Abstract
Non-invasive brain stimulations mainly consist of repetitive transcranial magnetic stimulation and transcranial direct current stimulation. Repetitive transcranial magnetic stimulation exhibits satisfactory outcomes in improving multiple sclerosis, stroke, spinal cord injury and cerebral palsy-induced spasticity. By contrast, transcranial direct current stimulation has only been studied in post-stroke spasticity. To better validate the efficacy of non-invasive brain stimulations in improving the spasticity post-stroke, more prospective cohort studies involving large sample sizes are needed.
Keywords: reviews, muscle spasticity, motor cortex, transcranial magnetic stimulation, transcranial direct current stimulation, central nervous system, stroke
Introduction
Spasticity is one of the features of upper motor neuron syndrome and an important cause of disability in patients with central nervous system (CNS) disorders affecting the motor cortex or corticospinal tract. Examples of such disorders include multiple sclerosis (MS) (Oreja-Guevara et al., 2013), spinal cord injury (SCI) (Levi et al., 1995; Skold et al., 1999), brain injury due to stroke (Wissel et al., 2013) or cerebral palsy (CP) (Reid et al., 2011). Spasticity is defined as a velocity-dependent increase in muscle tone, and presents with exaggerated tendon jerks, clonus, and spasms, which result from hyperexcitability of the stretch reflex (Lance, 1980). Spasticity may be generalized, focal or multifocal. It may restrict range of movements, dexterity and may cause abnormal postures and pain which may interfere with patients’ activity of daily living or maintenance of hygiene (Zorowitz et al., 2013). Other problems caused by spasticity like fatigue, psychosocial and behavioural limitations further increase the disability. Spasticity affects approximately 50–80% of patients with muscle spasticity (Barnes et al., 2003; Oreja-Guevara et al., 2013), 4–50% of patients with stroke (Wissel et al., 2013), 20–50% of patients with CP and 60–80% of patients with SCI patients (Levi et al., 1995; Skold et al., 1999). There are different pharmacological management strategies for spasticity control, and non-invasive brain stimulations could be one of various non-pharmacological management strategies for spasticity control (Centonze et al., 2007; Mori et al., 2009; Kumru et al., 2010).
Transcranial magnetic stimulation (TMS) is a neurostimulation and neuromodulation technique, based on the principle of electromagnetic induction of an electric field in the discrete brain regions (Kobayashi and Pascual-Leone, 2003; Rossi et al., 2009). Repetitive TMS (rTMS) is a non-invasive technique that induces changes in cortical excitability at the site of stimulation and transsynaptically at distant sites. Modulation of excitability at the directly targeted brain region depends on the frequency, intensity and the pattern of stimulation. The induced effects promote inhibition or excitation of the stimulated area (Berardelli et al., 1998; Pascual-Leone et al., 1994, 1998; Wassermann and Lisanby, 2001; Romero et al., 2002; Kobayashi and Pascual-Leone, 2003; Siebner and Rothwell, 2003; Rossi et al., 2009). Trains of rTMS pulses can induce modification of activity in the targeted brain region, which can last for minutes or even hours.
When multiple stimuli of TMS are delivered in trains, one can differentiate “conventional” and “patterned” protocols of repetitive stimulation (Rossi et al., 2009). The term ‘fast’ or ‘high-frequency’ rTMS refers to stimulus rates of more than 1 Hz, and the term ‘slow’ or ‘low-frequency’ rTMS refers to stimulus rates of 1 Hz or less. Such a classification is based on the different physiological effects and degrees of risk associated with low- and high-frequency rTMS stimulation.
Patterned rTMS refers to repetitive application of short rTMS bursts at a high inner frequency interleaved by short pauses of no stimulation. Theta burst stimulation protocols in which short bursts of 50 Hz rTMS are repeated at a rate in the theta range (5 Hz) as a continuous (cTBS), or intermittent (iTBS) train (Huang et al., 2005; Di Lazzaro et al., 2008).
rTMS or TBS can be directly used to facilitate recovery. Lasting inhibitory after-effects of 1 Hz rTMS and cTBS and facilitatory after-effects following high-frequency rTMS and iTBS were found on motor corticospinal output in healthy subjects (Kobayashi and Pascual-Leone, 2003; Rossi et al., 2009). Various mechanisms have been considered, including synaptic changes resembling experimental long term depression (LTD) and long term potentiation (LTP) mechanisms, as well as shifts in network excitability, activation of feedback loops, and activity-dependent metaplasticity (Gentner et al., 2008; Iezzi et al., 2008). LTP is a long-lasting enhancement in signal transmission between two neurons that results from stimulating them synchronously (Matsuzaki et al., 2004; Cooke and Bliss, 2006). LTP is widely considered to be one of the major cellular mechanisms that underlie learning and memory (Cooke and Bliss, 2006).
tDCS is a non-invasive technique of neuromodulation, which passes low amplitude direct electrical current (1–2 mA) through pad electrodes placed on the scalp to alter neuronal firing. The term ‘direct’ means the electrical flow travels from one electrode (anode, positive electrode) towards the other (cathode, negative electrode) (Nitsche and Paulus, 2000; Lefaucheur, 2008).
Neuronal changes in the underlying cerebral tissue start a few minutes after tDCS and the duration of the effects outlasts the period of stimulation (Nitsche and Paulus, 2000). Transcranial direct current stimulation (tDCS) is classified as anodal or cathodal according to the type of electrode applied over the target in cerebral cortex. While anodal tDCS elicits prolonged increases in the cortical excitability of the underlying brain area, cathodal stimulation shows opposite effects (Nitsche and Paulus, 2000, 2001). However, direction of polarising effects is strictly influenced by the orientation of dendrites and axons in the electrical field further (Nitsche and Paulus, 2000; Priori, 2003; Lefaucheur, 2008). Both animal and human studies showed approximately 50% of transcranially applied current passes through the skull (Rush and Driscoll, 1968; Dymond et al., 1975).
tDCS differs from other transcranial stimulation techniques as it does not trigger an action potential because of low current density. Therefore, it exerts modulation of spontaneous neuronal excitability. The mechanisms are believed to be changes in spontaneous neuronal firing rates, synaptic plasticity and non-synaptic plasticity and result in change of resting polarization of neurons. However, the evidence indicates that non-synaptic mechanisms, specifically shifts in resting membrane potential of pre- and post-synaptic neurons are responsible for the major effect (Nitsche et al., 2003). Overall, tDCS features highly portable and safe. It permits modulation of cortical excitability with reasonable topographic resolution and reliable experimental blinding. tDCS is a painless technique for electrically stimulating the brain with mild side effects such as a mild headache or itching on the electrode site.
Non-invasive brain stimulations could be one of various non-pharmacological management strategies for spasticity control (Centonze et al., 2007; Mori et al, 2009; Kumru et al., 2010). This review aimed to evaluate effectiveness and safety of non-invasive brain stimulations in neurological disorders.
Data Source and Methodology
A literature search was performed using the words “non-invasive brain stimulation (repetitive transcranial magnetic stimulation [rTMS] or theta burst stimulation or transcranial direct current stimulation [tDCS]” and “spasticity”. The search was limited to literatures published in English until October 2013 in Pubmed, Cochrane, MEDLINE, Embase, Scopus, Web of Science and PICARTA Databases. Included in this review were published papers that studied the effect of noninvasive brain stimulation on spasticity alone or in combination of any other treatment (e.g., physiotherapy and medication) in adult or pediatric patients.
The following information was extracted from each study: publication details, study design, patient population (age, aetiology, disease duration, and disability), details on spasticity (localization, severity), details of intervention, outcome measures, method of follow-up, and withdrawals.
There were several clinical and electrophysiological outcome measures, which were used by the authors in those reviewed papers.
For the clinical outcome measures:
(1) Spasticity evaluation: Ashworth or modified Ashworth scale (MAS), Spinal cord injury spasticity evaluation tool (SCI-SET); Spinal Cord Assessment Tool for Spastic Reflexes (SCAT); modified Penn spasm frequency scale (MPSFS); Leeds arm spasticity impact scale, visual analogue scale (VAS) for spasticity, Tardieu scale.
(2) Upper limb functioning: Fugl-Mayer Assessment (FMA), Wolf motor function test (WMFT).
(3) Gait: Timed up and go test, 10 meters walk test (10MWT), walking index for spinal cord injury (WISCI), Barthel test.
(4) Activities of daily living (ADL).
Electrophysiological measures were:
(1) Maximum H reflex and M wave amplitude ratio: gives information about the excitability changes in the spinal cord.
(2) T reflex: gives information about excitability changes in the spinal cord.
(3) Withdrawal reflex (nociceptive or flexor withdrawal reflex): spinal reflex intended to protect the body from damaging stimuli.
(4) Motor evoked potential (MEP) amplitude: shows integrity and excitability of corticospinal motor pathway.
Results
Effects of rTMS and tDCS on spasticity
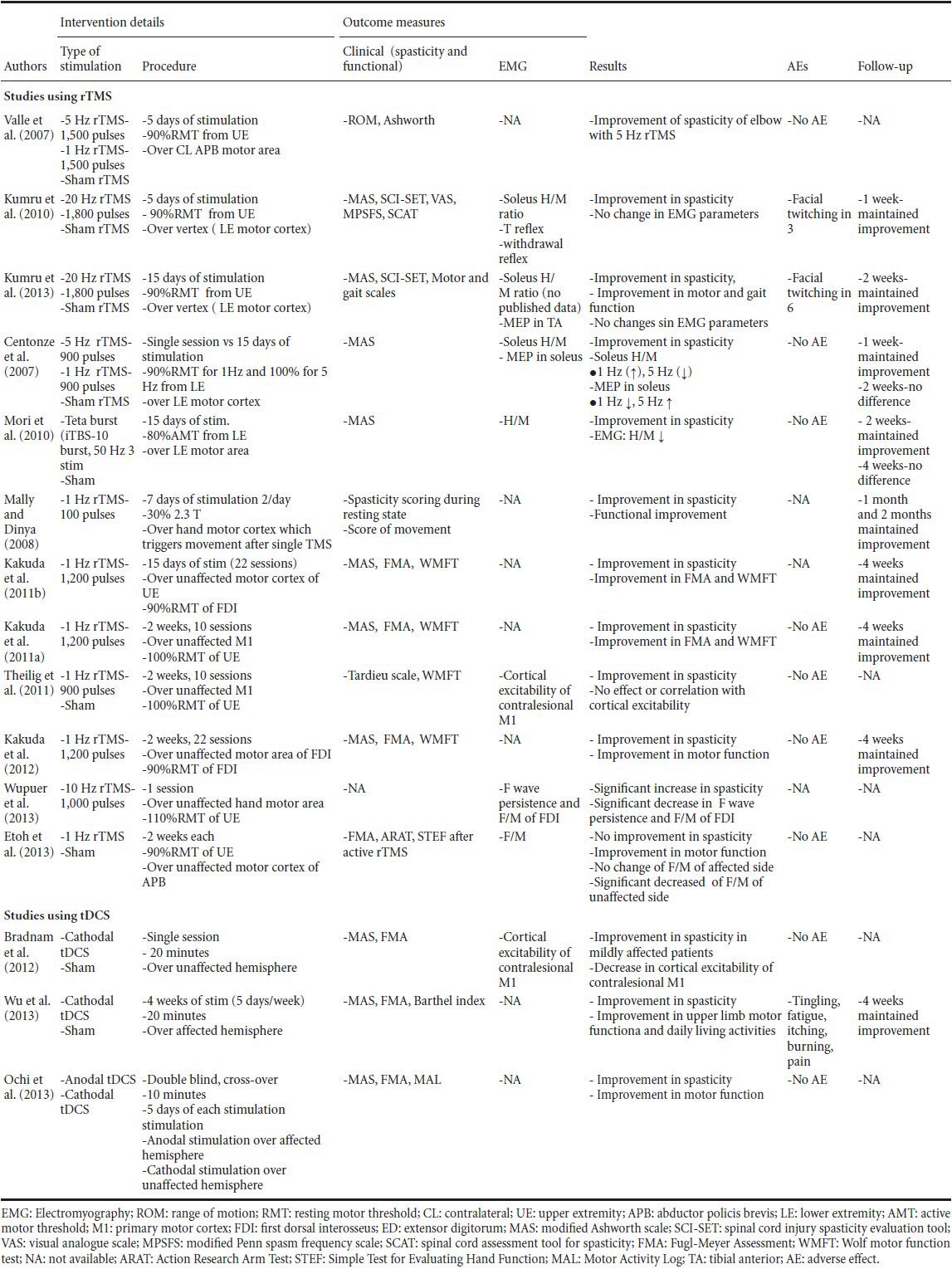
The effect of rTMS on spasticity was studied in patients with MS, stroke, SCI and CP. There were no studies regarding non-invasive brain stimulation on spasticity in ALS or after traumatic brain injury. Table 1 shows, patient population (age, aetiology, disease duration, and disability) and Table 2 shows details of non-invasive brain stimulation, outcome measures, results of changes especially in spasticity, side effects, follow-up, and withdrawals.
Table 1.
Demographical and clinical characteristics of the patients from the literature, which used non-invasive brain stimulation for spasticity
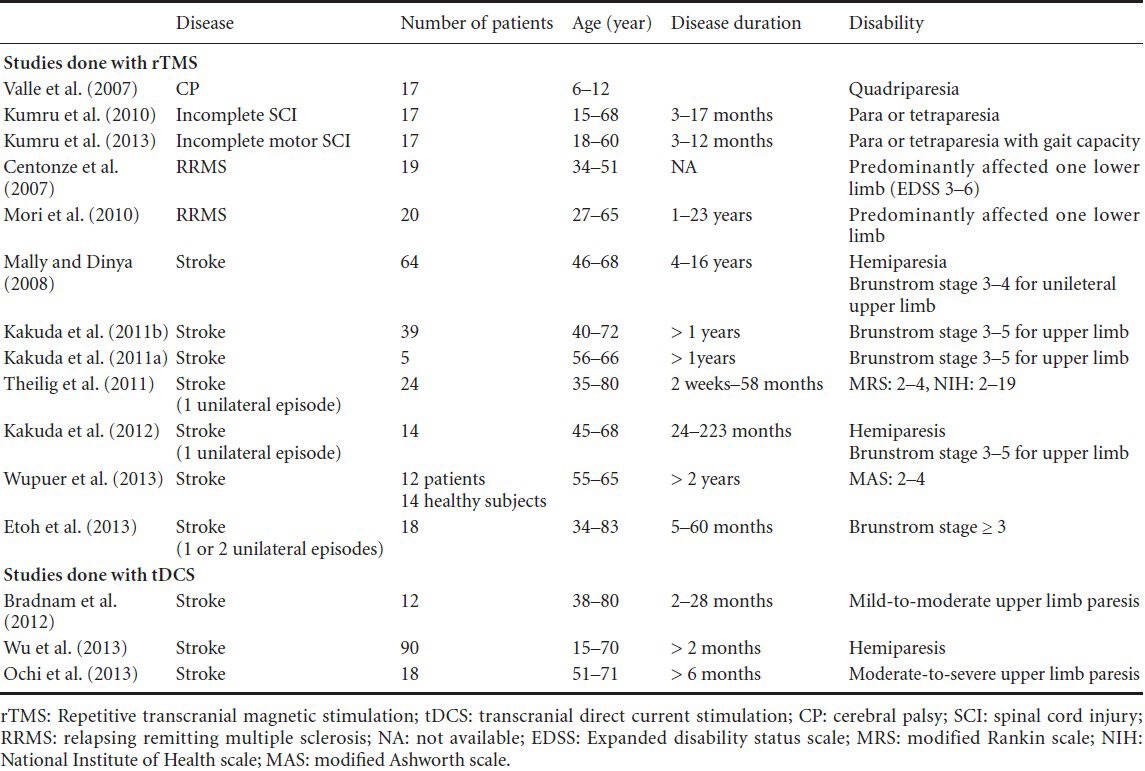
Table 2.
Non-invasive brain stimulation protocols and results of studies which used repetitive transcranial magnetic stimulation (rTMS) or transcranial direct current stimulation (tDCS) in spasticity
MS
Eight articles were found in the database after a search with the keywords: spasticity, MS and rTMS (Amatya et al, 2013; Centonze et al 2007; Mori et al, 2010; Mori et al; Nielsen et al, 1997; Nielsen et al, 1995a; Nielsen et al, 1995b; Nielsen et al, 1996). Only two of these articles explored the effect of rTMS in MS spasticity (Centonze et al., 2007; Mori et al., 2010). Centonze et al. (2007) evaluated the effect of rTMS in 19 patients with relapsing remitting MS and with spasticity which affected exclusively or predominantly one lower limb. rTMS protocol included stimulation with figure-of-eight coil over primary motor cortex. They showed improvement of MAS after the last session of 15-daily rTMS stimulation at 5 Hz which was maintained for 1 week. They also found that a single session of 5 Hz stimulation inhibited H reflex (reduction of reflex activity in the spinal cord level) and increased MEP amplitude whereas 1 Hz had the opposite effect.
Mori et al. (2010) studied effectiveness of iTBS in 20 relapsing remitting MS patients with spasticity affecting exclusively or predominantly one lower limb. Study protocol included 2-week daily sessions of real or sham iTBS (10 bursts, composed of three stimuli at 50 Hz, repeated at a theta frequency of 5 Hz) applied over primary motor cortex of lower extremity. The spasticity measured by MAS improved significantly and the suppression of H reflex was reported after the last session and those changes were maintained during 1 month of follow-up. Based on these two previous works, magnetic stimulation, either rTMS or iTBS might have a therapeutic effect in MS spasticity lasting at least for 1 week.
There are no studies about the effect of tDCS on spasticity in MS.
SCI
In two different studies, it has been shown that in incomplete SCI, high frequency rTMS induced significant reduction in the spasticity in the lower extremities (Kumru et al., 2010; Kumru et al., 2013). In the first study (Kumru et al., 2010), 20 Hz rTMS was applied on the vertex for 5 days (motor cortex area of legs) and the patients reported significant improvement in spasticity after the last rTMS session and during 1 week of follow-up in the lower extremity according to modified Ashworth scale (MAS), visual analogue scale (VAS) for spasticity, spinal cord assessment tool for spasticity (SCAT), modified Penn spasm frequency scale (MPSFS), spinal cord injury spasticity evaluation tool (SCI-SET).
The same authors in a recently published article (Kumru et al., 2013) found that the application of high-frequency rTMS for 15 days in SCI patients with gait capacity also induced reduction in spasticity. The authors reported significant reduction in spasticity according to MAS after first and last sessions of rTMS in addition to improvement in motor score in lower extremity and gait (Kumru et al., 2013). However, SCI-SET failed to show improvement in spasticity either after the last session of rTMS or during 2 weeks of follow-up. Those studies failed to show changes in neurophysiological examinations such as H and T reflexes or MEPs in the lower extremity.
There were no complications or major adverse effects except transient facial twitching during stimulation reported by few patients. Based on two studies (Kumru et al., 2010; Kumru et al., 2013) and a review article (Awad et al., 2013), it can be concluded that high-frequency rTMS could improve spasticity and motor function with minimal side effects and with good tolerability.
Although there are some studies regarding tDCS over motor cortex in patients with SCI, all the studies targeted pain reduction and none of them applied any measurement of functional improvement in spasticity. Therefore, it remains a promising area to investigate in SCI patients with spasticity.
CP
Valle et al. (2007) evaluated the effect of rTMS on spasticity in patients with CP. The authors compared the effect of five daily rTMS at 5 Hz and at 1 Hz vs. sham rTMS over upper extremity motor area in 17 children with CP. Repetitive transcranial magnetic stimulation at 5 Hz did not induce significant improvement in spasticity measured by MAS but led to a partial improvement in range of movement at assessments after the last session. Repetitive transcranial magnetic stimulation at 1 Hz did not induce any improvement in spasticity, neither in range of movement. There were no reported complications or adverse effects in children.
There is no study regarding the effects of tDCS on spasticity in CP.
Stroke
The effect of rTMS on spasticity was studied more frequently in patients with stroke than any other neurological disease and tDCS was analyzed only in stroke-induced spasticity. There are seven studies regarding spasticity in stroke and rTMS. Most of the studies investigated patients with severe spasticity in subacute or chronic phase which were generally defined as spasticity with Brunstrom stages 3 to 5 and duration from few months to almost 20 years. All studies used low-frequency rTMS (Mally and Dinya, 2008; Kakuda et al., 2010; Kakuda et al., 2011a; Kakuda et al., 2011b; Theilig et al., 2011; Kakuda et al., 2012; Etoh et al., 2013) except the study of Wupuer et al. (2013), who applied high-frequency rTMS at 10 Hz over unaffected hemisphere in stroke.
Málly and Dinya (Mally and Dinya, 2008) studied the effectiveness of rTMS in 64 chronic stroke patients (disease duration between 4 to 16 years). They evaluated the effect of low-frequency rTMS over either affected or unaffected hemisphere. They chose the area of rTMS according to induced visible movement by single TMS in the paretic arm and created four groups: (1) stimulation of both hemispheres (because single stimulation of TMS could induce movement from both sides of hemispheres), (2) stimulation of the intact pathway to the healthy extremities when visible movement could not be evoked stimulating either sides of the brain, (3) the stimulation of the contralateral hemisphere to the paresis when this side could induce movement in the paretic arm, (4) stimulation of the ipsilateral side of paresis when this side could induce movement in the paretic arm. Málly and Dinya (2008) found reduction in the spasticity which was maintained for more than 1 month after rTMS applied over either hemisphere whereas improvement of movement was only achieved by stimulation of intact hemisphere.
Kakuda et al. (2010; 2011a; 2011b; 2012) performed several investigations regarding the effect of rTMS in stroke spasticity. The study protocol included 15-daily sessions of rTMS at 1 Hz over unaffected hemisphere and combined with occupational therapy (Kakuda et al., 2010; 2011b). The authors found significant improvement in the spasticity according to MAS and motor functions, which was maintained for 1 month in the affected upper limb. In the other study, the authors (Kakuda et al., 2011a) applied low-frequency rTMS during 15 days over unaffected hemisphere combined with occupational therapy and 100 mg/d levodopa administration. The authors reported significant improvement of upper limb functions and spasticity measured by MAS scores of wrist and finger flexor muscles (Kakuda et al., 2011a). Yamada et al. (2013) applied low- and high-frequency rTMS simultaneous over both hemispheres (at 1 Hz and 50-second period over the unaffected hemisphere at 10 Hz and 5-second period over affected hemisphere). The authors showed significant improvement in spasticity measured by MAS in the elbow, wrist, and finger flexors in the affected limb (Yamada et al., 2013).
Two studies compared the effect of 15-daily rTMS at 1 Hz over unaffected hemisphere with sham stimulation in stroke patients (Theilig et al., 2011; Etoh et al., 2013). Theilig et al. (2011) showed significant improvement in spasticity measured with Tardieu scale in the upper extremity, whereas Etoh et al. (2013) failed to show change in spasticity measured with MAS.
In view of the foregoing, we may conclude that low-frequency rTMS over unaffected hemispheres could be effective in the reduction of spasticity in patients with stroke. There is one study regarding high-frequency rTMS (10 Hz at 110% of resting MT on primary motor cortex) in stroke. They found F-wave suppression and they also showed that one session of high-frequency stimulation was also found to be safe (Wupuer et al., 2013).
The effect of tDCS on spasticity was studied only in patients with stroke. Applying cathodal tDCS over unaffected motor cortex (M1), Bradnam et al., (2012) reported improvement in the control of the paretic proximal upper limb for mildly impaired patients and worsened control for moderate to severely impaired patients. The authors also reported variable improvement of selective proximal upper limb control which was strongly related with upper limb spasticity (Bradnam et al., 2012).
Wu et al. (2013) studied effect of cathodal tDCS over the affected primary motor cortex combined with conventional physical therapy on the upper limb spasticity in stroke patients. The authors reported significant improvement in the spasticity and in the daily living activities after the last session.
Using anodal tDCS and cathodal tDCS combined with robot-assisted arm training in a randomized, double-blinded, crossover study, Ochi et al. (2013) showed significant improvement in spasticity and in functional arm movement measured by FMA and Motor Activity Log for the upper limb. Anodal tDCS was applied over the affected hemisphere whereas cathodal tDCS was placed over the unaffected hemisphere for 5 days. For right hemispheric lesions, improvement in distal spasticity was greater in cathodal tDCS compared to anodal tDCS. However, this is not so for left hemispheric lesions.
Conclusions
According to published data, both high-frequency and low-frequency rTMS could decrease spasticity depending on the applied hemisphere and underlying neurological pathology as a unique intervention or combination with medical and/or physical therapy. Choice of rTMS frequency may depend on safety issues in neurological disorders (Rossi et al., 2009). Because of seizure risk with high-frequency rTMS, the authors generally prefer to use low-frequency rTMS which is hypothesized to have inhibitory effects over cortex. Therefore, it is applied over healthy hemisphere to suppress the inhibitory effect of the healthy hemisphere on the affected hemisphere. Significant changes in neurophysiological measurements (F wave persistence and F/M ratio in the first dorsal interosseus muscle) (Wupuer et al., 2013) were found in only study in which a high-frequency rTMS was performed, but just once because of safety issues.
Studies in pediatric patients (e.g., CP) are limited probably because of safety issues, in spite of the absence of important adverse effects (Valle et al., 2007). Although rTMS on spasticity in stroke patients studied more frequently, they did not study sham rTMS as control groups except two studies (Theilig et al., 2011; Etoh et al., 2013). Therefore, further studies may be needed to confirm its efficacy in stroke patients.
Intermittent theta burst stimulation was applied just for spasticity in patients with MS and improvement in spasticity was reported after the last session.
tDCS has been studied in management of pain and motor function but studies regarding tDCS application in spasticity are very limited and it has been studied only in stroke patients. Comparison between anodal tDCS and cathodal tDCS in two different studies showed similar results. Although it appeared to be safe and superior to sham stimulation, it may have a variable effect depending on the severity of spasticity and/or underlying neurological disorder.
Non-invasive brain stimulation seems to be a promising intervention to reduce spasticity in patients with MS, stroke, CP and SCI. The clinical applicability of the findings of this review needs to be confirmed in well-designed trials with bigger sample size and longer-term follow-up.
Footnotes
Funding: This work was supported in part by grants from Foundation La Marató TV3. No. PI110932.
Conflicts of interest: None declared.
Copyedited by Machado S, Yu HY, Li CH, Song LP, Zhao M
References
- 1.Amatya B, Khan F, La Mantia L, Demetrios M, Wade DT. Non pharmacological interventions for spasticity in multiple sclerosis. Cochrane Database Syst Rev. 2013;2:CD009974. doi: 10.1002/14651858.CD009974.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Awad BI, Carmody MA, Zhang X, Lin VW, Steinmetz MP. Transcranial Magnetic Stimulation After Spinal Cord Injury World Neurosurg. World Neurosurg. 2013 doi: 10.1016/j.wneu.2013.01.043. [DOI] [PubMed] [Google Scholar]
- 3.Barnes MP, Kent RM, Semlyen JK, McMullen KM. Spasticity in multiple sclerosis. Neurorehabil Neural Repair. 2003;17:66–70. doi: 10.1177/0888439002250449. [DOI] [PubMed] [Google Scholar]
- 4.Berardelli A, Inghilleri M, Rothwell JC, Romeo S, Curra A, Gilio F, Modugno N, Manfredi M. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res. 1998;122:79–84. doi: 10.1007/s002210050493. [DOI] [PubMed] [Google Scholar]
- 5.Bradnam LV, Stinear CM, Barber PA, Byblow WD. Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb Cortex. 2012;22:2662–2671. doi: 10.1093/cercor/bhr344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centonze D, Koch G, Versace V, Mori F, Rossi S, Brusa L, Grossi K, Torelli F, Prosperetti C, Cervellino A, Marfia GA, Stanzione P, Marciani MG, Boffa L, Bernardi G. Repetitive transcranial magnetic stimulation of the motor cortex ameliorates spasticity in multiple sclerosis. Neurology. 2007;68:1045–1050. doi: 10.1212/01.wnl.0000257818.16952.62. [DOI] [PubMed] [Google Scholar]
- 7.Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- 8.Di Lazzaro V, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, Insola A, Ranieri F, Meglio M, Tonali PA, Rothwell JC. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J Physiol. 2008;586:3871–3879. doi: 10.1113/jphysiol.2008.152736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dymond AM, Coger RW, Serafetinides EA. Intracerebral current levels in man during electrosleep therapy. Biol Psychiatry. 1975;10:101–104. [PubMed] [Google Scholar]
- 10.Etoh S, Noma T, Ikeda K, Jonoshita Y, Ogata A, Matsumoto S, Shimodozono M, Kawahira K. Effects of repetitive trascranial magnetic stimulation on repetitive facilitation exercises of the hemiplegic hand in chronic stroke patients. J Rehabil Med. 2013;45:843–847. doi: 10.2340/16501977-1175. [DOI] [PubMed] [Google Scholar]
- 11.Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex. 2008;18:2046–2053. doi: 10.1093/cercor/bhm239. [DOI] [PubMed] [Google Scholar]
- 12.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 13.Iezzi E, Conte A, Suppa A, Agostino R, Dinapoli L, Scontrini A, Berardelli A. Phasic voluntary movements reverse the aftereffects of subsequent theta-burst stimulation in humans. J Neurophysiol. 2008;100:2070–2076. doi: 10.1152/jn.90521.2008. [DOI] [PubMed] [Google Scholar]
- 14.Kakuda W, Abo M, Kobayashi K, Momosaki R, Yokoi A, Fukuda A, Ishikawa A, Ito H, Tominaga A. Low-frequency repetitive transcranial magnetic stimulation and intensive occupational therapy for poststroke patients with upper limb hemiparesis: preliminary study of a 15-day protocol. Int J Rehabil Res. 2010;33:339–345. doi: 10.1097/MRR.0b013e32833cdf10. [DOI] [PubMed] [Google Scholar]
- 15.Kakuda W, Abo M, Kobayashi K, Momosaki R, Yokoi A, Fukuda A, Ito H, Tominaga A. Combination treatment of low-frequency rTMS and occupational therapy with levodopa administration: an intensive neurorehabilitative approach for upper limb hemiparesis after stroke. Int J Neurosci. 2011a;121:373–378. doi: 10.3109/00207454.2011.560314. [DOI] [PubMed] [Google Scholar]
- 16.Kakuda W, Abo M, Kobayashi K, Momosaki R, Yokoi A, Fukuda A, Ito H, Tominaga A, Umemori T, Kameda Y. Anti-spastic effect of low-frequency rTMS applied with occupational therapy in post-stroke patients with upper limb hemiparesis. Brain Inj. 2011b;25:496–502. doi: 10.3109/02699052.2011.559610. [DOI] [PubMed] [Google Scholar]
- 17.Kakuda W, Abo M, Momosaki R, Yokoi A, Fukuda A, Ito H, Tominaga A, Umemori T, Kameda Y. Combined therapeutic application of botulinum toxin type A low-frequency rTMS, and intensive occupational therapy for post-stroke spastic upper limb hemiparesis. Eur J Phys Rehabil Med. 2012;48:47–55. [PubMed] [Google Scholar]
- 18.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 19.Kumru H, Murillo N, Samso JV, Valls-Sole J, Edwards D, Pelayo R, Valero-Cabre A, Tormos JM, Pascual-Leone A. Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury. Neurorehabil Neural Repair. 2010;24:435–441. doi: 10.1177/1545968309356095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumru H, Benito J, Murillo N, Valls-Sole J, Valles M, Lopez-Blazquez R, Costa U, Tormos JM, Pascual-Leone A, Vidal J. Effects of high-frequency repetitive transcranial magnetic stimulation on motor and gait improvement in incomplete spinal cord injury patients. Neurorehabil Neural Repair. 2013;27:421–429. doi: 10.1177/1545968312471901. [DOI] [PubMed] [Google Scholar]
- 21.Lance JW. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology. 1980;30:1303–1313. doi: 10.1212/wnl.30.12.1303. [DOI] [PubMed] [Google Scholar]
- 22.Lefaucheur JP. Principles of therapeutic use of transcranial and epidural cortical stimulation. Clin Neurophysiol. 2008;119:2179–2184. doi: 10.1016/j.clinph.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Levi R, Hultling C, Nash MS, Seiger A. The Stockholm spinal cord injury study: 1. Medical problems in a regional SCI population. Paraplegia. 1995;33:308–315. doi: 10.1038/sc.1995.70. [DOI] [PubMed] [Google Scholar]
- 24.Mally J, Dinya E. Recovery of motor disability and spasticity in post-stroke after repetitive transcranial magnetic stimulation (rTMS) Brain Res Bull. 2008;76:388–395. doi: 10.1016/j.brainresbull.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori F, Koch G, Foti C, Bernardi G, Centonze D. The use of repetitive transcranial magnetic stimulation (rTMS) for the treatment of spasticity. Prog Brain Res. 2009;175:429–439. doi: 10.1016/S0079-6123(09)17528-3. [DOI] [PubMed] [Google Scholar]
- 27.Mori F, Codeca C, Kusayanagi H, Monteleone F, Boffa L, Rimano A, Bernardi G, Koch G, Centonze D. Effects of intermittent theta burst stimulation on spasticity in patients with multiple sclerosis. Eur J Neurol. 2010;17:295–300. doi: 10.1111/j.1468-1331.2009.02806.x. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen JF, Sinkjaer T. Long-lasting depression of soleus motoneurons excitability following repetitive magnetic stimuli of the spinal cord in multiple sclerosis patients. Mult Scler. 1997;3:18–30. doi: 10.1177/135245859700300103. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen JF, Klemar B, Hansen HJ, Sinkjaer T. A new treatment of spasticity with repetitive magnetic stimulation in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1995a;58:254–255. doi: 10.1136/jnnp.58.2.254-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen JF, Klemar B, Kiilerich H. A new high-frequency magnetic stimulator with an oil-cooled coil. J Clin Neurophysiol. 1995b;12:460–467. doi: 10.1097/00004691-199509010-00005. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen JF, Sinkjaer T, Jakobsen J. Treatment of spasticity with repetitive magnetic stimulation; a double-blind placebo-controlled study. Mult Scler. 1996;2:227–232. doi: 10.1177/135245859600200503. [DOI] [PubMed] [Google Scholar]
- 32.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 1996;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 34.Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ochi M, Saeki S, Oda T, Matsushima Y, Hachisuka K. Effects of anodal and cathodal transcranial direct current stimulation combined with robotic therapy on severely affected arms in chronic stroke patients. J Rehabil Med. 2013;45:137–140. doi: 10.2340/16501977-1099. [DOI] [PubMed] [Google Scholar]
- 36.Oreja-Guevara C, Gonzalez-Segura D, Vila C. Spasticity in multiple sclerosis: results of a patient survey. Int J Neurosci. 2013;123:400–408. doi: 10.3109/00207454.2012.762364. [DOI] [PubMed] [Google Scholar]
- 37.Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- 38.Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Priori A. Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin Neurophysiol. 2003;114:589–595. doi: 10.1016/s1388-2457(02)00437-6. [DOI] [PubMed] [Google Scholar]
- 40.Reid SM, Carlin JB, Reddihough DS. Classification of topographical pattern of spasticity in cerebral palsy: a registry perspective. Res Dev Disabil. 2011;32:2909–2915. doi: 10.1016/j.ridd.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Romero JR, Anschel D, Sparing R, Gangitano M, Pascual-Leone A. Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin Neurophysiol. 2002;113:101–107. doi: 10.1016/s1388-2457(01)00693-9. [DOI] [PubMed] [Google Scholar]
- 42.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rush S, Driscoll DA. Current distribution in the brain from surface electrodes. Anesth Analg. 1968;47:717–723. [PubMed] [Google Scholar]
- 44.Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148:1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- 45.Skold C, Levi R, Seiger A. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch Phys Med Rehabil. 1999;80:1548–1557. doi: 10.1016/s0003-9993(99)90329-5. [DOI] [PubMed] [Google Scholar]
- 46.Theilig S, Podubecka J, Bosl K, Wiederer R, Nowak DA. Functional neuromuscular stimulation to improve severe hand dysfunction after stroke: does inhibitory rTMS enhance therapeutic efficiency? Exp Neurol. 2011;230:149–155. doi: 10.1016/j.expneurol.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Valle AC, Dionisio K, Pitskel NB, Pascual-Leone A, Orsati F, Ferreira MJ, Boggio PS, Lima MC, Rigonatti SP, Fregni F. Low and high frequency repetitive transcranial magnetic stimulation for the treatment of spasticity. Dev Med Child Neurol. 2007;49:534–538. doi: 10.1111/j.1469-8749.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 48.Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clin Neurophysiol. 2001;112:1367–1377. doi: 10.1016/s1388-2457(01)00585-5. [DOI] [PubMed] [Google Scholar]
- 49.Wissel J, Manack A, Brainin M. Toward an epidemiology of poststroke spasticity. Neurology. 2013;80:S13–19. doi: 10.1212/WNL.0b013e3182762448. [DOI] [PubMed] [Google Scholar]
- 50.Wu D, Qian L, Zorowitz RD, Zhang L, Qu Y, Yuan Y. Effects on decreasing upper-limb poststroke muscle tone using transcranial direct current stimulation: a randomized sham-controlled study. Arch Phys Med Rehabil. 2013;94:1–8. doi: 10.1016/j.apmr.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 51.Wupuer S, Yamamoto T, Katayama Y, Motohiko H, Sekiguchi S, Matsumura Y, Kobayashi K, Obuchi T, Fukaya C. F-wave suppression induced by suprathreshold high-frequency repetitive trascranial magnetic stimulation in poststroke patients with increased spasticity. Neuromodulation. 2013;16:206–211. doi: 10.1111/j.1525-1403.2012.00520.x. [DOI] [PubMed] [Google Scholar]
- 52.Yamada N, Kakuda W, Kondo T, Shimizu M, Mitani S, Abo M. Bihemispheric repetitive transcranial magnetic stimulation combined with intensive occupational therapy for upper limb hemiparesis after stroke: a preliminary study. Int J Rehabil Res. 2013;36:323–329. doi: 10.1097/MRR.0b013e3283624907. [DOI] [PubMed] [Google Scholar]
- 53.Zorowitz RD, Gillard PJ, Brainin M. Poststroke spasticity: sequelae and burden on stroke survivors and caregivers. Neurology. 2013;80:S45–52. doi: 10.1212/WNL.0b013e3182764c86. [DOI] [PubMed] [Google Scholar]


