Abstract
The brain is highly plastic after stroke or epilepsy; however, there is a paucity of brain plasticity investigation after traumatic brain injury (TBI). This mini review summarizes the most recent evidence of brain plasticity in human TBI patients from the perspective of advanced magnetic resonance imaging. Similar to other forms of acquired brain injury, TBI patients also demonstrated both structural reorganization as well as functional compensation by the recruitment of other brain regions. However, the large scale brain network alterations after TBI are still unknown, and the field is still short of proper means on how to guide the choice of TBI rehabilitation or treatment plan to promote brain plasticity. The authors also point out the new direction of brain plasticity investigation.
Keywords: traumatic brain injury, brain plasticity, neuroplasticity, neuroimaging, magnetic resonance imaging, fMRI, diffusion tensor imaging
Introduction
Traumatic brain injury (TBI) is a significant public health care burden, with over 1.7 million incidents every year in the United States alone (Kay, 1993; National Institutes of Health, 1999). Currently, over 5.7 million Americans live in the shadow of TBI-induced disability (Kay, 1993; National Institutes of Health, 1999). Design of proper rehabilitation program is urgently needed to help them cope with TBI-induced challenges on daily basis. Evidence demonstrates that, even with damage in certain functional structures or networks, e.g., motor control and somatosensory networks, many patients can still pick up these functionalities during their recovery. This leads to the possibility that the brain could reorganize itself either through natural recovery or acquired training experience to cope with deficits.
More evidence also suggests a cumulative effect of brain injury, which may depend on changes in the baseline status of the brain at each injury. Most recently, the TRACK TBI study reported that TBI patients who had a previous head injury history, even just a brief loss of consciousness, tend to have worse outcomes than those without a history of brain injury (Dams-O’Connor et al., 2013). This evidence suggests that, after TBI, the brain does not return to its previous state. The neurocognitive recovery could be largely due to the brain remodeling to compensate for impaired function, called brain plasticity. In addition, the advent of advanced magnetic resonance imaging (MRI) techniques has significantly changed the view of our brain in both normal and pathological conditions. High resolution structural imaging enables improved quantification of even small anatomical changes; diffusion tensor imaging (DTI) allows us to probe the brain's physiological conditions at microstructural level that is invisible on structural MRI; and functional MRI (fMRI) detects brain functional changes in response to external stimuli. The use of advanced MRI to detect brain plasticity has expanded drastically in cognitive neuroscience. From an imaging point of view, brain plasticity could be broken down into structural reorganization and functional compensation (Voss and Schiff, 2009).
Previously, Chen et al. (2010) gave a systematic review of brain plasticity after stroke. Dijkhuizen (2012) summarized recent DTI and fMRI findings of brain plasticity in animal stroke models. Kolb et al. (2010) summarized factors that influence cerebral plasticity in the normal and injured brain based on animal studies. Staudt (2010) gave insights into brain plasticity following early life brain injury (at the perinatal stage) from an imaging perspective. This mini-review adds to these efforts by summarizing the most recent imaging evidence of brain plasticity in TBI patients, from synaptic, microstructural, to functional network levels of the brain, particularly focusing on advanced MRI. At the end of the paper, the authors also point out the future directions for further investigation.
Brain plasticity at the synaptic level
Brain plastic changes are age-, gender-, time-, experience-, and region-dependent (Chen et al., 2010; Kolb et al., 2010; Staudt, 2010). Among all regions, the cortex is the most widely reported as the most plastic region after injury. These plastic changes can be seen as reorganization in regions surrounding the damaged area and recruitment of new regions or use of alternative networks (Muñoz-Cespedes et al., 2001; Chen et al., 2010; Nishibe et al., 2010). In experimental animal models, extensive synaptic remodeling can be seen in the ipsilateral neocortex at the acute stage, as shown by a decrease in the density of pedunculated spines on apical dendrites (Campbell et al., 2012). Depending on the injury type, animals with focal injury show reinnervation of damaged tissue by surrounding tissue, and animals with diffuse injury show regenerative responses in damaged tissue areas at the acute stage (Hall and Lifshitz, 2010). At the chronic stage, there is a drag during neuroinflammatory microglial activation, which could be detrimental to brain recovery, along with continuous expression of plasticity-relevant proteins in promotion of cortical plasticity, including microtubule-associated protein-2 (MAP2) and synaptophysin (SYN) (Hall and Lifshitz, 2010; Jones et al., 2012; Morris et al., 2013).
Another important region of brain plasticity investigation is the striatum, including the thalamus. The thalamus serves as a relay station of somatosensory and motor function, and the striatum is full of dopaminergic neurotransmitters and plays an important role in motor control, motivation, arousal, cognition, and reward. Using fMRI, manganese-enhanced MRI and histological validation, Yu et al. (2012) demonstrated that thalamocortic inputs may represent a major site for adult plasticity, in contrast to the consensus that adult plasticity mainly occurs at cortico-cortical connections. Synaptic plasticity in the striatum is also linked to the activation of dopamine receptors with DARPP-32 as well as glutamatergic transmission (Calabresi et al., 2000), and tyrosine hydroxylase serves to catalyze the formation of dopamine precursors (van Bregt et al., 2012). van Bregt et al. (2012) reported lasting alterations in dopamine metabolism in association with neuronal degeneration in the substantia nigra at the acute stage but no tyrosine hydroxylase change. At the chronic stage, 28 days after injury in rodent TBI models, both van Bregt et al. (2012) and Yan et al. (2007) reported alternations of tyrosine hydroxylase in the striatum as a compensatory mechanism to counteract dopamine defficiency after TBI.
Additionally, the hippocampus in particular has been an important region of interest for plasticity investigation in animal models, due to its theorized role in memory modulation, which are widely reported across different populations and can persist for long time. Early after TBI, at 24 hours, a substantial increase in spine density on dendrites bilaterally in CA1 and CA3 and the dorsal dentate gyrus could be seen, indicative of an increase in excitatory synapses (Campbell et al., 2012). Later, from the first week up to 2 months after injury, aberrant mossy fiber prouting in the dentate gyrus at the ipsilateral side of brain injury can be seen, which is associated with spontaneous convulsive seizures (Hunt et al., 2009). Therefore, mossy fiber sprouting may play a role, along with neuron loss, neurogenesis, gliosis, and morphological changes that alter sensitivity to stimulation after TBI in animal models (Hunt et al., 2009).
Brain plasticity at microstructural level
It has been reported that brain injury in early life, at the perinatal stage, can trigger structural and functional reorganization of the brain, which can be seen in both structural and functional MRI. Among structural MRI, DTI has been reported being sensitive to white matter (WM) injury at a microstructural level that is invisible on conventional MRI (Niogi et al., 2008a, 2008b).
Evidence of brain plasticity includes reorganizations of the motor system, somatosensory system, and language regions detected by structural T1, DTI tractography and task oriented fMRI (Staudt, 2010). In the early childhood, a patient who was born prematurely with serious injury on the arcuate fasciculus, the critical pathway for language processing, could still have preserved language and reading functions (Yeatman and Feldman, 2013). Instead, DTI tractography demonstrated intact ventral connections between the temporal and frontal lobes through the extreme capsule fiber system and uncinate fasciculus. This connection is likely to take over the language processing functionality as a compensational mechanism (Yeatman and Feldman, 2013). In adult brain injury patients, Yogarajah et al. (2010) conducted a DTI study of 46 epilepsy patients with anterior temporal lobe resection. They reported increased fractional anisotropy (FA) in the ventro-medial language network, in suggestion of structural reorganization in response to the resection (Yogarajah et al., 2010). In a case study of a patient suffering from tuberous meningitis at the age of 12 years, Jan et al. reported a new motor pathway posterior to the lesion in the midbrain and upper pons, evidenced by diffusion tensor tractography, task oriented fMRI, and transcranial magnetic stimulation (Ndode-Ekane et al., 2010). Laitinen et al. (2010) also reported increased fractional anisotropy in the hippocampal dentate gyrus in ananimal model induced with status epilepticus, and the FA changes are correlated (P < 0.01) with histologically verified axonal plasticity of myelinated and non-myelinated neuronal fibers. This evidence demonstrated that morphological structural is the foundation of brain plasticity. In case of injury at one location, other possible alternative routes must be intact to compensate the impaired function. Zhou et al. (2014) demonstrated that rats with partial corpus callosotomy can have restored functional connectivity between hemispheres at 28 days after injury, but rats with complete corpus callosotomy cannot, likely due to the compensation that occurred through the remaining interhemispheric axonal pathways. Their data suggest that axonal connections are the indispensable foundation for resting state functional connectivity.
Brain plasticity at functional network level
Task-oriented fMRI findings
fMRI is the most widely used modality to investigate brain functional network alternations in pathological conditions. Task-based fMRI is considered the gold standard for identifying corresponding functional activations. In a case study of two chronic TBI patients, Sacco et al. (2011) reported greater activation in the sensorimotor and supplementary motor cortices after a combined robotic and cognitive rehabilitation training, as well as enhanced functional connectivity within the motor network (Figure 1). Improvements in balance and, to a lesser extent, in gait outcomes were also found in these two patients. In another study of 20 patients at 1 month after mild TBI (mTBI) and 18 healthy controls, Chen et al. (2012) demonstrated that brain activation patterns differed between mTBI patients and controls in response to increasing working memory loads (P < 0.01, uncorrected). mTBI patients were impaired in their ability to increase activation in working memory circuitry under both moderate and high working memory load conditions in contrast with controls. However, mTBI patients showed cerebral plasticity, as evidenced by more activation in some areas outside and inside the working memory circuitry as compared with control subjects (P < 0.01, uncorrected). This work is in line with the earlier study by McAllister et al. (1999) on mTBI. In addition, Tivarus et al. (2012) reported the reorganization of language network to the right hemisphere after early left hemisphere injury by using task-based fMRI. In a study of a severe and persistent speech disorder, dysarthria, Morgan et al. (2013) reported functional reorganization involved over-recruitment of left-hemisphere motor regions for language processing. In contrast with these beneficial findings of brain plasticity, Hampshire et al. (2013) studied 13 retired National Football League players by using task-based fMRI. They reported that the players showed pronounced hyperactivation and hypoconnectivity of the dorsolateral frontal and frontopolar cortices. Critically, abnormal frontal lobe function was correlated with the self-reported number of times of the players’ being removed from play due to head injury (Hampshire et al., 2013). This might be a direct evidence of pathological plasticity due to maladaptive recruitment of brain resources and activations.
Figure 1.
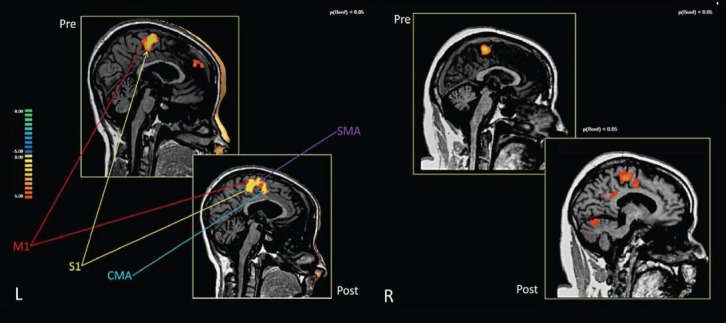
Brain activations in the pre- and post-training conditions of two chronic traumatic brain injury patients receiving a combined robotic and cognitive training for rehabilitation.
The highlighted warm areas designate signal intensity of brain activation. For the first patient at left side (L), combined training increased the recruitment of cingulate motor area and supplemental motor area in compensation of brain function. For the second case at right (R), combined training increased recruitment of more brain regions for compensation. M1: Primary motor cortex; S1: primary somatosensory cortex; CMA: cingulate motor area; SMA: supplemental motor area. Image courtesy Sacco et al. (2011) and Frontiers in Human Neurosciences.
Resting state functional connectivity MRI (fcMRI) findings
Despite the popularity of task-based fMRI in mapping functional activations, it is not possible to use it to identify all functional networks of the brain. As a complement, resting state fcMRI is the most widely used technique in recent years to identify the brain network connectivity. Among all brain networks in the resting state, the “Default Mode Network” (DMN) is the most studied in mTBI (Mayer et al., 2011; Johnson et al., 2012; Stevens et al., 2012). The DMN is assumed to control passive mental activities in which individuals are awake and alert but do not specifically perform any “goal-directed task” (Broyd et al., 2009). The posterior cingulate cortex (PCC) and the medial prefrontal cortex (MPFC) are two important components of the DMN. PCC and MPFC are associated with self-referential and emotional processing, as well as semantic processing, and their activity is attenuated when attention to the “goal-directed task” increases (Gilbert et al., 2007). The DMN's important role in several cognitive functions and its disruption in several neurocognitive disorders both highlight the importance of the DMN's connections and possible alterations in its activity as a possible diagnostic marker of injury-related neural damage. In mTBI investigation, most studies demonstrated decreased functional connectivity among the regions of the default mode network (DMN) and increased connectivity of DMN regions with brain regions that belong to other functional networks, in suggestion of functional network remodeling of the brain after injury. Mayer et al. (2011) studied mTBI patients at the semi-acute stage (within 2 weeks after injury) by choosing the rostral anterior cingulate gyrus (rACC) as the seed region and demonstrated a reduction in connectivity within DMN for mTBI and an increase in connectivity within a task-related network (TRN) relative to matched controls. Similarly, Johnson et al. (2012) scanned sports athletes with mTBI at the sub-acute phase (10 ± 2 days). By using voxel-based and region of interest (ROI)-based analysis, they focused on DMN regions such as PCC, MPFC, the lateral parietal lobes, and the parahippocampal gyrus. They indicated that both the number and strength of connections decreased in the PCC and the lateral parietal cortices but increased in the medial prefrontal cortex following mTBI. With an independent component analysis (ICA) analysis, Stevents et al. (2012) explored different ICA components to find alterations between healthy subjects and mTBI patients who were scanned 61 days after injury. They found diminished functional connectivity in the DMN and many other networks in the patient group, such as the primary visual processing circuit, the motor system, the left-lateralized frontoparietal circuit, the dorsomedial circuit, the frontoparietal executive system, and the frontostriatal network. They found that the precuneus had greater connectivity with the default mode network, while the connectivity between the cingulate and the DMN was diminished.
In a severe TBI study, Nakamura et al. (2009) studied six TBI patients at 3 months and then followed them up at 6 months after injury. By using a graph theory-based analysis of whole brain networks, they reported reduced overall strength in connectivity and increased “small-worldness” of TBI patients at 3 months after injury. At 6 months after injury, these parameters tend to return back to normal level as healthy controls (Nakamura et al., 2009). Despite their obvious structural damage on conventional MRI in these severe TBI patients, their brain overall connectivity could still come back to normal level as controls at the chronic stage. This is another piece of direct evidence for brain plasticity after injury as an important determiner of recovery.
Combining structural imaging with fcMRI
A combinational analysis of DTI (white matter structural damage) and fcMRI (functional network alternation) could offer more meaningful insights of the brain plasticity to cope with injury. We acquired MRI data in a preliminary dataset from 7 healthy controls (27.71 ± 10.43 years old) and 7 patients (33.57 ± 8.44 years old) with mTBI within 24 hours after injury. Both DTI probabilistic tractography and resting state fcMRI analysis have been performed to investigate the structural damage on DTI and functional alternatives on fcMRI. Our fcMRI analysis showed a decreased strength of connectivity within the DMN but an increased connectivity between posterior cingulate cortex (PCC) and other brain networks (especially supplementary motor cortex) in mTBI patients, which is consistent with our previous work and other studies. The DTI probabilistic tractography analysis also showed structural damage in the cingulate bundle. Statistical analysis on the fMRI functional connectivity map demonstrated significantly decreased connectivity in the angular gyrus (P = 0.0005) with cluster size = 64, PCC (P = 0.005), and orbitofrontal gyrus (P = 0.005) (Figure 2). In other brain areas, such as in a big cluster in the supplementary motor cortex (Brodmann area 6), connectivity was significantly increased (P = 0.0005) with cluster size = 116, which may be related to motor disabilities after injury. The PCC also showed higher connectivity with the middle cingulate gyrus in the patient group than in the control group. This region of high functional connectivity on fcMRI closely opposes a region of lower structural connectivity in DTI probabilistic measurements in the middle part of cingulum bundle in patients (P = 0.05) (Figure 2). Our data represent the first report regarding the relationship between structural damage and functional response in mTBI at the hyperacute stage. It suggests that, along with structural damage in white matter tract after TBI, the brain is trying to recruit more neuronal resources to compensate for changes in functionalities.
Figure 2.
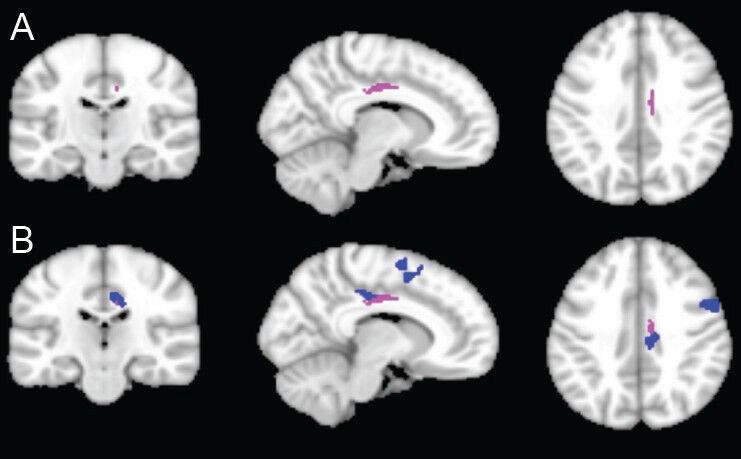
Functional compensation for structural damage after traumatic brain injury.
(A) Structural connectivity of white matter by using poster cingulate bundle as the seed region for probabilistic tractography analysis: The pink color shows the areas in which the patient group has lower connectivity (two sample t-test, P < 0.05). (B) Overlay of functional connectivity onto DTI structural connectivity. The blue color shows the areas in which the patient group has higher functional connectivity by using posterior cingulate cortex as the seed region (two sample t-test, P < 0.005). The figure clearly shows that the increased functional connectivity on resting state functional MRI (fMRI) tends to compensate for structural damage in the cingulate bundle in TBI patients. This demonstrates the complementary role of DTI and fMRI in delineating brain plasticity after TBI.
Brain plasticity at connectomic scale
Unlike stroke or epilepsy, in which the injury lesion tends to be restricted to a focal region, TBI pathology is much more heterogeneous and tends to have multi-focal lesions. One example is diffuse axonal injury, which always has more than one WM tract involved in TBI on DTI findings (Kou et al., 2010; Niogi and Mukherjee, 2010; Kou and VandeVord, 2014). Despite the plasticity findings in memory, sensorimotor and other functions, the extents of brain injury and responsive plasticity at a large scale brain network level or connectome level are still unknown. By using ICA analysis, Stevens et al. (2012) identified the reduced connectivity in several brain networks and increased connectivity in other networks. However, ICA may not be the ideal approach to analyze whole brain connectivity: it is not clear which ICA network represents which brain function and using different numbers of components will result in different results. The combination of DTI, for the detection of structural damage, with fcMRI, for functional network identification, offers a unique opportunity to investigate the functional brain plasticity in response to structural damage over the whole brain. However, due to the lack of dense and corresponding cortical landmarks across individuals, the systematical alterations of functional connectivities in large-scale brain networks and the alteration of structural brain architecture in TBI brain are largely unknown. A newly developed data-driven strategy, called Dense Individualized and Common Connectivity-Based Cortical Landmarks (DICCCOL), might hold the promise in this avenue (Zhu et al., 2013a). It has been reported as being sensitive to brain network alterations at a connectomics level in mild cognitive impairment patients (Zhu et al., 2013b) as well as prenatal cocaine expose (PCE) patients (Li et al., 2013). It is based on the hypothesis that the common cortical architecture can be effectively represented by group-wise consistent structural fiber connections. Each DICCCOL node is a functional land marker on cortical area, defined by group-wise consistent white matter fiber connection patterns derived from DTI data (Zhu et al., 2013a). The analysis of PCE patients discovered informative functional connectomics signatures among consistent landmarks that distinctively differentiate PCE brains from their matched controls (Li et al., 2013). Taking it one step further, this approach could be used in TBI patients to detect their connectomics signature, including both structural deficit and functional connectivity remodeling.
Brain plasticity as a treatment target
Training promoted brain plasticity
It has been well recognized in both animal models and human studies that brain plasticity is experience dependent, even in adulthood (Chen et al., 2010; Kolb et al., 2010). The brain can be altered by a wide variety of experiences across the lifespan as summarized by Kolb et al. (2010). These include sensory and motor experience, e.g., sports training; psychomotor stimulus, e.g., drug use; and career related training experience. These changes can also be detected in modern MRI techniques. As an example, London taxi drivers tend to have increased hippocampal volumes with the length of their career, which is thought to be a result of navigating the streets of London on daily basis because of the hippocampus role in spatial navigation (Maguire et al., 2000). Another fMRI study of subjects who learned sequential finger movements demonstrated changes in the motor cortex, cerebellum and basal ganglia following the training (Ungerleider et al., 2002). Therefore, brain plasticity could be used as a treatment target even in TBI patients, by prescribing a method called training-induced recovery (Chen et al., 2010). Specific rehabilitation programs could be designed to address certain deficit aspects of brain injury to stimulate the reorganization of functional brain regions. Advanced MRI, particularly fMRI, could play a significant role in assessing the treatment effect. Successful examples include language training in aphasia patients after stroke, which shows right-hemisphere activation shifted to left hemisphere activation at the chronic stage (Meinzer et al., 2007; Richter et al., 2008), and robot-based hand motor therapy, which promotes motor cortex activation and reorganization in chronic stroke patients (Luft et al., 2001; Takahashi et al., 2008).
Proactive neurorehabilitation, which targets specific impaired neurocognitive domain(s) in TBI patients, holds the promise of improving patients’ recovery by stimulating brain remodeling of functional regions. Evidence also demonstrated that TBI patients who actively returned back to work that requires highly intellectual involvement tend have a favorable outcome. As a matter of fact, the highly intellectually involved work itself provides an ideal environment of neurorehabilitation that may promote brain reorganization. To date, its underlying brain plasticity mechanism is still yet to be investigated.
The role of microglial activation
One important aspect of TBI at the chronic stage is the persistent microglial activated neuroinflammation, which may contradict the effort of treatment plans that promote brain plasticity. Recent positron emission tomography studies using 11C-(R)-PK11195 (PK) tracer have demonstrated persistent microglial activation even 17 years after injury (Ramlackhansingh et al., 2011). This work is also confirmed in TBI animal model using PK binding (Folkersma et al., 2011). An autopsy study of brain injury patients also demonstrated that persistent microglial activation could be a major cause of progressive atrophy of the brain, indicating that one single brain injury can result in microglial activation persisting for decades (Ungerleider et al., 2002). However, we are still short of means to non-invasively quantify microglial activation in TBI patients (Kou and VandeVord, 2014). The field is still lacking in knowledge about the role of microglial activation and how it can negatively affect the efforts towards brain plasticity (Kou and VandeVord, 2014). As a matter of fact, the neuroscience community has been dramatically changing the view about the role of microglia in recent years (Eyo and Dailey, 2013). A recent systemic review reported on microglial activation and white matter injury, a potential treatment target (Kou and VandeVord, 2014). Under the notion of combinational therapy (Margulies et al., 2009), anti-inflammatory treatments may also need to be considered along with the brain plasticity stimulation plan to achieve better patient outcomes over the long run.
The caveat
Not all plasticity is good
Despite its overall beneficial role in brain functional compensation in the literature, not all brain plasticity is beneficial to the brain function (Chen et al., 2010; Kolb et al., 2010; Li et al., 2014). Some plasticity can induce pathological conditions, or called pathological plasticity (Li et al., 2014). Examples include pathological pain (Baranauskas, 2001), pathological response to sickness (Raison et al., 2006), epilepsy (Teskey, 2001), schizophrenia (Black et al., 2004) and dementia (Mattson et al., 2001). One well-known example is that some drug treatments can cause maladaptive brain plasticity for drug addiction (Kolb et al., 2010). Another example is the mossy fiber sprouting and reorganization in hippocampus after TBI that may lead to epilepsy due to pathological firing of neurons and reduced synaptic inhibition in the region (Hunt et al., 2009). In addition, maladaptive changes during plasticity can have detrimental effects on the brain itself (Carey and Seitz, 2007; Chen et al., 2010) and interfere with regaining of normal functions (Murphy and Corbett, 2009). In stroke, recruitment of the contralateral region is compensatory in the early stage but may become maladaptive in later stages (Carey and Seitz, 2007; Chen et al., 2010). One challenge during pharmacological treatment or in the designing of rehabilitation programs for TBI patients is to minimize the side effects of treatment so that it will not induce maladaptive plastic changes that could actually interfere with recovery. Particular caution has to be taken during drug treatment to avoid maladaptive behavior of drug addiction (Kolb et al., 2010).
The spurious fibers may not be fibers
Another example is the spurious WM fibers after TBI on DTI either in contralateral or ipsilateral side of injury. It has been recognized that reactive astroglial activation occurs in either ipsilateral or contralateral side of brain injury (Budde et al., 2011; Zhuo et al., 2012). In the DTI signature, the cellular infiltration of reactive astroglial cells will cause reduced radial diffusivity and increased FA, which is similar to neuronal regeneration or reorganization. DTI fiber tractography will show spurious WM fibers (Figure 3). As demonstrated by Budde et al. (2011), the spurious WM fibers on DTI tractography are caused by reactive astrocytes, as validated histologically, instead of axonal regeneration or reorganization. Particularly in neuroimaging of clinical patients, due to the lack of histological validation, caution should be taken before drawing any conclusion of axonal regeneration or reorganization after TBI, especially in cortical regions.
Figure 3.
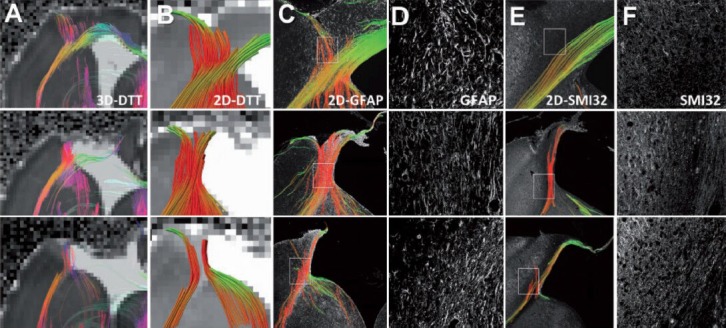
Comparison of diffusion tensor tractography and histology-derived tractography demonstrates that the spurious fibers can be caused by reactive astrogliosis.
Three representative controlled cortical impact animals are shown. (A) Ex vivo 3D diffusion tensor tractography (DTT) maps depict tracts propagating in and along the lesion periphery similar to those observed in vivo. (B) 2D diffusion tensor tractography was subsequently performed to allow direct comparison to the 2D histology-derived tractography. (C) 2D tractography maps from the glial fibrillary acidic protein (GFAP)-stained sections revealed similarities to the DTI-derived maps near the lesion border. (D) The coherent orientation of astrocytes is shown on confocal images from selected regions. (E) 2D tractography maps from the Sternberger monoclonal antibody (SMI) 32-stained sections revealed fewer, if any, tracts propagating into the cortex near the lesion periphery. (F) A loss of structural integrity is noted for both the injured white matter and grey matter along the lesion border. These three examples demonstrate spurious WM tracts that are actually caused by reactive astrogliosis instead of fiber reorganization during brain plasticity. Image reproduced with permission from Budde et al. (2011) and Brain journal.
Summary
As the most important organ of human body, the brain is highly plastic even in the adulthood in response to external stimuli, either favorably or unfavorably. Damage to certain areas of brain can be compensated for, either structurally or functionally. Due to the heterogeneity and multi-focal nature of TBI, the brain network alternations in response to injury at a connectomic scale are still largely unknown. Further, the choice of pharmacological treatment plan and the design of proper rehabilitation programs hold the promise to stimulate functional brain plasticity for a favorable recovery. Methods to avoid pathological plasticity are an important area of investigation which can be strongly aided by the use of advanced MRI.
Acknowledgments:
The authors thank Ms. Natalie Wiseman, MD/PhD candidate, for her proof reading of the manuscript.
Footnotes
Funding: The work was supported by the Department of Defense, grant number W81XWH-11-1-0493.
References
- 1.Baranauskas G. Pain-induced plasticity in the spinal cord. In: Shaw CA, McEachern J, editors. Toward a Theory of Neuroplasticity. Philadelphia: PA: Psychology Press; 2001. [Google Scholar]
- 2.Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, Uranova N, Greenough WT. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- 3.Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Budde MD, Janes L, Gold E, Turtzo LC, Frank JA. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain. 2011;134:2248–2260. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calabresi P, Gubellini P, Centonze D, Picconi B, Bernardi G, Chergui K, Svenningsson P, Fienberg AA, Greengard P. Dopamine and cAMP-regulated phosphoprotein 32kDa controls both striatal long-term depression and long-term potentiation, opposingforms of synaptic plasticity. J Neurosci. 2000;20:8443–8451. doi: 10.1523/JNEUROSCI.20-22-08443.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell JN, Register D, Churn SB. Traumatic brain injury causes an FK506-sensitive loss and an overgrowth of dendritic spines in rat forebrain. J Neurotrauma. 2012;29:201–217. doi: 10.1089/neu.2011.1761. [DOI] [PubMed] [Google Scholar]
- 7.Carey LM, Seitz RJ. Functional neuroimaging in stroke recovery and neurorehabilitation: conceptual issues and perspectives. Int J Stroke. 2007;2:245–264. doi: 10.1111/j.1747-4949.2007.00164.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen CJ, Wu CH, Liao YP, Hsu HL, Tseng YC, Liu HL, Chiu WT. Working memory in patients with mild traumatic brain injury: functional MR imaging analysis. Radiology. 2012;264:844–851. doi: 10.1148/radiol.12112154. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Epstein J, Stern E. Neural plasticity after acquired brain injury: evidence from functional neuroimaging. PM R. 2010;2:S306–312. doi: 10.1016/j.pmrj.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Dams-O’Connor K, Spielman L, Singh A, Gordon WA, Lingsma HF, Maas AI, Manley GT, Mukherjee P, Okonkwo DO, Puccio AM, Schnyer DM, Valadka AB, Yue JK, Yuh EL, Track-Tbi Investigators, Casey SS, Cooper SR, Cheong M, Hricik AJ, Knight EE, et al. The impact of previous traumaticbrain injury on health and functioning: a TRACK-TBI study. J Neurotrauma. 2013;30:2014–2020. doi: 10.1089/neu.2013.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dijkhuizen RM, van der Marel K, Otte WM, Hoff EI, van der Zijden JP, van der Toorn A, van Meer MP. Functional MRI and diffusion tensor imaging of brain reorganization after experimental stroke. Transl Stroke Res. 2012;3:36–43. doi: 10.1007/s12975-011-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eyo UB, Dailey ME. Microglia: key elements in neural development plasticity and pathology. J Neuroimmune Pharmacol. 2013;8:494–509. doi: 10.1007/s11481-013-9434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folkersma H, Foster Dingley JC, van Berckel BN, Rozemuller A, Boellaard R, Huisman MC, Lammertsma AA, Vandertop WP, Molthoff CF. Increased cerebral (R)-[(11)C]PK11195 uptake and glutamate release in a rat model of traumatic brain injury: a longitudinal pilot study. J Neuroinflammation. 2011;8:67. doi: 10.1186/1742-2094-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW. Comment on “Wanderingminds: The default network and stimulus-independent thought”. Science. 2007;317:43. doi: 10.1126/science.317.5834.43. [DOI] [PubMed] [Google Scholar]
- 15.Hall KD, Lifshitz J. Diffuse traumatic brain injury initially attenuates and later expands activation of the rat somatosensory whisker circuit concomitant with neuroplastic responses. Brain Res. 2010;1323:161–173. doi: 10.1016/j.brainres.2010.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hampshire A, MacDonald A, Owen AM. Hypoconnectivity and hyperfrontality in retired American football players. Sci Rep. 2013 doi: 10.1038/srep02972. doi:10.1038/srep02972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt RF, Scheff SW, Smith BN. Post traumatic epilepsy after controlled cortical impact injury in mice. Exp Neurol. 2009;215:243–252. doi: 10.1016/j.expneurol.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson B, Zhang K, Gay M, Horovitz S, Hallett M, Sebastianelli W, Slobounov S. Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. Neuroimage. 2012;59:511–518. doi: 10.1016/j.neuroimage.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones TA, Liput DJ, Maresh EL, Donlan N, Parikh TJ, Marlowe D, Kozlowski DA. Use-dependent dendritic regrowth is limited after unilateral controlled cortical impact to the forelimb sensorimotor cortex. J Neurotrauma. 2012;29:1455–1468. doi: 10.1089/neu.2011.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kay T. Neuropsychological treatment of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8:74–85. [Google Scholar]
- 21.Kolb B, Teskey GC, Gibb R. Factors influencing cerebral plasticity in the normal and injured brain. Front Hum Neurosci. 2010 doi: 10.3389/fnhum.2010.00204. doi:10.3389/fnhum.2010.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kou Z, VandeVord PJ. Glia: 2014. Traumatic white matter injury and glial activation: Review. In press. DOI. [DOI] [PubMed] [Google Scholar]
- 23.Kou Z, Wu Z, Tong KA, Holshouser B, Benson RR, Hu J, EM H. The role of advanced MR imaging findings as biomarkers of traumatic brain injury. J Head Trauma Rehabil. 2010;25:267–282. doi: 10.1097/HTR.0b013e3181e54793. [DOI] [PubMed] [Google Scholar]
- 24.Laitinen T, Sierra A, Pitkänen A, Gröhn O. Diffusion tensor MRI of axonal plasticity in the rat hippocampus. Neuroimage. 2010;51:521–530. doi: 10.1016/j.neuroimage.2010.02.077. [DOI] [PubMed] [Google Scholar]
- 25.Li K, Zhu D, Guo L, Li Z, Lynch ME, Coles C, Hu X, Liu T. Connectomics signatures of prenatal cocaine exposure affected adolescent brains. Hum Brain Mapp. 2010;34:2494–2510. doi: 10.1002/hbm.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li N, Yang Y, Glover DP, Zhang J, Saraswati M, Robertson C, Pelled G. Evidence forimpaired plasticity after traumatic brain injury in the developing brain. J Neurotrauma. 2010;31:395–403. doi: 10.1089/neu.2013.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luft AR, McCombe-Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, Schulz JB, Goldberg AP, Hanley DF. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA. 2001;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margulies S, Hicks R. Combination Therapies for Traumatic Brain Injury Workshop Leaders (2009) Combination therapies for traumatic brain injury: prospective considerations. J Neurotrauma. 26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattson MP, Duan W, Chan SL, Guo Z. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. In: Shaw CA, McEachern J, editors. Toward a Theory of Neuroplasticity. Philadelphia, PA: Psychology Press; 2001. [Google Scholar]
- 31.Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Hum Brain Mapp. 2011;32:1825–1835. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McAllister TW, Saykin AJ, Flashman LA, Sparling MB, Johnson SC, Guerin SJ, Mamourian AC, Weaver JB, Yanofsky N. Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology. 1999;53:1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- 33.Meinzer M, Elbert T, Djundja D, Taub E, Rockstroh B. Extending the Constraint-Induced Movement Therapy (CIMT) approach to cognitive functions: Constraint-Induced AphasiaTherapy (CIAT) of chronic aphasia. NeuroRehabilitation. 2007;22:311–318. [PubMed] [Google Scholar]
- 34.Morgan AT, Masterton R, Pigdon L, Connelly A, Liégeois FJ. Functional magnetic resonance imaging of chronic dysarthric speech after childhood brain injury: reliance ona left-hemisphere compensatory network. Brain. 2013;136:646–657. doi: 10.1093/brain/aws355. [DOI] [PubMed] [Google Scholar]
- 35.Morris GP, Clark IA, Zinn R, Vissel B. Microglia: a new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiol Learn Mem. 2013;105:40–53. doi: 10.1016/j.nlm.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Muñoz-Cespedes JM, Rios-Lago M, Paul N, Maestu F. Functional neuroimaging studies of cognitive recovery after acquired brain damage in adults. Neuropsychol Rev. 2001;15:169–183. doi: 10.1007/s11065-005-9178-5. [DOI] [PubMed] [Google Scholar]
- 37.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura T, Hillary FG, Biswal BB. Resting network plasticity following brain injury. PLoS One. 2009;4:e8220. doi: 10.1371/journal.pone.0008220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Institutes of Health. NIH consensus development panel on rehabilitation of persons with traumatic brain injury. JAMA. 1999;282:974–983. [PubMed] [Google Scholar]
- 40.Ndode-Ekane XE, Hayward N, Gröhn O, Pitkänen A. Vascular changes in epilepsy: functional consequences and association with network plasticity in pilocarpine-induced experimental epilepsy. Neuroscience. 2010;166:312–332. doi: 10.1016/j.neuroscience.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Niogi SN, Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil. 2010;25:241–255. doi: 10.1097/HTR.0b013e3181e52c2a. [DOI] [PubMed] [Google Scholar]
- 42.Niogi SN, Mukherjee P, Ghajar J, Johnson C, Kolster RA, Sarkar R, Lee H, Meeker M, Zimmerman RD, Manley GT, McCandliss BD. Extent of microstructural white matter injury in post concussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol. 2008a;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niogi SN, Mukherjee P, Ghajar J, Johnson CE, Kolster R, Lee H, Suh M, Zimmerman RD, Manley GT, McCandliss BD. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008b;131:3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- 44.Nishibe M, Barbay S, Guggenmos D, Nudo RJ. Reorganization of motor cortex after controlled cortical impact in rats and implications for functional recovery. J Neurotrauma. 2010;27:2221–2232. doi: 10.1089/neu.2010.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raison C, Capuron L, Miller AH. Cytokines sing the blues: inflammation and thepathogenesis of depression. Trends Immunol. 2010;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 47.Richter M, Miltner WH, Straube T. Association between therapy outcome and right hemispheric activation in chronic aphasia. Brain. 2008;131:1391–1401. doi: 10.1093/brain/awn043. [DOI] [PubMed] [Google Scholar]
- 48.Sacco K, Cauda F, D’Agata F, Duca S, Zettin M, Virgilio R, Nascimbeni A, Belforte G, Eula G, Gastaldi L, Appendino S, Geminiani G. A combined robotic and cognitive training for locomotor rehabilitation: evidences of cerebral functional reorganization in two chronic traumatic brain injured patients. Front Hum Neurosci. 2011 doi: 10.3389/fnhum.2011.00146. doi:10.3389/fnhum.2011.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Staudt M. Brain plasticity following early life brain injury: insights from neuroimaging. Semin Perinatol. 2010;34:87–92. doi: 10.1053/j.semperi.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Stevens MC, Lovejoy D, Kim J, Oakes H, Kureshi I, Witt ST. Multiple resting state network functional connectivity abnormalities in mild traumatic brain injury. Brain Imaging Behav. 2012;6:293–318. doi: 10.1007/s11682-012-9157-4. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi CD, Der-Yeghiaian L, Le V, Motiwala RR, Cramer SC. Robot-based handmotor therapy after stroke. Brain. 2008;131:425–437. doi: 10.1093/brain/awm311. [DOI] [PubMed] [Google Scholar]
- 52.Teskey GC. Using kindling to model the neuroplastic changes associated with learning and memory, neuropsychiatric disorders, and epilepsy. In: Shaw CA, McEachern J, editors. Toward a Theory of Neuroplasticity. Philadelphia, PA: Psychology Press; 2001. [Google Scholar]
- 53.Tivarus ME, Starling SJ, Newport EL, Langfitt JT. Homotopic language reorganization in the right hemisphere after early left hemisphere injury. Brain Lang. 2012;123:1–10. doi: 10.1016/j.bandl.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem. 2002;78:553–564. doi: 10.1006/nlme.2002.4091. [DOI] [PubMed] [Google Scholar]
- 55.van Bregt DR, Thomas TC, Hinzman JM, Cao T, Liu M, Bing G, Gerhardt GA, Pauly JR, Lifshitz J. Substantia nigra vulnerability after a single moderate diffuse brain injury in the rat. Exp Neurol. 2012;234:8–19. doi: 10.1016/j.expneurol.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voss HU, Schiff ND. MRI of neuronal network structure, function and plasticity. Prog Brain Res. 2009;175:483–496. doi: 10.1016/S0079-6123(09)17532-5. [DOI] [PubMed] [Google Scholar]
- 57.Yan HQ, Ma X, Chen X, Li Y, Shao L, Dixon CE. Delayed increase of tyrosinehydroxylase expression in rat nigrostriatal system after traumatic brain injury. Brain Res. 2009;1134:171–179. doi: 10.1016/j.brainres.2006.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeatman JD, Feldman HM. Neural plasticity after pre-linguistic injury to the arcuate and superior longitudinal fasciculi. Cortex. 2013;49:301–311. doi: 10.1016/j.cortex.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yogarajah M, Focke NK, Bonelli SB, Thompson P, Vollmar C, McEvoy AW, Alexander DC, Symms MR, Koepp MJ, Duncan JS. The structural plasticity of white matter networks following anterior temporal lobe resection. Brain. 2010;133:2348–2364. doi: 10.1093/brain/awq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu X, Chung S, Chen DY, Wang S, Dodd SJ, Walters JR, Isaac JT, Koretsky AP. Thalamocortical inputs show post-critical-period plasticity. Neuron. 2012;74:731–742. doi: 10.1016/j.neuron.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou IY, Liang YX, Chan RW, Gao PP, Cheng JS, Hu Y, So KF, Wu EX. Brain resting state functional MRI connectivity: morphological foundation and plasticity. Neuroimage. 2014;84:1–10. doi: 10.1016/j.neuroimage.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 62.Zhu D, Li K, Guo L, Jiang X, Zhang T, Zhang D, Chen H, Deng F, Faraco C, Jin C, Wee CY, Yuan Y, Lv P, Yin Y, Hu X, Duan L, Hu X, Han J, Wang L, Shen D, et al. DICCCOL: dense individualized and common connectivity-based cortical landmarks. Cereb Cortex. 2013a;23:786–800. doi: 10.1093/cercor/bhs072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu D, Li K, Terry DP, Puente AN, Wang L, Shen D, Miller LS, Liu T. Connectome-scale assessments of structural and functional connectivity in MCI. Hum Brain Mapp. 2013b doi: 10.1002/hbm.22373. doi:10.1002/hbm.22373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhuo J, Xu S, Proctor JL, Mullins RJ, Simon JZ, Fiskum G, Gullapalli RP. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage. 2012;59:467–477. doi: 10.1016/j.neuroimage.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]


