Idiopathic Parkinson's disease (IPD) is a progressive, neurodegenerative movement disorder characterized by bradykinesia, muscular rigidity, postural instability and tremor. IPD is usually diagnosed based on clinical findings, but diagnoses are only 75–90% accurate when compared with autopsy results. Improving diagnostic accuracy is critical for the early differentiation of IPD from other Parkinsonism-related disorders because of differences in their prognoses and treatment. Furthermore, IPD is clinically heterogeneous, with variable prognosis. Although the biological function of neuromelanin has not yet been determined, the selective vulnerability of neuromelanin-containing neurons in patients with IPD suggests a role for this pigment in neurodegeneration. Recently developed ultra-high-field magnetic resonance imaging (MRI) systems produce T1-weighted neuromelanin-sensitive images with very high spatial resolution, enabling the depiction of tissue containing neuromelanin. Here we review recent advances in neuromelanin-sensitive MRI in IPD and related conditions suggesting that neuromelanin may be a potential diagnostic biomarker for IPD.
Neuromelanin is a dark polymer produced in specific populations of catecholaminergic neurons in the brain. Three main regions of the brain contain neuromelanin-producing cells: the substantia nigra (SN) of the midbrain, the locus coeruleus (LC) within the pons and the ventrolateral reticular formation and nucleus of the solitary tract in the medulla oblongata. Moreover, two of these regions, the SN and the LC, contain large clusters of pigmented neurons that appear macroscopically as darkened areas (Fedorow et al., 2005).
Parkinsonian syndrome is a heterogeneous group of movement disorders, which can be subdivided into IPD, genetic forms of Parkinson's disease (PD) and atypical parkinsonian syndrome. In addition, several other neurodegenerative disorders may show clinical signs of parkinsonism. The etiology, histopathology, clinical manifestation and disease course of these disorders vary significantly. The loss of neuromelanin-containing cells within the SN and LC is a primary pathological diagnostic criterion for IPD. The presence of neuromelanin in these vulnerable cells suggests a role for this pigment in the neurodegenerative process of IPD. The amount of neuromelanin contained within the dopaminergic neurons of the midbrain has been reported to be inversely related to the relative vulnerability of these cells to IPD (Hirsch et al., 1988). In addition to the degree of neuromelanin pigmentation, the position of neuromelanin-containing cells within the nigral complex was also found to be a key factor for neuronal survival in IPD (Damier et al., 1999). Neuromelanin has a high chelating ability for iron, and has been shown to bind neurotoxic and toxic metals that could promote neurodegeneration, such as pesticides and MPP +, suggesting a neuroprotective role of neuromelanin (Zecca et al., 2001). Cell death observed in IPD may be partly due to oxidative stress. This oxidation may also be relieved by neuromelanin.
Following its binding of iron, neuromelanin acts as a paramagnetic agent. Recently developed ultra-high-field magnetic resonance imaging (MRI) systems produce T1-weighted neuromelanin-sensitive images of very high spatial resolution (Enochs et al., 1997; Sasaki et al., 2006; Bolding et al., 2013). On these images, neuromelanin-containing tissues appear as foci of high signal intensity, with the intensity proportional to the neuromelanin concentration. Neuromelanin-sensitive MRI can be used to directly measure the volume or concentration of neuromelanin in the SN and LC, suggesting that this modality may be able to distinguish IPD from other related conditions. We review here the usefulness of neuromelanin MRI for the diagnosis of parkinsonian syndrome, with special reference to neuropathological findings.
Parkinson's disease
Using a 3-T system, neuromelanin-sensitive MRI can visualize changes associated with neuronal loss in the SN and LC in patients with IPD (Figure 1), even during early stages, and can distinguish these patients from healthy individuals with high sensitivity and specificity (Sasaki et al., 2006; Ogisu et al., 2013; Ohtsuka et al., 2013). Within the SN, the most significant signal attenuation in patients with IPD was detected in the lateral part, but was less evident in the central part and insignificant in the medial part (Ohtsuka et al., 2013). The topological deletion of neurons in the SN differs in IPD and normal aging, with the ventrolateral tier of neurons being most severely affected in IPD; in contrast, the dorsal tier shows the heaviest cell losses in normal aging while neurons in the ventral tier are relatively well preserved (Damier et al., 1999; Jellinger, 2012). In patients with IPD, signal changes in the LC were greater than in the central and medial parts of the SN and were comparable with those in the lateral part of the SN (Ohtsuka et al., 2013). These findings support the hypothesis of Braak staging of Lewy body pathology in IPD patients, which states that neuronal pathological alterations in the LC precede those in the SN (Braak et al., 2003). However, the actual process of cell death in the LC and SN in patients with IPD has not yet been determined. Neuromelanin MRI may provide an opportunity to distinguish between the neurodegenerative processes observed in patients with Braak stages 2 and 3.
Figure 1.
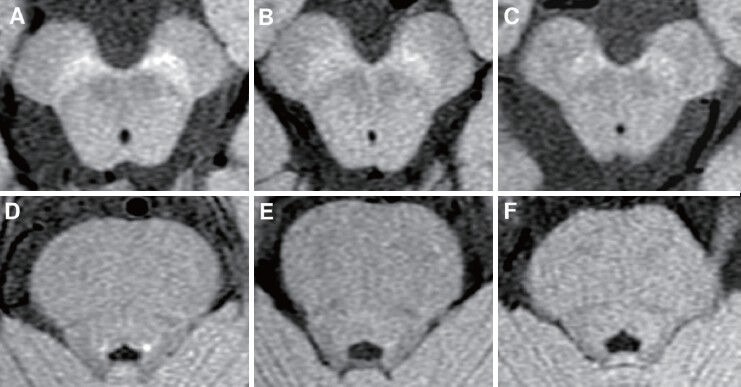
Stage-dependent signal loss of neuromelanin-sensitive MRI in patients with IPD.
Axial images of the SN (top) and LC (bottom) were obtained by 3-Tesla MRI scanning. (A, D) A healthy control. (B, E) A patient with early stage of IPD (Hoehn and Yahr stage II). (C, F) A patient with advanced stage of IPD (Hoehn and Yahr stage IV). IPD: Idiopathic Parkinson's disease; SN: Substantia nigra; LN: locus coeruleus.
Benign tremulous PD (BTP)
BTP is a tremor dominant syndrome with a relatively slow rate of deterioration; its pathological basis is not well understood. BTP can be distinguished from IPD by several clinical features. For example, BTP is characterized by a prominent resting tremor, one of the first signs of this condition, which persistently overshadows other aspects of parkinsonism throughout the course of disease. In addition, BTP is accompanied by non-tremor components of parkinsonism that remain mild; and by an absence of gait disorder, apart from a reduced arm swing or mild stooping. Furthermore, patients with BTP show no more than mild progression, except for tremor, even after having parkinsonism eight years, plus an absence of disability apart from tremor (Josephs et al., 2006). The slower rate of clinical progression in BTP correlates with less severe nigral cell loss on postmortem examinations (Selikhova et al., 2013). We have encountered several patients diagnosed with probable IPD who showed detectable signal intensities in the LC, but also showed normal or mild reduction of hyperintense areas in the SN on neuromelanin-sensitive MRI. Retrospectively, these patients had clinical features consistent with BTP. Neuromelanin MRI results may therefore be good in differentiating BTP from IPD.
Genetic forms of PD
Many recent studies have reported pathology data from autopsies of patients genotyped for PD-related mutations in the alpha-synuclein, Parkin, PINK1, LRRK2 and glucocerebrosidase genes. Postmortem neuropathological findings in patients with genetic forms of PD associated with Parkin, PINK1, and POLG1 mutations showed that the relative preservation of LC neurons compared to the severe loss of SN neurons (Poulopoulos et al., 2012). Interestingly, all of these conditions are considered mitochondrial parkinsonism. The signal intensity changes we observed on neuromelanin imaging in patients with mutations in POLG1 or Parkin provided further evidence that neurons in the SN are profoundly affected while those in the LC are relatively preserved, consistent with the neuropathological findings in each disease (Mukai et al., 2013). In contrast, postmortem neuropathological findings in patients with mutations in alpha-synuclein or LRRK2 showed severe neuronal loss in the LC as well as in the SN, results similar to those in patients with IPD (Poulopoulos et al., 2012). These findings suggest that neuromelanin-sensitive MRI may help differentiate mitochondrial parkinsonism from IPD.
Other parkinsonism-related disorders
Parkinsonism is clinically defined as motor slowness, along with muscle rigidity and/or tremor and/or postural instability. IPD may be misdiagnosed as atypical parkinsonian syndromes, such as multiple system atrophy (MSA), progressive supranuclear palsy (PSP) and corticobasal syndrome. Pathologically, these disorders are characterized by progressive neuronal cell loss in the SN and LC, similar to findings in patients with IPD. Patients with MSA and IPD showed differences in the contrast ratios of the LC and SN on neuromelanin MRI (Matsuura et al., 2013). The contrast ratios of LC and SN were decreased in patients with both MSA and IPD, but most prominently in the LC in patients with MSA. These results suggest that neuromelanin MRI has potential diagnostic value in distinguishing IPD from MSA. In patients with PSP or dementia with Lewy bodies (LBD), neuromelanin-sensitive MRI can show signal loss associated with neuronal loss in the SN and LC, similar to findings in patients with IPD (Kashihara et al., 2011; Kitao et al., 2013). It is therefore necessary to determine whether neuromelanin imaging can be used to differentiate PSP or LBD from IPD.
Neuromelanin MRI may also be useful in distinguishing among other neurodegenerative diseases such as spinocerebellar ataxia (SCA). Postmortem neuropathological findings in patients with SCA2 and SCA3 showed severe neuronal loss in the SN (Yamada et al., 2008). The one patient with SCA2 in our study showed signal attenuation in both the SN and LC on neuromelanin-sensitive MRI.
Conclusion
The signal changes observed on neuromelanin-sensitive MRI in patients with Parkinsonism-related disorders and IPD were consistent with postmortem neuropathological findings. Indeed, comparisons of postmortem neuromelanin MR imaging with neuropathological findings showed a significant positive correlation between signal intensity and the density of neuromelanin-containing neurons in the SN of patients with IPD or LBD (Kitao et al., 2013). Thus, we describe here PD-related conditions in which neuromelanin-sensitive MRI is fairly expected to be useful in differentiating these conditions from IPD, but it is clinically difficult. However, neuromelanin imaging could be used in other disorders, including Alzheimer's disease and schizophrenia (Miyoshi et al., 2013).
A major advance in neuromelanin imaging is the potential to assess disease severity or possibly even disease progression. Although neuromelanin-sensitive MRI showed stage-dependent signal loss in the SN of patients with IPD (Schwarz et al., 2011; Miyoshi et al., 2013), there has been no longitudinal study using neuromelanin imaging. Thus, it remains unclear whether this technique can be used as a biomarker for disease progression in IPD or PD-related disorders.
Although neuromelanin imaging using a 3-T system may become a powerful tool in IPD and related disorders, caution should be exercised when interpreting these data. The iron concentration within the SN can strongly affect neuromelanin-generated T1 contrast. In advanced-stages IPD, augmented iron content in the SN may enhance neuromelanin-related contrast (Wallis et al., 2008). Although postmortem neuromelanin-sensitive MRI in a patient with IPD showed that signal intensity in the SN was not influenced by iron deposition (Kitao et al., 2013), this technique may be needed to improve quantitative evaluation of neuromelanin in the SN. Furthermore, histological studies have revealed that the number of neuromelanin-containing neurons and the amounts of intraneuronal neuromelanin in the LC and SN change with age (Zucca et al., 2006), suggesting that LC and SN signals on neuromelanin MRI imaging reflect changes in neuronal number and intracellular neuromelanin concentration that occur during normal aging (Shibata et al., 2006). Patient age should also be considered when evaluating signal abnormalities. Importantly, the UK PD Society Brain Bank criteria for diagnosis of probable PD do not exclude BTP or genetic forms of PD from IPD. It remains to be determined whether neuromelanin MRI imaging can differentiate early stage IPD from other related disorders, and whether this technique can predict subtypes of PD and patient outcomes.
References
- 1.Bolding MS, Reid MA, Avsar KB, Roberts RC, Gamlin PD, Gawne TJ, White DM, den Hollander JA, Lahti AC. Magnetic transfer contrast accurately localizes substantia nigra confirmed by histology. Biol Psychiatry. 2013;73:289–294. doi: 10.1016/j.biopsych.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 3.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122(Pt 8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 4.Enochs WS, Petherick P, Bogdanova A, Mohr U, Weissleder R. Paramagnetic metal scavenging by melanin: MR imaging. Radiology. 1997;204:417–423. doi: 10.1148/radiology.204.2.9240529. [DOI] [PubMed] [Google Scholar]
- 5.Fedorow H, Tribl F, Halliday G, Gerlach M, Riederer P, Double KL. Neuromelanin in human dopamine neurons: comparison with peripheral melanins and relevance to Parkinson's disease. Prog Neurobiol. 2005;75:109–124. doi: 10.1016/j.pneurobio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature. 1988;334:345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- 7.Jellinger KA. Neuropathology of sporadic Parkinson's disease: evaluation and changes of concepts. Mov Disord. 2012;27:8–30. doi: 10.1002/mds.23795. [DOI] [PubMed] [Google Scholar]
- 8.Josephs KA, Matsumoto JY, Ahlskog JE. Benign tremulous parkinsonism. Arch Neurol. 2006;63:354–357. doi: 10.1001/archneur.63.3.354. [DOI] [PubMed] [Google Scholar]
- 9.Kashihara K, Shinya T, Higaki F. Reduction of neuromelanin-positive nigral volume in patients with MSA, PSP and CBD. Intern Med. 2006;50:1683–1687. doi: 10.2169/internalmedicine.50.5101. [DOI] [PubMed] [Google Scholar]
- 10.Kitao S, Matsusue E, Fujii S, Miyoshi F, Kaminou T, Kato S, Ito H, Ogawa T. Correlation between pathology and neuromelanin MR imaging in Parkinson's disease and dementia with Lewy bodies. Neuroradiology. 2013;55:947–953. doi: 10.1007/s00234-013-1199-9. [DOI] [PubMed] [Google Scholar]
- 11.Matsuura K, Maeda M, Yata K, Ichiba Y, Yamaguchi T, Kanamaru K, Tomimoto H. Neuromelanin magnetic resonance imaging in Parkinson's disease and multiple system atrophy. Eur Neurol. 2013;70:70–77. doi: 10.1159/000350291. [DOI] [PubMed] [Google Scholar]
- 12.Miyoshi F, Ogawa T, Kitao SI, Kitayama M, Shinohara Y, Takasugi M, Fujii S, Kaminou T. Evaluation of Parkinson disease and Alzheimer disease with the use of neuromelanin MR imaging and (123)I-metaiodobenzylguanidine scintigraphy. AJNR Am J Neuroradiol. 2013;34:2113–2118. doi: 10.3174/ajnr.A3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukai M, Sugaya K, Yabe I, Goto Y, Yokochi F, Miyamoto K, Cai H, Sasaki H, Matsubara S. Neuromelanin MRI in a family with mitochondrial parkinsonism harboring a Y955C mutation in POLG1. Parkinsonism Relat Disord. 2013;19:821–824. doi: 10.1016/j.parkreldis.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Ogisu K, Kudo K, Sasaki M, Sakushima K, Yabe I, Sasaki H, Terae S, Nakanishi M, Shirato H. 3D neuromelanin-sensitive magnetic resonance imaging with semi-automated volume measurement of the substantia nigra pars compacta for diagnosis of Parkinson's disease. Neuroradiology. 2013;55:719–724. doi: 10.1007/s00234-013-1171-8. [DOI] [PubMed] [Google Scholar]
- 15.Ohtsuka C, Sasaki M, Konno K, Koide M, Kato K, Takahashi J, Takahashi S, Kudo K, Yamashita F, Terayama Y. Changes in substantia nigra and locus coeruleus in patients with early-stage Parkinson's disease using neuromelanin-sensitive MR imaging. Neurosci Lett. 2013;541:93–98. doi: 10.1016/j.neulet.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Poulopoulos M, Levy OA, Alcalay RN. The neuropathology of genetic Parkinson's disease. Mov Disord. 2012;27:831–842. doi: 10.1002/mds.24962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki M, Shibata E, Tohyama K, Takahashi J, Otsuka K, Tsuchiya K, Takahashi S, Ehara S, Terayama Y, Sakai A. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson's disease. Neuroreport. 2006;17:1215–1218. doi: 10.1097/01.wnr.0000227984.84927.a7. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz ST, Rittman T, Gontu V, Morgan PS, Bajaj N, Auer DP. T1-weighted MRI shows stage-dependent substantia nigra signal loss in Parkinson's disease. Mov Disord. 2011;26:1633–1638. doi: 10.1002/mds.23722. [DOI] [PubMed] [Google Scholar]
- 19.Selikhova M, Kempster PA, Revesz T, Holton JL, Lees AJ. Neuropathological findings in benign tremulous parkinsonism. Mov Disord. 2013;28:145–152. doi: 10.1002/mds.25220. [DOI] [PubMed] [Google Scholar]
- 20.Shibata E, Sasaki M, Tohyama K, Kanbara Y, Otsuka K, Ehara S, Sakai A. Age-related changes in locus ceruleus on neuromelanin magnetic resonance imaging at 3 Tesla. Magn Reson Med Sci. 2013;5:197–200. doi: 10.2463/mrms.5.197. [DOI] [PubMed] [Google Scholar]
- 21.Wallis LI, Paley MN, Graham JM, Grunewald RA, Wignall EL, Joy HM, Griffiths PD. MRI assessment of basal ganglia iron deposition in Parkinson's disease. J Magn Reson Imaging. 2008;28:1061–1067. doi: 10.1002/jmri.21563. [DOI] [PubMed] [Google Scholar]
- 22.Yamada M, Sato T, Tsuji S, Takahashi H. CAG repeat disorder models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:71–86. doi: 10.1007/s00401-007-0287-5. [DOI] [PubMed] [Google Scholar]
- 23.Zecca L, Tampellini D, Gerlach M, Riederer P, Fariello RG, Sulzer D. Substantia nigra neuromelanin: structure, synthesis, and molecular behaviour. Mol Pathol. 2008;54:414–418. [PMC free article] [PubMed] [Google Scholar]
- 24.Zucca FA, Bellei C, Giannelli S, Terreni MR, Gallorini M, Rizzio E, Pezzoli G, Albertini A, Zecca L. Neuromelanin and iron in human locus coeruleus and substantia nigra during aging: consequences for neuronal vulnerability. J Neural Transm. 2006;113:757–767. doi: 10.1007/s00702-006-0453-2. [DOI] [PubMed] [Google Scholar]


