Abstract
Traumatic brain injury (TBI) is the leading cause of death and disability of persons under 45 years old in the United States, affecting over 1.5 million individuals each year. It had been thought that recovery from such injuries is severely limited due to the inability of the adult brain to replace damaged neurons. However, recent studies indicate that the mature mammalian central nervous system (CNS) has the potential to replenish damaged neurons by proliferation and neuronal differentiation of adult neural stem/progenitor cells residing in the neurogenic regions in the brain. Furthermore, increasing evidence indicates that these endogenous stem/progenitor cells may play regenerative and reparative roles in response to CNS injuries or diseases. In support of this notion, heightened levels of cell proliferation and neurogenesis have been observed in response to brain trauma or insults suggesting that the brain has the inherent potential to restore populations of damaged or destroyed neurons. This review will discuss the potential functions of adult neurogenesis and recent development of strategies aiming at harnessing this neurogenic capacity in order to repopulate and repair the injured brain.
Keywords: traumatic brain injury, adult neurogenesis, neural stem cells, cell proliferation, hippocampus, subventricular zone, learning and memory function, regeneration, cognition
Introduction
Traumatic brain injury (TBI) is the leading cause of death and disability worldwide. TBI is caused largely by motor vehicle accidents, violence, combat injuries, fall or sport related injures. Each year, many individuals suffer from TBI, those survived often left with disabilities ranging from long-term sensorimotor deficits, cognitive impairment to vegetative state. To date, there is no effective treatment for TBI.
TBI consists of two distinct phases: primary and secondary insults. The primary insult, completed within seconds of impact, is due to mechanical tissue damage. Secondary insults consist of a complex cascade of interrelated events including ischemia, excitotoxicity, and metabolic failure which result in further cell death and dysfunction. Currently, therapies are primarily focused on reducing the extent of secondary insult rather than repairing the damage from the primary injury. Clinical evidence indicates that the hippocampus is particularly vulnerable to the secondary insults. Hippocampal injury associated learning and memory deficits are the hallmarks of brain trauma (Hilton, 1994). The cognitive sequelae are the most enduring and debilitating of TBI deficits because they prevent reintegration of patients into a normal lifestyle by impairing employment and social interaction. Spontaneous cognitive improvement is not uncommon but is greatly limited and not normally seen past the second year post-injury (Schmidt et al., 1999). This natural recovery, however, does suggest that innate mechanisms for repair and regeneration are present within the brain.
Despite the high frequency and severity of TBI, relatively little is known about the biological basis of the cognitive deficits associated with insults to the brain or the innate repair processes that occur in the brain in response to insults. Consequently, no cure is available for the enduring deficits induced by TBI. Nevertheless, recent findings reveal that multipotent neural stem/progenitor cells (NS/NPCs) persist in selected regions of the brain throughout the lifespan of an animal, rendering the brain capable of generating new neurons and glia (Lois and varez-Buylla, 1993; Gage et al., 1998). Furthermore, increasing evidence indicates that these endogenous NS/NPCs may play regenerative and reparative roles in response to CNS injuries or diseases. In support of this notion, heightened levels of cell proliferation and neurogenesis have been observed in response to brain trauma or insults suggesting that the brain has the inherent potential to restore populations of damaged or destroyed neurons. This raises the possibility of developing therapeutic strategies aiming at harnessing this neurogenic capacity in order to repopulate and repair the damaged brain.
Adult neurogenesis in the mammalian brain and its function
The region of neurogenesis in the mature mammalian brain is primarily confined to the subventricular zone (SVZ) surrounding the lateral ventricle and the dentate gyrus (DG) of the hippocampus (Altman and Das, 1965; Lois and Alvarez-Buylla, 1993). The majority of the SVZ progeny are neuroblasts which undergo chain migration along the rostral migratory stream to the olfactory bulb, where they differentiate into olfactory interneurons (Doetsch and Alvarez-Buylla, 1996). Another sub-population of these cells migrate into cortical regions for reasons yet to be identified, but evidence suggests they may be involved in repair or cell renewal mechanisms (Parent, 2002). Likewise, the newly generated cells of the DG migrate laterally into the granule cell layer and exhibit properties of fully integrated mature dentate granule neurons (Kempermann and Gage, 2000; van Praag et al., 2002b). Most importantly, the newly generated DG granule neurons form synapses and extend axons into their correct target area, the CA3 region (Hastings and Gould, 1999).
Multiple studies have quantified the degree of cytogenesis occurring in these regions and have clearly shown that large numbers of new cells are regularly produced (Lois and Alvarez-Buylla, 1993; Cameron and McKay, 2001). Specifically, the rat dentate gyrus produces ~9,000 new cells per day which equates to ~270,000 cells per month (Cameron and McKay, 2001). Considering that the total granule cell population in the rat is 1–2 million cells, this degree of new cell addition is certainly large enough to affect network function. A more recent study has found that in the olfactory bulb almost the entire granule cell population in the deep layer and half of the super layer was replaced by new neurons over a 12-month period (Imayoshi et al., 2008). The same study also reported that in the hippocampus, the adult generated neurons comprised about 10% of the total number of dentate granule cells and they were equally present along the anterior-posterior axis of the DG (Imayoshi et al., 2008). However, studies have also found that in normal adult rodent brains, many newly generated neurons in the DG and non-olfactory bound SVZ cells have a transient existence of two weeks or less (Gould et al., 2001). While this interval is long enough for supportive glial roles; neuron formation and integration into an existing network takes approximately 10–14 days (Alvarez-Buylla and Nottebohm, 1988; Kirn et al., 1999). It must be noted, however, that a small population of these cells are sustained for months to years (Altman and Das, 1965; Eriksson et al., 1998; Gould et al., 2001), strongly supporting the theory of network integration. Furthermore, this dramatic loss of newly generated cells might be a recapitulation of network pruning seen in early mammalian development. Whether the limited life-span represents network pruning or merely distinct cell specific roles is yet to be understood.
In the normal hippocampus, the newly generated granular cells in the adult dentate gyrus (DG) can become functional neurons by displaying passive membrane properties, generate action potentials and functional synaptic inputs as seen in mature DG neurons (van Praag et al., 2002). Increasing evidence has also shown that adult hippocampal neurogenesis is involved in learning and memory function (Clelland et al., 2009; Deng et al., 2009). For example, mouse strains with genetically low levels of neurogenesis perform poorly on learning tasks when compared to those with higher level of baseline neurogenesis (Kempermann et al., 1997a; Kempermann et al., 1998). Conversely, physical activity stimulates a robust increase in the generation of new neurons and subsequently enhances spatial learning and long-term potentiation (van Praag et al., 1999a). Additionally, diminished hippocampal neurogenesis, as observed following the administration of anti-mitotic drugs such as methylazoxymethanol acetate (MAM), cytosine-β-D-arabinofuranoside (AraC), by irradiation or by genetic manipulation, was associated with worse performance on hippocampus-dependent trace eyeblink conditioning (Shors et al., 2001), contextual fear conditioning (Shors et al., 2002; Saxe et al., 2006) and long term spatial memory function tests (Rola et al., 2004; Snyder et al., 2005). Collectively, these studies provide compelling evidence that adult born neurons in the hippocampus play a critical role in many important hippocampal-dependent functions in normal adult brain. Compared to the evident role of hippocampal neurogenesis in hippocampal-dependent functions, the function of SVZ-olfactory neurogenesis is less certain. Thus far, limited studies have found that adult generated neurons in the olfactory bulb have a critical role in olfactory tissue maintenance and are involved in olfactory discrimination and olfactory perceptual learning functions (Gheusi et al., 2000; Moreno et al., 2009; Kageyama et al., 2012).
The proliferation and maturational fate of cells within the SVZ and DG is modulated by a number of physical and chemical cues. For example, biochemical factors such as serotonin, glucocorticoids, ovarian steroids, and growth factors tightly regulate the proliferative response, suggesting that cell proliferation within these regions have physiologic importance (Cameron and Gould, 1994; Kuhn et al., 1997; Tanapat et al., 1999; Banasr et al., 2001). In addition, certain physical stimuli produce alterations in cell production suggesting a role in network adaptation (Gould et al., 1997; Kempermann et al., 1997b; van Praag et al., 1999b). For example, environments that are cognitively and physically enriched increase cell proliferation and neurogenesis in both the SVZ and DG, while stress reduces this type of cellular response (Kempermann et al., 1997b; Gould and Tanapat, 1999). Nevertheless, a functional role for these new cells is dependent upon a significant number of cells being generated, their survival, differentiation, and ultimate integration into existing neuronal circuitry.
Compared to rodent brains, the degree of adult neurogenesis in human brain is less clear. Similar to rodent brains, the SVZ and the hippocampus in human brains are the active neurogenic regions (Eriksson et al., 1998; Sanai et al., 2004). Proliferating NS/NPCs have been found in these areas from autopsy brain samples. Under culture conditions, cells isolated from the adult human brain are capable of generating both neurons and glia (Kukekov et al., 1999; Nunes et al., 2003; Murrell et al., 2013). However, despite the presence of mitotic active cells in the adult human brain, the generation and migration of new neurons is very much limited to the early childhood (Curtis et al., 2012). It is not clear whether this relative resistance of neurogenesis in adult human brain is due to the evolutional repression induced specific regulatory mechanisms or other unknown cellular and molecular mechanisms.
Post-TBI neurogenesis and strategies to enhance endogenous neurogenesis following TBI
The regenerative capacity of the SVZ and DG is of particular interest with regards to TBI. As adult generated neurons from both regions have functional roles, harnessing this endogenous population of stem cells to repopulate the damaged brain is an attractive strategy to repair and regenerate the injured brain.
In the injured brain, studies from our lab and others have shown that TBI significantly increases cell proliferation in both SVZ and DG in adult mice and rats in varying TBI models including fluid percussive injury (Chirumamilla et al., 2002; Rice et al., 2003), controlled cortical impact injury (Dash et al., 2001; Gao et al., 2009), and diffuse weight drop injury (Dash et al., 2001; Chirumamilla et al., 2002). We have also found that the juvenile hippocampus presents a more robust neurogenic response following injury than the adult and aged brain (Sun et al., 2005). Such increased levels of cell proliferation, particularly generation of new neurons, likely contribute to the better recovery in juvenile animals after TBI. Furthermore, we and other have found that injury-induced newly generated granular cells integrate into the existing hippocampal circuitry (Emery et al., 2005; Sun et al., 2007), and this endogenous neurogenesis is associated with innate cognitive recovery following injury (Sun et al., 2007). In human brain specimens, a recently study has found an increased number of cells expressing NS/NPCs markers in the perilesion cortex in the injured brain (Zheng et al., 2013). These studies strongly indicated the inherent attempts of the brain to repair and regenerate following injury.
As the innate recovery capacity is rather limited, it is imperative to augment this endogenous process via exogenous means. As mentioned, many factors have been identified to be able to enhance neurogenesis particularly in the hippocampus. Following traumatic brain injury, thus far, studies have found that varying types of growth factors and drugs can enhance neurogenesis and improve functional recovery of the injured brain following trauma. For example, studies from our lab have shown that direct administration of growth factors bFGF, or EGF through intraventricular infusion can significantly enhance TBI-induced cell proliferation in the hippocampus and the SVZ, and drastically improve cognitive functional recovery of the injured adult animals (Figure 1; Sun et al., 2009, 2010). Other studies have found that infusion of S100β or VEGF can also enhance neurogenesis in the hippocampus and improve the functional recovery of animals following TBI (Kleindienst et al., 2005; Lee and Agoston, 2010; Thau-Zuchman et al., 2010). Several drugs that are currently in clinical trials for treating TBI or other conditions have shown effects in enhancing neurogenesis and cognitive function in TBI animals including statins (Lu et al., 2007), erythropoietin (Lu et al., 2005; Xiong et al., 2010), progesterone (Barha et al., 2011) and anti-depressant imipramine (Han et al., 2011), etc. Other strategies which have beneficial effect for TBI such as hypothermia, environment enrichment also enhanced hippocampal neurogenesis in injured animals (Kovesdi et al., 2011; Bregy et al., 2012). Collectively, these studies suggested the therapeutic potential of augmenting the endogenous repair response for treating TBI.
Figure 1.
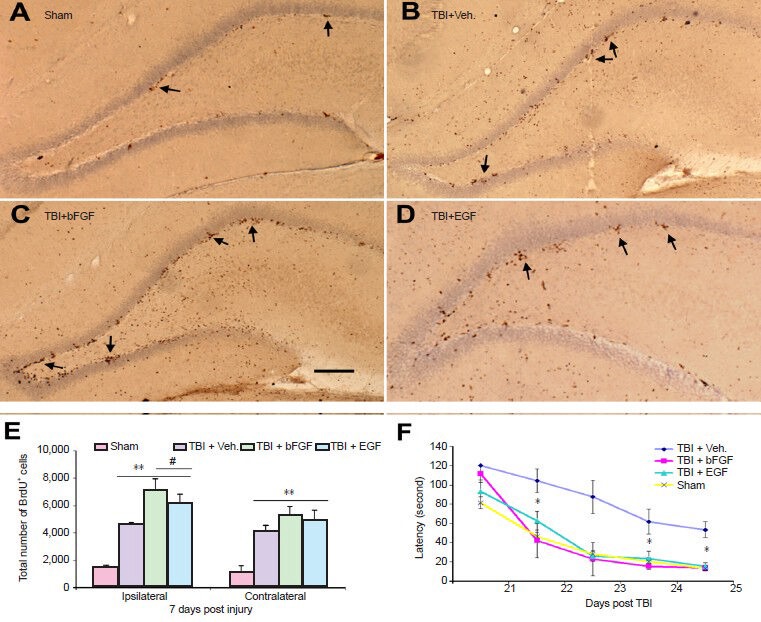
Growth factor basic fibroblast growth factor (bFGF) or epidermal growth factor (EGF) infusion enhances injury-induced cell proliferation in the dentate gyrus (DG) and improves cognitive function in rats following fluid percussive injury.
(A–D) Coronal sections of the ipsilateral DG taken from the following animals at 7 days post injury: sham with vehicle infusion (A), injured with vehicle infusion (B), injured with bFGF infusion (C) and injured with EGF infusion (D). Increased numbers of BrdU+ cells were observed in the injured animals with either vehicle or growth factor infusions compared to the sham (brown dots indicated by arrows). BrdU+ cells in the DG were clustered and mainly located in the subgranular zone. Bar = 100 μm. (E) Stereological quantification analysis of the degree of cell proliferation in the DG. Compared to sham, injured animals with vehicle or growth infusion had significantly more proliferating cells in the granular zone in both ipsilateral and contralateral side (**P < 0.01). Compared to injured with vehicle infusion, injured animals which received bFGF or EGF infusion had significantly higher number of BrdU+ cells in the ipsilateral granular zone (#P < 0.05). (F) Graph compares Morris water maze performance of injured rats infused with bFGF, EGF or vehicle to sham animals. Injured rats infused with bFGF or EGF had a significant improvement of cognitive recovery as compared to injured rats with vehicle (*P < 0.01, n = 10 in each group). This cognitive recovery, as characterized by shorter goal latency in the water maze performance reached similar levels to that observed in sham animals through days 21–25 following injury. TBI: Traumatic brain injury.
Summary
The adult mammalian brain harbors mitotic active cells. These cells have potential to become functional neurons participating neural network repair. Modulating these adult neural stem/progenitor cell pools in the injured brain to treat the injured brain has significant advantages because it makes use of endogenous repair mechanisms to facilitate regeneration which are superior, both in an immunologic and physiological standpoint, than those which rely on exogenous cell replacement.
References
- 1.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–354. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- 3.Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur J Neurosci. 2001;14:1417–1424. doi: 10.1046/j.0953-816x.2001.01763.x. [DOI] [PubMed] [Google Scholar]
- 4.Bregy A, Nixon R, Lotocki G, Alonso OF, Atkins CM, Tsoulfas P, Bramlett HM, Dietrich WD. Posttraumatic hypothermia increases doublecortin expressing neurons in the dentate gyrus after traumatic brain injury in the rat. Exp Neurol. 2012;233:821–828. doi: 10.1016/j.expneurol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 6.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 7.Chirumamilla S, Sun D, Bullock MR, Colello RJ. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J Neurotrauma. 2002;19:693–703. doi: 10.1089/08977150260139084. [DOI] [PubMed] [Google Scholar]
- 8.Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis MA, Low VF, Faull RL. Neurogenesis and progenitor cells in the adult human brain: a comparison between hippocampal and subventricular progenitor proliferation. Dev Neurobiol. 2012;72:990–1005. doi: 10.1002/dneu.22028. [DOI] [PubMed] [Google Scholar]
- 10.Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emery DL, Fulp CT, Saatman KE, Schutz C, Neugebauer E, McIntosh TK. Newly born granule cells in the dentate gyrus rapidly extend axons into the hippocampal CA3 region following experimental brain injury. J Neurotrauma. 2005;22:978–988. doi: 10.1089/neu.2005.22.978. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 14.Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Gao X, Enikolopov G, Chen J. Moderate traumatic brain injury promotes proliferation of quiescent neural progenitors in the adult hippocampus. Exp Neurol. 2009;219:516–523. doi: 10.1016/j.expneurol.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci U S A. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 19.Gould E, Vail N, Wagers M, Gross CG. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci U S A. 2001;98:10910–10917. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han X, Tong J, Zhang J, Farahvar A, Wang E, Yang J, Samadani U, Smith DH, Huang JH. Imipramine treatment improves cognitive outcome associated with enhanced hippocampal neurogenesis after traumatic brain injury in mice. J Neurotrauma. 2011;28:995–1007. doi: 10.1089/neu.2010.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Hilton G. Behavioral and cognitive sequelae of head trauma. Orthop Nurs. 1994;13:25–32. doi: 10.1097/00006416-199407000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 24.Kageyama R, Imayoshi I, Sakamoto M. The role of neurogenesis in olfaction-dependent behaviors. Behav Brain Res. 2012;227:459–463. doi: 10.1016/j.bbr.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 25.Kempermann G, Brandon EP, Gage FH. Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr Biol. 1998;8:939–942. doi: 10.1016/s0960-9822(07)00377-6. [DOI] [PubMed] [Google Scholar]
- 26.Kempermann G, Gage FH. Neurogenesis in the adult hippocampus. Novartis Found Symp. 2000;231:220–235. [PubMed] [Google Scholar]
- 27.Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997a;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997b;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 29.Kirn JR, Fishman Y, Sasportas K, Alvarez-Buylla A, Nottebohm F. Fate of new neurons in adult canary high vocal center during the first 30 days after their formation. J Comp Neurol. 1999;411:487–494. [PubMed] [Google Scholar]
- 30.Kleindienst A, McGinn MJ, Harvey HB, Colello RJ, Hamm RJ, Bullock MR. Enhanced hippocampal neurogenesis by intraventricular S100B infusion is associated with improved cognitive recovery after traumatic brain injury. J Neurotrauma. 2005;22:645–655. doi: 10.1089/neu.2005.22.645. [DOI] [PubMed] [Google Scholar]
- 31.Kovesdi E, Gyorgy AB, Kwon SK, Wingo DL, Kamnaksh A, Long JB, Kasper CE, Agoston DV. The effect of enriched environment on the outcome of traumatic brain injury; a behavioral, pro-teomics and histological study. Front Neurosci. 2011;5:42. doi: 10.3389/fnins.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, O’Brien TF, Kusakabe M, Steindler DA. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156:333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- 34.Lee C, Agoston DV. Vascular endothelial growth factor is involved in mediating increased de novo hippocampal neurogenesis in response to traumatic brain injury. J Neurotrauma. 2010;27:541–553. doi: 10.1089/neu.2009.0905. [DOI] [PubMed] [Google Scholar]
- 35.Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu D, Mahmood A, Qu C, Goussev A, Schallert T, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- 37.Lu D, Qu C, Goussev A, Jiang H, Lu C, Schallert T, Mahmood A, Chen J, Li Y, Chopp M. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma. 2007;24:1132–1146. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreno MM, Linster C, Escanilla O, Sacquet J, Didier A, Mandairon N. Olfactory perceptual learning requires adult neurogenesis. Proc Natl Acad Sci U S A. 2009;106:17980–17985. doi: 10.1073/pnas.0907063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murrell W, Palmero E, Bianco J, Stangeland B, Joel M, Paulson L, Thiede B, Grieg Z, Ramsnes I, Skjellegrind HK, Nygard S, Brandal P, Sandberg C, Vik-Mo E, Palmero S, Langmoen IA. Expansion of multipotent stem cells from the adult human brain. PLoS One. 2013;8:e71334. doi: 10.1371/journal.pone.0071334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 41.Parent JM. The role of seizure-induced neurogenesis in epileptogenesis and brain repair. Epilepsy Res. 2002;50:179–189. doi: 10.1016/s0920-1211(02)00078-5. [DOI] [PubMed] [Google Scholar]
- 42.Rice AC, Khaldi A, Harvey HB, Salman NJ, White F, Fillmore H, Bullock MR. Proliferation and neuronal differentiation of mitotically active cells following traumatic brain injury. Exp Neurol. 2003;183:406–417. doi: 10.1016/s0014-4886(03)00241-3. [DOI] [PubMed] [Google Scholar]
- 43.Rola R, Otsuka S, Obenaus A, Nelson GA, Limoli CL, VandenBerg SR, Fike JR. Indicators of hippocampal neurogenesis are altered by 56Fe-particle irradiation in a dose-dependent manner. Radiat Res. 2004;162:442–446. doi: 10.1667/rr3234. [DOI] [PubMed] [Google Scholar]
- 44.Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia VJ, Berger MS, varez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 45.Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt RH, Scholten KJ, Maughan PH. Time course for recovery of water maze performance and central cholinergic innervation after fluid percussion injury. J Neurotrauma. 1999;16:1139–1147. doi: 10.1089/neu.1999.16.1139. [DOI] [PubMed] [Google Scholar]
- 47.Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 48.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Sun D, Bullock MR, Altememi N, Zhou Z, Hagood S, Rolfe A, McGinn MJ, Hamm R, Colello RJ. The effect of epidermal growth factor in the injured brain after trauma in rats. J Neurotrauma. 2010;27:923–938. doi: 10.1089/neu.2009.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun D, Bullock MR, McGinn MJ, Zhou Z, Altememi N, Hagood S, Hamm R, Colello RJ. Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp Neurol. 2009;216:56–65. doi: 10.1016/j.expneurol.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun D, Colello RJ, Daugherty WP, Kwon TH, McGinn MJ, Harvey HB, Bullock MR. Cell proliferation and neuronal differentiation in the dentate gyrus in juvenile and adult rats following traumatic brain injury. J Neurotrauma. 2005;22:95–105. doi: 10.1089/neu.2005.22.95. [DOI] [PubMed] [Google Scholar]
- 53.Sun D, McGinn MJ, Zhou Z, Harvey HB, Bullock MR, Colello RJ. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp Neurol. 2007;204:264–272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thau-Zuchman O, Shohami E, Alexandrovich AG, Leker RR. Vascular endothelial growth factor increases neurogenesis after traumatic brain injury. J Cereb Blood Flow Metab. 2010;30:1008–1016. doi: 10.1038/jcbfm.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999a;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 57.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999b;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiong Y, Mahmood A, Meng Y, Zhang Y, Qu C, Schallert T, Chopp M. Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J Neurosurg. 2010;113:598–608. doi: 10.3171/2009.9.JNS09844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng W, Zhuge Q, Zhong M, Chen G, Shao B, Wang H, Mao X, Xie L, Jin K. Neurogenesis in adult human brain after traumatic brain injury. J Neurotrauma. 2013;30:1872–1880. doi: 10.1089/neu.2010.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]


