopulmonary resuscitation compared with epinephrine.
Immunohistochemical results demonstrated that expression of c-Jun N-terminal kinase and p38 was increased in the rat hippocampus after cardiopulmonary resuscitation, and indicated that mitogen-activated protein kinase signaling pathway was activated after ischemia and hypoxia, resulting in overexpression of c-Jun N-terminal kinase and p38. These changes contributed to neuronal apoptosis and aggravated ischemia/reperfusion injury. c-Jun N-terminal kinase expression showed a tendency to be increased and peaked in cardiac arrest rats at 6 hours after cardiopulmonary resuscitation. Vasopressin could more effectively suppress c-Jun N-terminal kinase and p38 overexpression compared with epinephrine. This study further verified that cardiopulmonary resuscitation activated the c-Jun N-terminal kinase/mitogen-activated protein kinase signaling pathway and worsened ischemia/reperfusion injury. In addition, vasopressin decreased c-Jun N-terminal kinase expression and protected the brain, consistent with previous results[30]. Li et al.[31] suggested that activation of the p38 signaling pathway induced the injury to hippocampal neurons. Preadministration of a p38 inhibitor effectively lessened this kind of injury, and protected the brain from ischemia/reperfusion injury. The p38 expression level also increased with prolonged time after cardiopulmonary resuscitation in each group. Vasopressin could more effectively suppress the activation of the p38 signaling pathway compared with epinephrine. The present study further confirmed that the p38 signaling pathway was activated after cells were subjected to ischemia and hypoxia, resulting in cell apoptosis, which would further aggravate the brain injury.
In summary, the therapeutic effects of vasopressin were better than or at least equal to those of epinephrine, especially the protective effect of vasopressin on the brain after resuscitation. This study is the first to show that vasopressin more effectively inhibits the overactivation of p38 and c-Jun N-terminal kinase during cardiopulmonary resuscitation compared with epinephrine, and that it exerts cerebroprotective effects.
Materials and Methods
Design
A randomized controlled animal study.
Time and setting
Experiments were performed in the Animal Experimental Center, Jilin University, China in November 2012.
Materials
Animals
Two-hundred and fifty healthy, specific pathogen-free, Wistar rats of both sexes, weighing 270 ± 20 g and aged 8–10 weeks, were provided by the Experimental Animal Center, Jilin University, China, license No. SCXK (Ji) 2007-0003. They were housed at 25°C with humidity of 50%. The disposal of experimental animals was in accordance with Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[32] and this stady was appooved by Animals Ethics Committee, the First Hospital, Jilin University, China.
Drugs
Epinephrine, parenteral solution, 1 mL:1 mg, 1 g/L, was purchased from Grand Pharmaceutical (China) Co., Ltd., Wuhan, Hubei Province, China; lot No. 130203, approval No. GYZZ H42021700.
Vasopressin, parenteral solution, 1 mL: 6 U, 6,000 U/L, was purchased from Shanghai Harvest Pharmaceutical Co., Ltd., Shanghai, China; lot No. 6A01008, approval No. GYZZ H31022751.
Methods
Preparation of asphyxial cardiac arrest and cardiopulmonary resuscitation
In accordance with the method of Kono et al.[23], the rats were deprived of food, but not deprived of water for 12 hours before the experiment. After weighing, the rats were intraperitoneally anesthetized with 10% chloral hydrate (0.3 mL/100 g; Shanghai Harvest Pharmaceutical Co., Ltd.), and then fixed on an operating table. A Lead II limb lead electrocardiogram monitoring system was used for recordings. Under sterilized conditions, a median anterior incision was made on the rat's neck using eye scissors. The muscular layer was bluntly isolated and the trachea was cut open. A tracheal cannula was set for connecting to a respirator (Model DH-140; Medical Instrument Factory, Zhejiang Medical University, China). The right inguinal region was exposed and an arterial cannula was set for filling with heparin sodium (5 IU/mL; Changzhou Qianhong Biochemistry Pharmaceutical Joint Stock Co., Ltd., Changzhou, Jiangsu Province, China). A pressure transducer and a blood pressure monitor (Hewlett-Packard, Qingdao, Shandong Province, China) were connected. The femoral vein was isolated and a transfusion needle was placed, with the presence of a syringe in the distal end for aspirating physiological saline. Spontaneous breathing was stabilized at room temperature for 10 minutes. In animals in the sham surgery group, under anesthesia, arterial, venous and tracheal cannulae were set for 10 minutes without touching.
The tracheal cannula was occluded for 5 minutes. When arterial blood pressure decreased below 10 mmHg, it was considered to represent cardiac arrest[33]. Cardiopulmonary resuscitation was performed. Cardiac compression was done at a frequency of 180 times/min and with a depth of 1/3 the anteroposterior diameter of rat thorax. Simultaneously, an animal respirator was rapidly connected at an oxygen concentration of 100%, a frequency of 70 times/min, tidal volume of 6 mL/kg and inspiratory/expiratory ratio of 1:1.5. Alterations in heart rate and blood pressure were monitored. When the rats suffered from supraventricular rhythm (sinus rhythm, atrial rhythm and junctional rhythm) and their mean arterial blood pressure was > 20 mmHg for at least 5 minutes, it was considered to represent the return of spontaneous circulation[3]. If spontaneous circulation returned within 10 minutes, the resuscitation was successful.
Injection of epinephrine and/or vasopressin
The rats in the epinephrine and vasopressin groups were intraperitoneally administered 0.2 mg/kg epinephrine and 0.8 U/kg vasopressin, respectively, at 1, 4 and 7 minutes after resuscitation. The rats in the combination group were administered epinephrine (0.2 mg/kg) and 3 minutes later given vasopressin (0.8 U/kg) via intraperitoneal injection. The rats in the model group were injected with 1 mL physiological saline. The success rate of resuscitation and the return rate of spontaneous circulation in each group were recorded. Changes in heart rate and blood pressure were monitored using an electrocardiogram monitor.
Sample collection
The rats in each group were fixed with 2.5% pentanediol at 1, 3, 6 and 12 hours after resuscitation, and then sacrificed. Brain tissues were obtained, fixed in 10% formaldehyde for 24 hours, and made into paraffin blocks. After paraffin embedding, the samples were sliced into 4-μm-thick sections.
Hematoxylin-eosin staining for pathological changes in rat brain tissues
In accordance with previous methods[34], sections were dewaxed, hydrated through a graded alcohol series, washed with distilled water, stained with hematoxylin for 5 minutes, washed with running water for 5 minutes, and then treated with 1% acidic alcohol for several seconds. After a wash with running water for 5 minutes, the sections were treated with weak aqueous ammonia, washed with running water for 5 minutes, stained with 0.5% eosin for 10 minutes, dehydrated through a graded alcohol series, permeabilized with xylene, and mounted with neutral resin.
Immunohistochemistry for c-Jun N-terminal kinase and p38 expressions in rat brain tissues
Paraffin sections were baked in a baking oven at 60°C for 3 hours, dewaxed with xylene, hydrated through a graded alcohol series, washed with distilled water, and boiled for antigen retrieval. These sections were treated with 3% H2O2 for 10 minutes at room temperature to eliminate endogenous peroxidase, washed three times with PBS, for 5 minutes each time, and incubated with normal goat serum for 15 minutes at room temperature. Serum was discarded. Without washing, these sections were incubated with rabbit anti-c-Jun N-terminal kinase polyclonal antibody (1:200; Boster, Wuhan, Hubei Province, China) and rabbit anti-p38 polyclonal antibody (1:200; Boster) overnight at 4°C. After washing three times with PBS for 5 minutes each time, the sections were incubated with biotinylated goat anti-rabbit IgG (1:200; Boster) for 10 minutes at 37°C, washed three times again with PBS, incubated with peroxidase-conjugated streptavidin for 10 minutes at 37°C, visualized with 3,3′-diaminobenzidine (Maxin Biotech Inc., Fuzhou, Fujian Province, China) for 1–2 minutes, terminated with distilled water, counterstained with hematoxylin, treated with acidic alcohol, washed with running water for developing blue for 1 minute, dehydrated through a graded alcohol series, permeabilized in xylene, mounted with neutral resin, and observed under a microscope (BX51; Olympus, Nanjing, Jiangsu Province, China). The rats in the sham surgery group served as a negative control. Ten fields were collected from each section (400 ×), and 100 cells were quantified in each field. Calculation of c-Jun N-terminal kinase and p38 expression levels was performed as follows: target protein expression levels were expressed as percentage of positive cells in each section together with the staining intensity for each section. Percentage of positive cells: 0: 0; 1: ≤ 25%; 2: 26–50%; 3: 50–75%; 4: > 75%. Staining intensity: 0: no staining; 1: light yellow; 2: dark yellow; 3: brown.
Statistical analysis
The data were analyzed using SPSS 18.0 software (SPSS, Chicago, IL, USA), and were expressed as mean ± SD. Intergroup comparisons were performed using one-way analysis of variance, and paired comparisons were performed using the Student-Newman-Keuls test. Measurement data were analyzed using the independent-samples t-test, and numeration data were analyzed using the chi-square test. A value of P < 0.05 was considered statistically significant.
Acknowledgments:
We are very grateful to teacher Huang M from Department of Physiology of Basic Medical College, Jilin Univesity, China, and teacher Chen from Department of Morphology, Jilin Univesity, China for their help and guidance during the experiment.
Footnotes
Conflicts of interest: None declared.
Peer review: This study confirmed that the application of vasopressin alone or combined with epinephrine better increased cerebral blood flow, reduced neuronal apoptosis, suppressed the activation of mitogen-activated protein kinase and c-Jun N-terminal kinase signaling pathways, and diminished ischemia/reperfusion injury.
Copyedited by McGowan D, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- [1].Ye RG, Lu ZY. Beijing: People's Medical Publishing House; 2001. Internal Medicine. [Google Scholar]
- [2].Meng QY. Thinking on the changes of epinephrine dose policy in cardiopulmonary resuscitation. Yixue Yu Zhexue: Linchuang Juece Luntan Ban. 2007;28(4):47–48. [Google Scholar]
- [3].van Walraven C, Stiell IG, Wells GA, et al. Do advanced cardiac life support drugs increase resuscitation rates from in-hospital cardiac arrest. The OTAC Study Group? Ann Emerg Med. 1998;32(5):544–553. doi: 10.1016/s0196-0644(98)70031-9. [DOI] [PubMed] [Google Scholar]
- [4].Wortsman J, Paradis NA, Martin GB, et al. Functional responses to extremely high plasma epinephrine concentrations in cardiac arrest. Crit Care Med. 1993;21(5):692–697. doi: 10.1097/00003246-199305000-00012. [DOI] [PubMed] [Google Scholar]
- [5].Ning JS, Jiang JH, Sui B. Developments of cardiopulmonary resuscitation. Linchuang Huicui. 2001;16(23):1102. [Google Scholar]
- [6].Hubloue I, Lauwaert I, Corne L. Adrenaline dosage during cardiopulmonary resuscitation: a critical review. Eur J Emerg Med. 1994;1(3):149–153. [PubMed] [Google Scholar]
- [7].Hazinski MF, Nadkarni VM, Hickey RW, et al. Major changes in the 2005 AHA Guidelines for CPR and ECC: reaching the tipping point for change. Circulation. 2005;112(24 Suppl):IV206–211. doi: 10.1161/CIRCULATIONAHA.105.170809. [DOI] [PubMed] [Google Scholar]
- [8].Treschan TA, Peters J. The vasopressin system: physiology and clinical strategies. Anesthesiology. 2006;105(3):599–612. doi: 10.1097/00000542-200609000-00026. [DOI] [PubMed] [Google Scholar]
- [9].Sirinek KR, Adcock DK, Levine BA. Simultaneous infusion of nitroglycerin and nitroprusside to offset adverse effects of vasopressin during portosystemic shunting. Am J Surg. 1989;157(1):33–37. doi: 10.1016/0002-9610(89)90416-9. [DOI] [PubMed] [Google Scholar]
- [10].Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab. 2002;22(6):631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- [11].Ravingerová T, Barancík M, Strnisková M. Mitogen-activated protein kinases: a new therapeutic target in cardiac pathology. Mol Cell Biochem. 2003;247(1-2):127–138. doi: 10.1023/a:1024119224033. [DOI] [PubMed] [Google Scholar]
- [12].Ravingerová T, Barancík M, Strnisková M. Mitogen-activated protein kinases: a new therapeutic target in cardiac pathology. Mol Cell Biochem. 2003;247(1-2):127–138. doi: 10.1023/a:1024119224033. [DOI] [PubMed] [Google Scholar]
- [13].Kolahgar G, Bardet PL, Langton PF, et al. Apical deficiency triggers JNK-dependent apoptosis in the embryonic epidermis of Drosophila. Development. 2011;138(14):3021–3031. doi: 10.1242/dev.059980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jeong CW, Yoo KY, Lee SH, et al. Curcumin protects against regional myocardial ischemia/reperfusion injury through activation of RISK/GSK-3β and inhibition of p38 MAPK and JNK. J Cardiovasc Pharmacol Ther. 2012;17(4):387–394. doi: 10.1177/1074248412438102. [DOI] [PubMed] [Google Scholar]
- [15].Chambers JW, Pachori A, Howard S, et al. Inhibition of JNK mitochondrial localization and signaling is protective against ischemia/reperfusion injury in rats. J Biol Chem. 2013;288(6):4000–4011. doi: 10.1074/jbc.M112.406777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15(1):11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- [17].Liu XL, Li HF, Sun ML. Chinese Medical Association. Chinese Medical Association Emergency Medicine Branch 13th National Emergency Medicine Conference Proceedings; 2010. Changes of TGF and p38MAPK during cardiopulmonary cerebral resuscitation. [Google Scholar]
- [18].Wenzel V, Krismer AC, Arntz HR, et al. A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. N Engl J Med. 2004;350(2):105–113. doi: 10.1056/NEJMoa025431. [DOI] [PubMed] [Google Scholar]
- [19].Stiell IG, Hébert PC, Wells GA, et al. Vasopressin versus epinephrine for inhospital cardiac arrest: a randomised controlled trial. Lancet. 2001;358(9276):105–109. doi: 10.1016/S0140-6736(01)05328-4. [DOI] [PubMed] [Google Scholar]
- [20].Wenzel V, Lindner KH, Prengel AW, et al. Vasopressin improves vital organ blood flow after prolonged cardiac arrest with postcountershock pulseless electrical activity in pigs. Crit Care Med. 1999;27(3):486–492. doi: 10.1097/00003246-199903000-00022. [DOI] [PubMed] [Google Scholar]
- [21].Lindner KH, Haak T, Keller A, et al. Release of endogenous vasopressors during and after cardiopulmonary resuscitation. Heart. 1996;75(2):145–150. doi: 10.1136/hrt.75.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lindner KH, Prengel AW, Pfenninger EG, et al. Vasopressin improves vital organ blood flow during closed-chest cardiopulmonary resuscitation in pigs. Circulation. 1995;91(1):215–221. doi: 10.1161/01.cir.91.1.215. [DOI] [PubMed] [Google Scholar]
- [23].Kono S, Bito H, Suzuki A, et al. Vasopressin and epinephrine are equally effective for CPR in a rat asphyxia model. Resuscitation. 2002;52(2):215–219. doi: 10.1016/s0300-9572(01)00447-6. [DOI] [PubMed] [Google Scholar]
- [24].Chen M, Xu JL, Hua HM, et al. Role of vasopressin on cardiopulmonary resuscitation in the cat. Xuzhou Yixueyuan Xuebao. 2007;27(9):584–588. [Google Scholar]
- [25].Ou H, Yang MS, Sun B, et al. Application of the combination of epinephrine and vasopressin in asphyxial rabbit models of cardiopulmonary resuscitation. Yixue Linchuang Yanjiu. 2006;23(7):1032–1035. [Google Scholar]
- [26].Mayr VD, Wenzel V, Voelckel WG, et al. Developing a vasopressor combination in a pig model of adult asphyxial cardiac arrest. Circulation. 2001;104(14):1651–1656. doi: 10.1161/hc3901.095896. [DOI] [PubMed] [Google Scholar]
- [27].Stadlbauer KH, Wagner-Berger HG, Wenzel V, et al. Survival with full neurologic recovery after prolonged cardiopulmonary resuscitation with a combination of vasopressin and epinephrine in pigs. Anesth Analg. 2003;96(6):1743–1749. doi: 10.1213/01.ANE.0000066017.66951.7F. table of contents. [DOI] [PubMed] [Google Scholar]
- [28].Wenzel V, Linder KH, Augenstein S, et al. Vasopressin combined with epinephrine decreases cerebral perfusion compared with vasopressin alone during cardiopulmonary resuscitation in pigs. Stroke. 1998;29(7):1462–1468. doi: 10.1161/01.str.29.7.1462. [DOI] [PubMed] [Google Scholar]
- [29].Voelckel WG, Lurie KG, McKnite S, et al. Effects of epinephrine and vasopressin in a piglet model of prolonged ventricular fibrillation and cardiopulmonary resuscitation. Crit Care Med. 2002;30(5):957–962. doi: 10.1097/00003246-200205000-00001. [DOI] [PubMed] [Google Scholar]
- [30].Ozawa H, Shioda S, Dohi K, et al. Delayed neuronal cell death in the rat hippocampus is mediated by the mitogen-activated protein kinase signal transduction pathway. Neurosci Lett. 1999;262(1):57–60. doi: 10.1016/s0304-3940(99)00034-8. [DOI] [PubMed] [Google Scholar]
- [31].Li GM, Li J, Cao H, et al. The role of MAPK in ischemia-reperfusion injury to the hippocampal neurons in gerbils. Xuzhou Yixueyuan Xuebao. 2004;24(4):285–289. [Google Scholar]
- [32].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [33].Liu XL, Sun ML, Yu YX, et al. Chinese Association of Integrative Medcine Disaster Medicine Speciality Committee. 4th National Disaster Medical Academic Conference; 2007. Optimal dose of epinephrine in cardiac arrest. [Google Scholar]
- [34].Paxinos G, Watson C. 5th ed. London: Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]


