Abstract
Several studies have demonstrated that selective serotonin reuptake inhibitor antidepressants can promote neuronal cell proliferation and enhance neuroplasticity both in vitro and in vivo. It is hypothesized that citalopram, a selective serotonin reuptake inhibitor, can promote the neuronal differentiation of adult bone marrow mesenchymal stem cells. Citalopram strongly enhanced neuronal characteristics of the cells derived from bone marrow mesenchymal stem cells. The rate of cell death was decreased in citalopram-treated bone marrow mesenchymal stem cells than in control cells in neurobasal medium. In addition, the cumulative population doubling level of the citalopram-treated cells was significantly increased compared to that of control cells. Also BrdU incorporation was elevated in citalopram-treated cells. These findings suggest that citalopram can improve the neuronal-like cell differentiation of bone marrow mesenchymal stem cells by increasing cell proliferation and survival while maintaining their neuronal characteristics.
Keywords: nerve regeneration, citalopram, stem cells, bone marrow mesenchymal stem cells, survival, proliferation, differentiation, neurons, neural regeneration
Introduction
Regeneration of damaged cells is the primary goal of cell therapy and one of the important reasons for the study of stem cells. In most cases of damaged central nervous system and neurodegenerative diseases, the neurons are lost (Rahmani et al., 2013a, 2013b; Croce et al., 2014; Sollberger et al., 2014). In these cases, the cell replacement is necessary to supply the function of lost cells. However, the ex vivo expansion of stem cells and their in vivo delivery are restricted by the limited availability of stem cell sources, the excessive cost of commercialization, and the difficulties of clinical approval (e.g., embryonic tissue grafts; Viswanathan et al., 2013). Ideally, a cell replacement source should be readily obtainable without technical and ethical problems (Huang et al., 2013; Shoae-Hassani et al., 2013b, 2013c; Qin et al., 2013). Adult bone marrow mesenchymal stem cells have great promise as a source for introducing new neurons (Scwaarz et al., 1999; Sasaki et al., 2001; Noureddini et al., 2012) to the damaged nervous system. They have the potential to substitute tissues lost by injury and diseases if lineage differentiation could be controlled exactly. On the other hand, a better understanding of the pharmacological manipulation of different cell types is important for the successful development of a new therapeutic strategy. Atkinson et al.(2012) described the important role of a pharmacological approach in controlling the differentiation of embryonic stem cells. However, the role of ‘adjunctive’ pharmacology in the clinical applications of stem cell therapy is underestimated. Studies have demonstrated the potential of stem cells through the implementation of efficient differentiation protocols, to alleviate symptoms in mouse models of human diseases. Such diseases include Parkinson's disease (Chung et al., 2011; Kriks et al., 2011; Kim et al., 2011b), spinal cord injury (Nori et al., 2011), Alzheimer's disease (Bissonnette et al., 2011) and efficient protocols for derivation of specific cell types to use of such cells to treat human disease. Up to now, multiple drugs that can modulate the differentiation of clinical-grade SCs (Ilic et al., 2011) have been discovered and may be used in the differentiation protocols to produce transplantable cells.
There are many reports showing that selective serotonin reuptake inhibitor antidepressants can promote neuronal cell proliferation and enhance neuroplasticity both in vitro and in vivo (Nandam et al., 2007; Wang et al., 2008; Boldrini et al., 2009; Rahmani et al., 2013a; Verdi et al., 2014). Citalopram, a selective serotonin reuptake inhibitor antidepressant is widely used for many disorders characterized by serotonergic dysfunctions such as depression, anxiety and panic disorders (Hashimoto et al., 2009). Considering this body of evidence, we hypothesized that a clinically effective antidepressant, citalopram can increase adult stem cell survival and differentiation in a neurobasal medium.
Materials and Methods
Bone marrow mesenchymal stem cell culture and differentiation
Human bone marrow mesenchymal stem cells (Lonza, Berkshire, UK) were cultured on collagen pre-coated plates with neurobasal media (Invitrogen Life Technologies, Glasgow, UK) supplemented with 5% fetal bovine serum in a humidified incubator with 5% CO2 at 37°C for 7 days. Stem cells that have grown to 70% confluence were pretreated with 1 μmol/L dimethyl-sulfoxide (DMSO; Sigma, St. Louis, MO, USA) and then were treated with citalopram (1, 5, and 10 μmol/L; Sigma) (Rahmani et al., 2013a) and/or 1 μmol/L retinoic acid (RA; Sigma). After treatment for 14 days, cells were subjected to reverse transcription PCR (RT-PCR) and immunocytochemistry assays to determine neuronal cell markers.
Immunofluorescence and quantification of immunoreactive neural cells
Immunocytochemistry experiment was performed as described previously (Noureddini et al., 2012). Briefly, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.05% Triton X-100. After blocking with 3% goat serum albumin, cells were incubated with primary antibodies for glial, neuronal and pre-neuronal markers at 37°C for 12 hours. The following primary antibodies and dilutions were used: mouse anti-β-tubulin-Tuj1 (1:500; Chemicon, Billerica, MA, USA); rabbit anti-glial fibrillary acidic protein (GFAP; 1:500; Sigma); rabbit anti-nestin (1:1,000; Sigma); mouse anti-microtubule-associated protein 2 (MAP-2; 1:500; Sigma). Then the cells were washed with PBS and reacted with the fluorescent isothiocyanate (FITC) conjugated secondary antibodies against rabbit and mouse Fc region (Sigma; 1:500) at room temperature for 2 hours. Finally, the cells were washed with PBS three times, and 4′,6-diamidino-2-phenylindole (DAPI) was used for DNA staining. The cells were visualized with Ceti immunofluorescence microscopy (Belgium).
RT-PCR
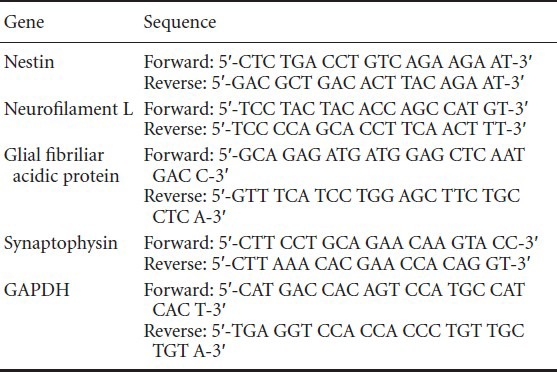
RT-PCR experiment was performed as described previously (Shoae-Hassani et al., 2013a). As a brief total RNA was extracted from differentiated cells before and after 2 weeks with and without citalopram, using the Qiagen RNA Isolation Kit and following the manufacturer's instructions (Qiagen, Valencia, CA, USA). After DNAse I digestion, cDNA was prepared from 1 μg total RNA, using the SuperScript III RT-PCR Kit (Invitrogen) as instructed by the manufacturer. Primer pair sequences are shown in Table 1. The amplification procedure consisted of 30 cycles (denaturation at 94°C for 30 seconds, annealing at 58°C for 40 seconds, and extension at 72°C for 45 seconds). Amplification reactions were conducted in a final volume of 25 μL containing 1.0 μL cDNA, 100 pmol each of forward and reverse primer and of PCR Master Mix (Promega). RT-PCR products were separated by electrophoresis on 1% agarose gels (Merck, Darmstadt, Germany) and stained with ethidium bromide (EB; Bio-Rad, Hercules, CA, USA).
Table 1.
Primer sequences specific for neurons and glial cells
MTT assay
Differentiated mesenchymal stem cells were tested for their survival time in the presence or absence of citalopram as described previously (Shoae-Hassani et al., 2013a). MTT assays were performed at 0, 1, 3, 7, 14 and 21 days and at 1 and 2 weeks after citalopram treatment. Cells growing without citalopram treatment were used as controls. Briefly, 5 × 103 mesenchymal stem cells were seeded on 96-well plates and grown in the presence of citalopram (10 μmol/L). MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) (400 μg/mL) was added to each well for a 4 hour incubation period. At the end of the incubation period, the medium was removed and 100 μL dimethyl sulfoxide (DMSO) (Sigma) was added into each well. To dissolve the formazan crystals, the supernatant was pipetted several times. Absorbance was measured on an ELISA plate reader (Perkin Elmer, Waltham, MA, USA) at a wavelength of 540 nm.
Cumulative population doubling level
Citalopram-treated stem cells were continuously passaged in neurobasal media with and without retinoic acid (RA) for 30 days, and there was a 5-day interval between each passage. The cumulative population doubling level was calculated to determine their proliferation potential. Non-treated cells, cultured in the same condition but without citalopram, were used as controls. The cumulative population doubling level at each passage was calculated from the cell count using the equation ln(Nf/Ni)/ln2, where Ni and Nf are the initial and final cell numbers, respectively, and ln is the natural log (Lin et al., 2005).
5-Bromo-2-deoxyuridine (BrdU) incorporation assay
The BrdU assay was performed as described previously (Rahmani et al., 2013a). Citalopram and/or RA-treated cells and control cells were cultured overnight with 30 μg/mL of BrdU prior to immunostaining. Cells were fixed with 4% paraformaldehyde and then incubated with blocking buffer (PBS containing 0.05% Triton X-100, 0.5% bovine serum albumin) for 1 hour at room temperature. Following incubation in 2.0 mol/L HCl for 15 minutes at room temperature, cells were washed with 0.1 mol/L sodium borate buffer. Immunostaining was done using primary mouse anti-BrdU antibody (1:100; Roche, Welwyn Garden, Hertfordshire, UK), and the secondary antibody was FITC anti-mouse antibody (Molecular Probes, USA; 1:500). The nuclei were counterstained with ethidium bromide for 10 minutes at room temperature. The number and the percentage of BrdU-immunoreactive cells were calculated in relation to the total number of ethidium bromide cells.
Statistical analysis
One-way analysis of variance (ANOVA) and two-tailed Student's t-test were used to analyze data using XLSTAT statistical software. Each experiment was conducted at least three times (n ≥ 3) and P-value of less than 0.05 was considered significant.
Results
Cell culture and differentiation
The in vitro study was conducted to determine whether the neurogenesis could be influenced by citalopram, a selective serotonin reuptake inhibitor antidepressant in cell culture system. After 7 days of culture, fibroblast-like cells with spindle-shaped morphology appeared on culture dishes (Figure 1A). After 7 days in vitro, stem cells growing in neuronal differentiation medium treated with citalopram displayed typical stem cell morphology (Figure 1B). Over the same time, stem cells treated with retinoic acid displayed neurite outgrowth (Figure 1C) and those treated with retinoic acid combined with citalopram increased neurite density (Figure 1D).
Figure 1.
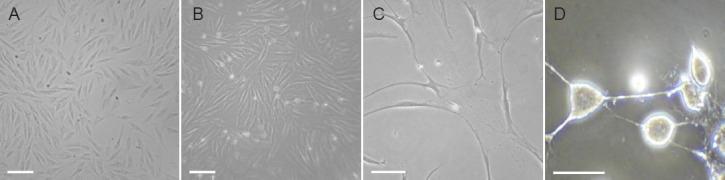
Morphological characteristics of bone marrow mesenchymal stem cells (BMSCs) and in vitro differentiation into neuronal-like cells.
Human BMSCs in dulbecco's modified eagle's medium (DMEM)/F12 with dimethyl sulfoxide showed spindle-shaped morphology before treatment (A), typical stem cell morphology after 7 days in neurobasal medium treated with 10 μmol/L citalopram (B), neurite growth after treatment with retinoic acid (C), and typical stem cell morphology after treatment with 1 μmol/L retinoic acid + 10 μmol/L citalopram (D) (Ceti immuno-fluorescence microscopy; × 400). Scale bars: 10 μm.
Immunofluorescence results
Citalopram and retinoic acid-treated human mesenchymal stem cells were visualized by fluorescence microscopy for detection of neuronal cell markers. Numerous cells immunoreactive for Tuj1, MAP-2 and nestin were seen in the immunostaining. There was no expression of glial fibrillary acidic protein (GFAP) in citalopram treated cells (Figure 2). The observed cellular characteristics and differentiation patterns demonstrate that citalopram enhanced the neuronal rather than the glial properties. The markers increased by citalopram were early neuronal cell markers, such as nestin.
Figure 2.
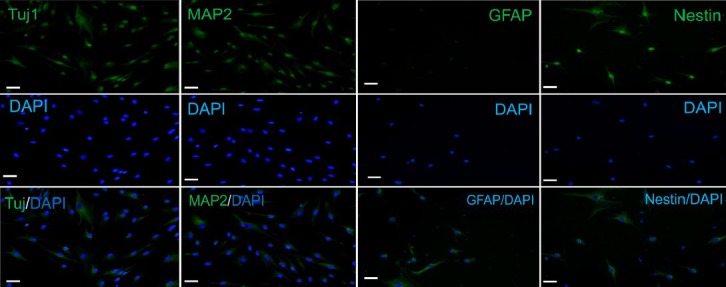
Expression of neuronal cell markers on the differentiated cells by BMSCs.
Immunostaining of Tuj1, MAP-2, nestin and GFAP in BMSCs treated with retinoic acid and citalopram. Numerous positive cells for Tuj1, MAP-2 and nestin were seen in the immunostaining. There was no expression of GFAP in citalopram treated cells (Ceti immunofluorescence microscopy; × 400; scale bars: 10 μm). The green color indicates fluorescein isothiocyanate (FITC) staining and the blue one indicates 4′,6-diamidino-2-phenylindole (DAPI) staining. BMSCs: Bone marrow mesenchymal stem cells; MAP-2: microtubule-associated protein 2; GFAP: glial fibrillary acidic protein.
RT-PCR analysis
RT-PCR detected the expression of neurofilament L, synaptophysin and nestin genes (Figure 3). There was no expression of glial cell marker GFAP as like as immunocytochemical staining. Expression of neuron-specific genes was up-regulated and remained elevated after 7 days in the citalopram-treated group.
Figure 3.
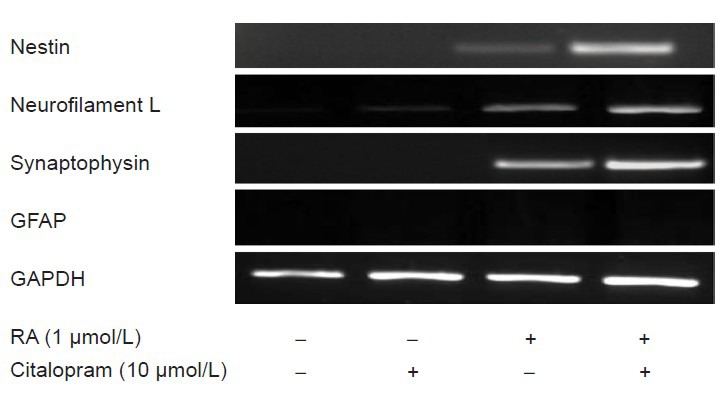
Expression of genes specific for neurons in differentiated cells by bone marrow mesenchymal stem cells.
Expression of nestin, neurofilament L, synaptophysin and glial fibrillary acidic protein (GFAP) was analyzed in negative control, retinoic acid (RA), and RA + citalopram treated cultures after 7 days. In the experiments, GAPDH was used as an internal control.
MTT assay
The effect of citalopram on the survival period of the differentiated stem cells was observed directly and investigated by MTT assay. As shown in Figure 4B, cell survival rate was significantly higher (P < 0.05) in the citalopram + retinoic acid group than in the retinoic acid group. Retinoic acid increased the cell survival rate of differentiated neurons in this assay, and citalopram exhibited a preservative effect on differentiated stem cells. The continuance of citalopram treatment increased the time and percentage of cell viability (Figure 4B).
Figure 4.
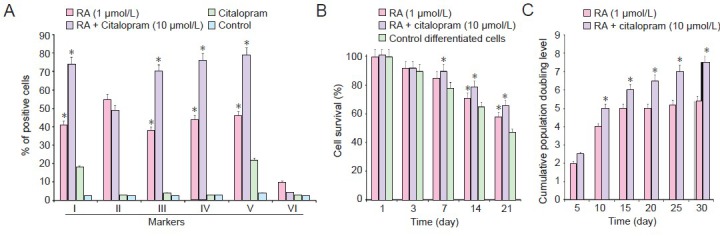
Characteristics of differentiated neuronal-like cells by BMSCs after 2 weeks of citalopram treatment.
Immunostaining showed that rates of nestin, MAP-2, β-tubulin and neurofilament L positive staining significantly increased compared to that in control (*P < 0.05) (A).rentiated by BMSCs in the presence of RA and citalopram compared to the only dimethyl sulfoxide pre-treatment (*P≤ 0.05) (B). Cell proliferation assays for neuronal-like cells with and without citalopram. Cumulative population doubling level of cells in cultures with and without citalopram. Cumulative population doubling level of citalopram-treated cells were significantly increased from day 15 until day 21 compared with RA-treated cells (*P < 0.05) (C). (A–C) Data are expressed as mean ± SEM. One-way analysis of variance (ANOVA) and two-tailed Student's t-test were used to analyze data. Each experiment was performed in triplicate. BMSCs: Bone marrow mesenchymal stem cells; MAP-2: microtubule-associated protein 2; GFAP: glial fibrillary acidic protein; RA: retinoic acid.
Cumulative population doubling level
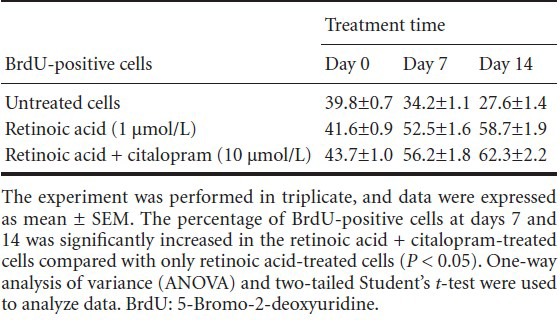
Cumulative population doubling level was measured in the presence and absence of citalopram to determine the proliferative potential of citalopram-treated NPs. After 30 days of continuous passages, 7.41 and 5.63 cumulative population doubling level units were found in retinoic acid + citalopram-treated and only retinoic acid cells, respectively (Figure 4C). When the cells were passaged at regular intervals (every 4–5 days), compared with only retinoic acid-treated cells, the cumulative population doubling level of retinoic acid + citalopram-treated cells was significantly increased at day 15 (P < 0.05; Figure 4C). To examine the citalopram effects on DNA synthesis, the numbers of BrdU-positive cells were counted at days 0, 7, and 14 after citalopram and RA treatment. The percentage of BrdU-positive cells at days 7 (56%) and 14 (62%) was significantly increased in the retinoic acid + citalopram-treated cells compared with only retinoic acid-treated cells (P < 0.05; Table 2).
Table 2.
BrdU-positive cells (percentage) derived from bone marrow mesenchymal stem cells after citalopram treatment versus control medium
Discussion
Considering the obtained evidence, we showed that citalopram, a clinically effective antidepressant, increases stem cell survival and differentiation into a neuronal-like cell. Degenerative diseases are increasingly epidemic. Regenerative pharmacology is a form of therapy aimed at repairing the progressive cell destruction in degenerative diseases. Stem cell-based regenerative pharmacology supports the use of stem cells for derivation of novel therapeutics. Adult stem cells can provide a useful model to test the therapeutic compounds and also can be used as models in regenerative pharmacology to test whether a specific drug promotes a lineage specific differentiation program (Shoae-Hassani et al., 2011a,c). Pharmacological agents could have inhibitory (Shoae-Hassani et al., 2011b) or stimulatory effect (Verdi et al., 2014) on the differentiation process of stem cells. There are many reports for selective serotonin reuptake inhibitor neurogenesis in vivo (Nandam et al., 2007; Newton and Duman 2007) but there was no study to survey the selective serotonin reuptake inhibitor effect on adult mesenchymal stem cell fate. The in vitro study was conducted to determine whether the neurogenesis could be influenced by citalopram, a selective serotonin reuptake inhibitor antidepressant in cell culture system.
Citalopram and retinoic acid-treated human mesenchymal stem cells were determined by fluorescence microscopy for detection of neuronal cell markers. Numerous cells immunoreactive for Tuj1, MAP-2 and nestin were seen in the immunostaining. There was no expression of GFAP in citalopram treated cells. The observed cellular characteristics and differentiation patterns demonstrate that citalopram enhanced the neuronal rather than the glial properties. The markers increased by citalopram were early neuronal cell markers, such as nestin. In 2011, Li et al. described rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. It is obvious that these molecules have direct or indirect effects on some cellular pathways. The works by Menendez et al. (2011) confirmed that Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Previous studies by Rahmani et al. (2013a) showed that 10 μmol/L of fluoxetine upregulated phosphorylation of Akt1 protein in induced stem cells significantly.
RT-PCR assay was used to detect the expression of neurofilament L, synaptophysin and nestin genes. There was no expression of glial cell marker GFAP, as confirmed by immunocytochemical staining. Expression of neuron-specific genes was up-regulated and remained elevated after 7 days in the retinoic acid + citalopram-treated group. Cells immunoreactive for neuronal cell markers showed neuronal-like cell appearance, i.e., large spherical cell body and extension. The percentage of differentiated neuronal cells reached to more than 70% in 14 days in the retinoic acid + citalopram-treated group compared to 50% of stem cells reaching a neuronal phenotype by 7 days in vitro induced just with retinoic acid (Figure 4A).
The effect of citalopram on the survival period of the differentiated stem cells was observed directly and investigated by MTT assay. We found that cell survival rate significantly higher following citalopram treatment than RA treated groups. While the retinoic acid increased differentiated neuronal cell survival in this assay, the effect of citalopram on differentiated stem cells was preservation. The continuance of citalopram treatment extended the time and percentage of cell viability. The obtained results are similar to those of Chen et al. (2007) who observed cell proliferation under in vitro conditions after 7 days, though they used different cell types and culture conditions. In 2001, Duman et al. reported that the new cell birth occurs in the brain and the rate of neurogenesis and survival of new neurons are regulated by a number of environmental and pharmacological treatments. The possibility that the cAMP signal transduction cascade and expression of brain derived neurotrophic factor (BDNF) contribute to the regulation of neurogenesis by antidepressants is supported by Thome et al. (2000). Up-regulation of brain derived neurotrophic factor could contribute to neurotrophic effects of antidepressants, including regulation of adult neurogenesis.
The cumulative population doubling level was measured with and without citalopram to determine the proliferative potential of citalopram-treated neural progenitors. After 30 days of continuous passages, 7.41 and 5.63 cumulative population doubling level units were found in retinoic acid + citalopram-treated and retinoic acid-treated cells, respectively. When the cells were passaged at regular intervals (every 4–5 days), the cumulative population doubling level of retinoic acid + citalopram-treated cells was significantly increased at day 15. For this analysis, the cells were passaged and they survived for up to 30 days. The proliferation rate of retinoic acid-treated cells exhibited a plateau from day 15 onward, while the plateau phase was on day 25 for retinoic acid + citalopram-treated cells. This finding suggests that the proliferation rate was higher in retinoic acid + citalopram-treated cells than in only retinoic acid-treated cells.
To examine the effects of citalopram on DNA synthesis, the numbers of BrdU-positive cells were counted at days 0, 7, and 14 after retinoic acid + citalopram treatment and only retinoic acid treatment. The percentage of BrdU-positive cells at days 7 (56%) and 14 (62%) was significantly increased in the retinoic acid + citalopram-treated cells compared to only retinoic acid-treated cells.
Studies of antidepressants have focused on cellular pathways, which are known to be activated by a number of extracellular signals, such as growth factors and neurotransmitters (Malberg 2004; Malberg and Blendy 2005). These signals regulate cellular processes associated with neuroplasticity and neurogenesis and seem necessary to mediate the therapeutic effects of citalopram.
To summary, our study elucidates the effect of citalopram on the neurogenesis of adult stem cells and confirms that it can increase the survival rate of the differentiated cells by adult stem cells as a therapeutic and regenerative drug.
Acknowledgments:
We would like to thank Shoa-Hasani A for the helpful comments and are also grateful for the time and patience of the anonymous referees.
Footnotes
Conflicts of interest: None declared.
Funding: This work was funded by the Research Center for Science and Technology in Medicine (RCSTiM), Tehran University of Medical Sciences, Tehran (TUMS), Tehran, Iran.
Copyedited by Widgerow A, Jin GH, Li YL, Li CH, Song LP, Zhao M
References
- 1.Atkinson S, Lako M, Armstrong I. Potential for pharmacological manipulation of embryonic stem cells. Br J Pharmacol. 2012;169:269–289. doi: 10.1111/j.1476-5381.2012.01978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bissonnette CJ, Lyass L, Bhattacharyya BJ, Belmadani A, Miller RJ, Kessler JA. The controlled generation of functional basal forebrain cholinergic neurons from human embryonic stem cells. Stem Cells. 2011;29:802–811. doi: 10.1002/stem.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen SJ, Kao CL, Chang YL, Yen CJ, Shui JW, Chien CS, Chen IL, Tsai TH, Ku HH, Chiou SH. Antidepressant administration modulates neural stem cell survival and serotoninergic differentiation through bcl-2. Curr Neurovasc Res. 2007;4:19–29. doi: 10.2174/156720207779940707. [DOI] [PubMed] [Google Scholar]
- 5.Chung S, Moon JI, Leung A, Aldrich D, Lukianov S, Kitayama Y. ES cell-derived renewable and functional midbrain dopaminergic progenitors. Proc Natl Acad Sci USA. 2011;108:9703–9708. doi: 10.1073/pnas.1016443108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croce N, Mathé AA, Gelfo F, Caltagirone C, Bernardini S, Angelucci F. Effects of lithium and valproic acid on BDNF protein and gene expression in an in vitro human neuron-like model of degeneration. J Psychopharmacol. 2014 doi: 10.1177/0269881114529379. doi: 10.1177/0269881114529379. [DOI] [PubMed] [Google Scholar]
- 7.Duman RS, Nakagawa S, Malberg J. Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology. 2001;25:836–844. doi: 10.1016/S0893-133X(01)00358-X. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto S, Inoue T, Muraki I, Koyama T. Effects of acute citalopram on the expression of conditioned freezing in naïve versus chronic citalopram-treated rats. Prog Neuropsychopharmacol Biol Psych. 2009;33:113–117. doi: 10.1016/j.pnpbp.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Huang B, Li K, Yu J, Zhang M, Li Y, Li W, Wang W, Guan L, Zhang W, Lin S, Huang X, Lin L, Lin Y, Zhang Y, Song X, Wang Z, Ge J. Generation of human epidermis-derived mesenchymal stem cell-like pluripotent cells (hEMSCPCs) Sci Reports. 2013 doi: 10.1038/srep01933. doi:10.1038/srep01933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilic D, Stephenson E, Wood V, Jacquet L, Stevenson D, Petrova A. Derivation and feeder-free propagation of human embryonic stem cells under xeno-free conditions. Cytotherapy. 2011;14:122–128. doi: 10.3109/14653249.2011.623692. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Su SC, Wang H, Cheng AW, Cassady JP, Lodato MA. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell. 2011;9:413–419. doi: 10.1016/j.stem.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Sun W, Zhang Y, Wei W, Ambasudhan R, Xia P. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc Natl Acad Sci U S A. 2011;108:8299–8304. doi: 10.1073/pnas.1014041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin TM, Tsai JL, Lin SD, Lai CS, Chang CC. Accelerated growth and prolonged lifespan of adipose tissue-derived human mesenchymal stem cells in a medium using reduced calcium and antioxidants. Stem Cells Dev. 2005;14:92–102. doi: 10.1089/scd.2005.14.92. [DOI] [PubMed] [Google Scholar]
- 15.Malberg JE. Implications of adult hippocampal neurogenesis in antidepressant action. J Psychiatry Neurosci. 2004;29:196–205. [PMC free article] [PubMed] [Google Scholar]
- 16.Malberg JE, Blendy JA. Antidepressant action: to the nucleus and beyond. Trends Pharmacol Sci. 2005;26:631–638. doi: 10.1016/j.tips.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menendez L, Yatskievych TA, Antin PB, Dalton S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci U S A. 2011;108:19240–19245. doi: 10.1073/pnas.1113746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nandam LS, Jhaveri D, Bartlett P. 5-HT7, neurogenesis and antidepressants: a promising therapeutic axis for treating depression. Clin Exp Pharmacol Physiol. 2007;34:546–551. doi: 10.1111/j.1440-1681.2007.04608.x. [DOI] [PubMed] [Google Scholar]
- 20.Newton SS, Duman RS. Neurogenic actions of atypical antipsychotic drugs and therapeutic implications. CNS Drugs. 2007;21:715–725. doi: 10.2165/00023210-200721090-00002. [DOI] [PubMed] [Google Scholar]
- 21.Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci U S A. 2011;108:16825–16830. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noureddini M, Verdi J, Mortazavi-Tabatabaei SA, Sharif S, Azimi A, Keyhanvar P, Shoae-Hassani A. Human endometrial stem cell neurogenesis in response to NGF and bFGF. Cell Biol Int. 2012;36:961–966. doi: 10.1042/CBI20110610. [DOI] [PubMed] [Google Scholar]
- 23.Qin Y, Lin J, Zhou C, Yin Q, Xie Z, Zhang X, Liu XY, Gao W, Li J. Mice cloned from white adipose tissue-derived cells. J Mol Cell Biol. 2013;5:348–350. doi: 10.1093/jmcb/mjt019. [DOI] [PubMed] [Google Scholar]
- 24.Rahmani A, Kheradmand D, Keyhanvar P, Shoae-Hassani A, Darbandi-Azar A. Neurogenesis and increase in differentiated neural cell survival via phosphorylation of Akt1 after fluoxetine treatment of stem cells. BioMed Res. 2013a doi: 10.1155/2013/582526. Int ID 582526. dx.doi.org/10.1155/2013/582526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahmani A, Shoae-Hassani A, Keyhanvar P, Kheradmand D, Darbandi-Azar A. Dehydroepiandrosterone stimulates nerve growth factor and brain derived neurotrophic factor in cortical neurons. Adv Pharmacol Sci. 2013b doi: 10.1155/2013/506191. ID 506191. dx.doi.org/10.1155/2013/506191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki M, Honmou O, Akiayama Y, Uede T, Hashi K, Kocsis JD. Transplantation of an acutely isolated bone marrow fraction repairs demyelinated adult rat spinal cord axons. Glia. 2001;35:26–34. doi: 10.1002/glia.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwaarz EJ, Alexander GM, Prockop DJ, Azizi SA. Multipotential marrow stromal cells transduced to produce L-DOPA: engraftment in a rat model of Parkinson disease. Hum Gene Ther. 1999;10:2539–2549. doi: 10.1089/10430349950016870. [DOI] [PubMed] [Google Scholar]
- 28.Shoae-Hassani A, Keyhanvar P, Seifalian AM, Mortazavi-Tabatabaei SA, Ghaderi N, Amirmozafari N. λ Phage nanobioparticle expressing apoptin efficiently suppress human breast carcinoma tumor growth in vivo. PLoS One. 2013a;8:e79907. doi: 10.1371/journal.pone.0079907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shoae-Hassani A, Mortazavi-Tabatabaei SA, Sharif S, Rezaei-Khaligh H, Verdi J. DHEA provides a microenvironment for endometrial stem cells neurogenesis. Med Hypotheses. 2011a;76:843–846. doi: 10.1016/j.mehy.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 30.Shoae-Hassani A, Mortazavi-Tabatabaei SA, Sharif S, Seifalian AM, Azimi A, Samadikuchaksaraei A, Verdi J. Differentiation of human endometrial stem cells into urothelial cells on a three-dimensional nanofibrous silk–collagen scaffold: an autologous cell resource for reconstruction of the urinary bladder wall. J Tissue Eng Regen Med. 2013 doi: 10.1002/term.1632. doi:101002/term1632. [DOI] [PubMed] [Google Scholar]
- 31.Shoae-Hassani A, Sharif S, Mortazavi-Tabatabaei SA, Verdi J. Could the endogenous opioid morphine prevent neural stem cell proliferation? Med Hypotheses. 2011b;76:225–229. doi: 10.1016/j.mehy.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Shoae-Hassani A, Sharif S, Seifalian AM, Mortazavi-Tabatabaei SA, Rezaie S, Verdi J. Endometrial stem cell differentiation into smooth muscle cell: a novel approach for bladder tissue engineering in women. BJU Int. 2013c;112:854–863. doi: 10.1111/bju.12195. [DOI] [PubMed] [Google Scholar]
- 33.Shoae-Hassani A, Sharif A, Verdi J. The neurosteroid dehydroepiandrosterone could improve somatic cell reprogramming. Cell Biol Int. 2011c;35:1037–1041. doi: 10.1042/CBI20100927. [DOI] [PubMed] [Google Scholar]
- 34.Sollberger M, Rosen HJ, Shany-Ur T, Ullah J, Stanley CM, Laluz V, Weiner MW, Wilson SM, Miller BL, Rankin KP. Neural substrates of socioemotional self-awareness in neurodegenerative disease. Brain Behav. 2014;4:201–214. doi: 10.1002/brb3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thome J, Sakai N, Shin K, Steffen C, Zhang YJ, Impey S, Storm D, Duman RS. cAMP response element mediated gene transcription is up-regulated by chronic antidepressant treatment. J Neurosci. 2000;20:4030–4036. doi: 10.1523/JNEUROSCI.20-11-04030.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verdi J, Shari S, Banafshe HR, Shoae-Hassani A. Sertraline increases the survival of retinoic acid induced neuronal cells but not glial cells from human mesenchymal stem cells. Cell Biol Int. 2014 doi: 10.1002/cbin.10283. doi:10.1002/cbin.10283. [DOI] [PubMed] [Google Scholar]
- 37.Viswanathan S, Rao M, Keating A, Srivastava A. Overcoming challenges to initiating cell therapy clinical trials in rapidly developing countries: India as a model. Stem Cells Transl Med. 2013;2:607–613. doi: 10.5966/sctm.2013-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


