Recent studies have shown that the glycation-associated damage is not limited to patients with diabetes. Because of their association with oxidative stress, advanced glycation end-products (AGEs) have also been implicated in many neurodegenerative diseases, such as Huntington disease, amyotrophic lateral sclerosis, and Alzheimer disease (Yan et al., 1996; Ma and Nicholson, 2004). As in other age-dependent neurodegenerative diseases of the brain, oxidative stress-associated age-dependent pathogenic processes are not unexpected in glaucoma because this disease is also more common in the elderly (Tezel et al. 2007).
Growing evidence indicates that neuronal abnormalities including neuronal cell death are associated with the pathogenesis of early diabetic retinopathy. Our previous studies of human retinas indicate that mitochondrial- and caspase-dependent cell death pathways are associated with retinal neuronal cell degeneration in patients with diabetes (Oshitari et al., 2008). Brain-derived neurotrophic factor, neurotrophin-4 (NT-4), vascular endothelial growth factor (VEGF)120, VEGF164, taurine-conjugated ursodeoxycholic acid and citicoline had been studied and showed a survival effect on damaged retinal neurons induced by diabetic stress (Oshitari et al. 2003, 2010). It was also found that NT-4 had the best neuroprotective and regenerative effect under high glucose conditions (Oshitari et al, 2010). Earlier study indicated that the maximum rescue ratio of caspase-1, -3, -8, and -9 inhibitors in cultured retinas was 60% in damaged retinal ganglion cells (RGCs) (Oshitari and Adachi-Usami, 2003). Thus, at least 40% of neuronal cell death in damaged RGCs in cultured retinas should be related to caspase-independent cell death mechanisms. However, no reports have focused on caspase-independent cell death pathways under diabetic stress including AGEs exposure. Apoptosis-inducing factor (AIF) was the first mitochondrial protein shown to mediate cell death independent of caspase (Susin et al., 1996, 1999). It was initially characterized as a mitochondrial protein confined in the intermembrane space of healthy cells. In healthy cells, AIF is a mitochondrial flavin adenine dinucleotide (FAD)-dependent oxidoreductase that plays roles in redox control and oxidative phosphorylation (Modjtahedi et al., 2006).
AGEs impact on neurite regeneration
The aim of our study was to determine the effect of low dose AGEs on neurite regeneration in isolated rat retinas and to estimate the regenerative effects of NT-4 in AGEs exposed retinas. In addition, immunohistochemistry was performed to examine whether AIF and active-forms of caspase-9 expression were related to the neuronal cell death induced by AGEs exposure in cultured retinas (Bikbova G et al, 2013).
For that purpose rat retinal explants were three-dimensionally cultured in collagen gel (Figure 1) and were incubated either in: (1) serum-free control culture media; (2) 10 µg/mL glucose-AGE-BSA media; (3) 10 µg/mL glycolaldehyde-AGE-BSA media; (4) 10 µg/mL glyceraldehyde-AGE-BSA media; (5) 10 µg/mL glucose-AGE-BSA+100 ng/mL NT-4 media; (6) 10 µg/mL glycolaldehyde-AGE-BSA+100 ng/mL NT-4 media; (7) 10 µg/mL glyceraldehyde-AGE-BSA+100 ng/mL NT-4 supplemented culture media; (8) 100 ng/mL NT-4 supplemented media without AGE-BSA; or (9) 10 µg/mL glucose-AGE-BSA+2 µM caspase-9 inhibitor supplemented culture media. The 10 µg/mL glycated BSA was selected to investigate the effect of a very low dose of AGEs on neurite regeneration (Bikbova et al, 2013). The results of earlier studies (Lecleire-Collet et al, 2005) demonstrated that the AGEs were neurotoxic, and 100 µg/mL and 250 µg/mL of AGEs increased the expression of VEGF in the retina (Green and Kroemer, 2004). For examining the dose-dependent effect of each AGE-BSA on neuronal cell death and neurite regeneration, 100 µg/mL glucose-AGE-BSA, 100 µg/mL glycolaldehyde-AGE-BSA, or 100 µg/mL glyceraldehyde-AGE-BSA were incubated in serum-free media.
Figure 1.
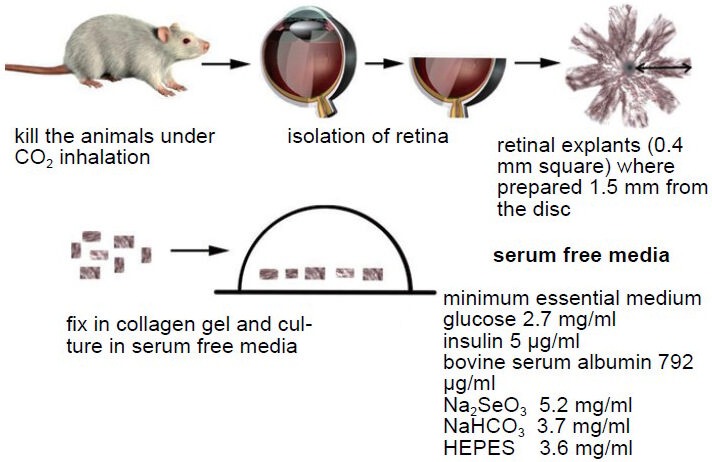
Three-dimensional collagen gel culture of retinal explants.
Retinas were isolated under sterile conditions, and cut into square pieces with a sharp razor blade. The retinal explants embedded into type I liquid collagen solution on ice. After warming at 37°C, the retinal explants were fixed in collagen gels. The explants were cultured in se-rum-free media at 37°C and exposed to 5% CO2.
The culture of retinal explants in a three-dimensional collagen gel culture system is accompanied by the axotomy of RGCs, together with the disturbance in the cellular environment during its manipulation (Oshitari and Adachi-Usami, 2003). Neurite regeneration from some RGCs of adult retinal tissue embedded in collagen gel has been observed. This finding indicated that some RGCs in adult retina have the capability of neural regeneration after axonal injury. The regeneration ability of damaged RGCs is used in our study to investigate the impact of different agents as AGEs.
Our results showed that even a low concentration of AGEs, e.g., 10 µg/mL, induces neuronal apoptosis in the ganglion cell layer (GCL) and decreases the number of regenerated neuritis (Bikbova et al, 2013). Earlier studies showed that between 1 to 120 µg/mL of AGEs (approximately 4–480 µg/mL of glycated BSA) was circulating in diabetic patients (Ono et al., 1998). Thus, the accumulation of AGEs in the retina could induce neuronal apoptosis in diabetic retinas more easily than in normal retinas. Endogenous neurotrophic factors such as VEGF are also up-regulated in diabetic patients. Several clinical trials have demonstrated that anti-VEGF therapies are helpful in the treatment of diabetic macular edema (Zechmeister-Koss and Huic, 2012). However, these studies did not evaluate the neuronal damage after repeated intravitreal injections of anti-VEGF drugs in diabetic patients. The imbalance between the toxic effect of the AGEs and the endogenous neurotrophic factors may cause retinal neuronal death in eyes with diabetic retinopathy. In high-dose (100 µg/mL) AGEs exposed retinas, the number of TUNEL-positive cells did not increase compared to in low-dose (10 µg/mL) AGEs exposed retinas. On the other hand, high-dose AGEs significantly impeded neurite regeneration compared to low-dose AGEs. Although the mechanisms of impeding neurite regeneration in high-dose AGEs exposed retinas are still unclear, our results suggest that the reduction of axon regrowth in high-dose AGEs exposed retinas could not be a consequence of increasing cell death and that regeneration is more sensitive than neuronal cell death in AGEs exposed retinal neurons. Our results also showed that the retinal neuronal death in AGE-BSA exposed rat retinas was correlated with the increased expression of active-form of caspase-9 and AIF. The majority of the cells that were immunopositive to AIF in the GCL were detected in the nucleus in cultured retinas. These results show that AIF is translocated in the nucleus and maybe involved in DNA fragmentation and chromatin condensation during the process of apoptosis in AGE-BSA exposed rat retinas. The active form of caspase-9 in the GCL was also detected mainly in the nucleus, and thus, both caspase-9 and AIF are translocated into the nucleus during the process of apoptosis in cultured retinas. To examine whether caspase-9 and AIF activation have biochemical links, we incubated caspase-9 inhibitor in cultured retinas and performed immunostaining of active form of caspase-9 and AIF. Caspase-9 inhibitor significantly reduced caspase-9-immunopositive cells but did not affect the number of AIF-immunopositive cells in the GCL. These results indicate that there maybe no biochemical links between AIF and caspase-9 activation in AGEs exposed retinas. Although caspase-9 is known to cleave Ring1B, one of the polycomb group proteins in the nucleus (Wong et al., 2007), further studies are needed to determine the precise role of AIF and caspase-9 in the nucleus during the process of apoptosis in AGEs exposed retinal neurons.
Perspectives
Finding a way to decrease the impact of the AGEs on the retina is important, and anti-AGEs drugs are now being intensively studied. Several studies suggested aminoguanidine as potential inhibitor of the conversion of early products to AGEs (Gul et al., 2008) or pyridoxamine as an efficacious and specific post-amadori inhibitor that reduces the accumulation of retinal AGEs (Stitt et al., 2002). AGEs cross-link breakers were described as attackers of AGEs-derived protein crosslinking, and treatment with this drug reduced vascular stiffening in experimental diabetes (Vasan et al., 2003). Although the effects of AGEs breakers on diabetic retinopathy have yet to be evaluated, the preliminary experimental studies suggested that AGEs formation and activation of AGEs-receptors represent important, interconnected pathogenic mechanisms in diabetic retinopathy. Thus, inhibition of these pathways could be a valid avenue for therapeutic exploitation.
Our study showed that NT-4 reduced the retinal neuronal apoptosis induced by AGEs and increased the number of regenerated neurites. The neuroprotective and regenerative effects of NT-4 were correlated with the reduction of caspase-9 and activation of AIF. Ghazi-Nouri et al. showed that NT-4, but not BDNF, was up-regulated in Müller cells of patients with proliferative vitreoretinopathy (Ghazi-Nouri et al., 2008). Their results including ours indicate that NT-4 may be one of suitable neurotrophic factors that protects retinal neurons from injury and is neuroprotective as well as neuroregenerative in the retina. Recent studies indicate that not only NT-4 but also other anti-apoptotic factors such as 70 kDa heat-shock protein (Sabirzhanov et al., 2012) or TAT-Bcl-xL fusion protein (Yin et al., 2006) attenuate both caspase-dependent and caspase-independent cell death pathways. NT-4 binds the neurotrophin-receptor TrkB and activates signal transduction pathways including phosphatidyl inositol-3 (PI3)-kinase pathway (PI3-kinase-Akt pathway) (Reichardt, 2006). The protein kinase Akt phosphorylates BAD, a proapoptotic Bcl-2-family member that binds Bcl-xL followed by preventing Bcl-xL and inhibiting the activity of Bax. The previous study indicates that TrKB receptor is colocalized with NT-4 on the mitochondrial membrane and that NT-4 plays a crucial role for mitochondrial function (Wiedemann et al., 2006). Although it is a speculation, NT-4 may be involved in regulating mitochondrial membrane permeability and preventing the release of AIF and procaspase-9 from mitochondria in AGEs exposed retinal neurons. Further studies should be established to determine the NT-4 promoted neuronal survival mechanism and regeneration under AGEs stress.
In conclusion, even low concentrations of AGEs are neurotoxic and inhibit regeneration retinal neuron's neurites. High-dose AGEs significantly impede neurite regeneration independent of increasing neuronal cell death. Although the mechanisms of impeding neurite regenerartion of high-dose AGEs are still unclear, the neurotoxic effect of AGEs is, in part, associated with increased expression of the active form of caspase-9 and AIF. NT-4 significantly enhances neurite regeneration in retinas exposed to AGE. The neuroprotective and regenerative effects of NT-4 are correlated with the reduction of AIF and caspase-9 expression.
Footnotes
Funding: This study is supported by a Grant-in Aid from the Ministry of Education, Science, Sports and Culture of the Japanese Government.
Conflicts of interest: None declared.
References
- 1.Bikbova G, Oshitari T, Yamamoto S. Neurite regeneration in advanced glycation end-products exposed adult rat retinas and regenerative effects of neurotrophin-4. Brain Res. 2013;1543:33–45. doi: 10.1016/j.brainres.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 2.Ghazi-Nouri S, Ellis J, Moss S, Limb G, Charteris D. Expression and localization of BDNF, NT4 and TrkB in proliferative vitreoretinopathy. Exp Eye Res. 2008;86:819–827. doi: 10.1016/j.exer.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 4.Ma L, Nicholson L. Expression of the receptor for advanced glycation end products in Huntington's disease caudate nucleus. Brain Res. 2004;1018:10–17. doi: 10.1016/j.brainres.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 5.Modjtahedi N, Giordanetto F, Madeo F, Kroemer G. Apoptosis-inducing factor: vital and lethal. Trends Cell Biol. 2006;16:264–272. doi: 10.1016/j.tcb.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Lecleire-Collet A, Tessier L, Massin P, Forster V, Brasseur G, Sahel J, Picaud S. Advanced glycation end products can induce glial reaction and neuronal degeneration in retinal explants. Br J Ophthalmol. 2005;89:1631–1633. doi: 10.1136/bjo.2005.079491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ono Y, Aoki S, Ohnishi K, Yasuda T, Kawano K, Tsukada Y. Increased serum levels of advanced glycation end-products and diabetic complications. Diabetes Res Clin Pract. 1998;41:131–137. doi: 10.1016/s0168-8227(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 8.Oshitari T, Adachi-Usami E. The effect of caspase inhibitors and neurotrophic factors on damaged retinal ganglion cells. Neuroreport. 2003;14:289–292. doi: 10.1097/00001756-200302100-00027. [DOI] [PubMed] [Google Scholar]
- 9.Oshitari T, Hanawa K, Adachi-Usami E. Changes of macular and RNFL thick-nesses measured by Stratus OCT in patients with early stage diabetes. Eye. 2009;23:884–889. doi: 10.1038/eye.2008.119. [DOI] [PubMed] [Google Scholar]
- 10.Oshitari T, Yamamoto S, Hata N, Roy S. Mitochondria- and caspase-dependent cell death pathway involved in neuronal degeneration in diabetic retinopathy. Br J Ophthalmol. 2008;92:552–556. doi: 10.1136/bjo.2007.132308. [DOI] [PubMed] [Google Scholar]
- 11.Oshitari T, Yoshida-Hata N, Yamamoto S. Effect of neurotrophic factors on neuronal apoptosis and neurite regeneration in cultured rat retinas exposed to high glucose. Brain Res. 2010;1346:43–51. doi: 10.1016/j.brainres.2010.05.073. [DOI] [PubMed] [Google Scholar]
- 12.Reichardt L. Neurotrophin-regulated signaling pathways. Philos Trans R Soc London B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabirzhanov B, Stoica B, Hanscom M, Piao C, Faden A. Over-expression of HSP70 attenuates caspase- dependent and caspase-independent pathways and inhibits neuronal apoptosis. J Neurochem. 2012;123:542–554. doi: 10.1111/j.1471-4159.2012.07927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Susin SA, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996;184:1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 16.Stitt A, Gardiner TA, Alderson NL, Canning P, Frizzell N, Duffy N, Boyle C, Januszewski AS, Chachich M, Baynes JW, Thorpe SR. The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes. 2002;51:2826–2832. doi: 10.2337/diabetes.51.9.2826. [DOI] [PubMed] [Google Scholar]
- 17.Tezel G, Chauhan B, LeBlanc R, Wax M. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Invest Ophthalmol Vis Sci. 2003;44:3025–3033. doi: 10.1167/iovs.02-1136. [DOI] [PubMed] [Google Scholar]
- 18.Vasan S, Foiles P, Founds H. Therapeutic potential of breakers of advanced glycation end product-protein crosslinks. Arch Biochem Biophys. 2003;419:89–96. doi: 10.1016/j.abb.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Wiedemann F, Siemen D, Mawrin C, Horn T, Dietzmann K. The neurotrophin receptor TrkB is colocalized to mitochondrial membranes. Int J Biochem Cell Biol. 2006;38:610–620. doi: 10.1016/j.biocel.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 21.Yin W, Cao G, Johnnides M, Signore A, Luo Y, Hickey R, Chen J. TAT-mediated delivery of Bcl-xL proteinis neuroprotective against neonatal hypoxic-ischemic brain injury via inhibition of caspases and AIF. Neurobiol Dis. 2006;21:358–371. doi: 10.1016/j.nbd.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Wong C, Chen Z, So K, Li D, Li P. Polycombgroup protein RING1 B is a direct substrate of Caspases-3 and-9. Biochim Biophys Acta. 2007;1773:844–852. doi: 10.1016/j.bbamcr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Zechmeister-Koss I, Huic M. Vascular endothelial growth factor inhibitors (anti-VEGF) in the management of diabetic macular oedema: as systematic review. Br J Ophthalmol. 2012;96:167–178. doi: 10.1136/bjophthalmol-2011-300674. [DOI] [PubMed] [Google Scholar]


