Experimental stroke research commonly employs focal cerebral ischemic rat models (Bederson et al., 1986a; Longa et al., 1989). In human patients, ischemic stroke typically results from thrombotic or embolic occlusion of a major cerebral artery, usually the middle cerebral artery (MCA). Experimental focal cerebral ischemia models have been employed to mimic human stroke (Durukan and Tatlisumak, 2007). Rodent models of focal cerebral ischemia that do not require craniotomy have been developed using intraluminal suture occlusion of the MCA (MCA occlusion, MCAO) (Rosamond et al., 2008). Furthermore, mouse MCAO models have been widely used and extended to genetic studies of cell death or recovery mechanisms (Liu and McCullough, 2011). Genetically engineered mouse stroke models are particularly useful for evaluation of ischemic pathophysiology and the design of new prophylactic, neuroprotective, and therapeutic agents and interventions (Armstead et al., 2010). During the past two decades, MCAO surgical techniques have been developed that do not reveal surgical techniques for mouse MCAO model engineering. Therefore, we compared MCAO surgical methods in rats and mice.
Forty-five male Sprague-Dawley rats, weighing 240–260 g, and thirty-four male C57BL/6 mice, weighing 20–25 g, were selected for this study. 4-0 and 6-0 monofilament nylon (AILEE Co., Busan, Korea) was used in MCAO surgery.
The monofilament was cut into 5-cm (for rats) or 2-cm (for mice) pieces, and the tip of the monofilament was blunted by heating or poly-L-lysine coating (Sigma-Aldrich, St. Louis, MO, USA) (outer diameter of monofilament: 0.4–0.45 mm for rats and 0.15–0.18 mm for mice). All surgical instruments and materials were autoclaved, and the surgical procedure was performed under sterile conditions. This study was approved by the Ethics Committee for Animal Care and Use at Inje University (Approval No. 2012-29), which is certified by the Korean Association of Laboratory Animal Care.
The animals were anesthetized with an intraperitoneal injection of Zoletil (tiletamine + zolazepam cocktail at 40 mg/kg or ketamine at 80 mg/kg) and xylazine (10 mg/kg) (Lee et al., 2012). After anesthetic induction, the animals were placed on a heating pad on a surgical table. During the surgical procedure, the body temperature was continuously monitored with a rectal probe and maintained at 36.5–37.0°C. The surgical region was disinfected with povidone-iodine or 70% alcohol. A midline neck incision was made, and the soft tissues over the trachea were gently retracted with a retractor. The common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were carefully isolated from the vagus nerve. Typically, the CCA bifurcates into the ECA and ICA, which flow toward the cranial and facial regions, respectively, and then the ICA bifurcates into the MCA and pterygopalatine artery (PPA). The occipital artery (OA) originates from the bifurcation point of the ECA which is placed on the side of the ICA (Figure 1A). Two closely spaced permanent knots were then placed at the distal part of the ECA (below the suprathyroid artery) to prevent the backflow of blood. A microvascular clamp was placed in the ICA and transiently proximal to the CCA junction. The tied section of the ECA was dissected using microscissors to insert the monofilament and reach the CCA junction, and a knot was placed below the arteriotomy in the ECA. The microvascular clamp placed in the ICA was removed to allow for filament insertion. The filament was carefully inserted, up to 18 to 20 mm for rats and 9 to 11 mm for mice, into the MCA from the CCA junction (Figure 1A; captured image). After confirmation of MCA blockage, the rat model allowed a blood supply from the CCA, whereas the mouse model allowed a blood supply after the occlusion period. After 60–90 minutes, the filament was carefully withdrawn until the tip was near the arteriotomy. Following removal of the filament, the knot was tightened in the ECA. When reperfusion was confirmed, the neck was sewn using surgical thread. To relieve pain and discomfort in the postoperative period, topical lidocaine gel was applied to the incision region, and the animal received 1.0 mL of normal saline subcutaneously as volume replenishment after the surgery. At 24 hours after the surgery, the animals were sacrificed and analyzed for brain infarction. All procedures had to be finished within 15 minutes, excluding the occlusion and reperfusion time (Figure 1). After 24 hours after reperfusion, infarct volume was calculated using 2% TTC staining method (Figure 1C–E) (Bederson et al., 1986b; Park et al., 2012).
Figure 1.
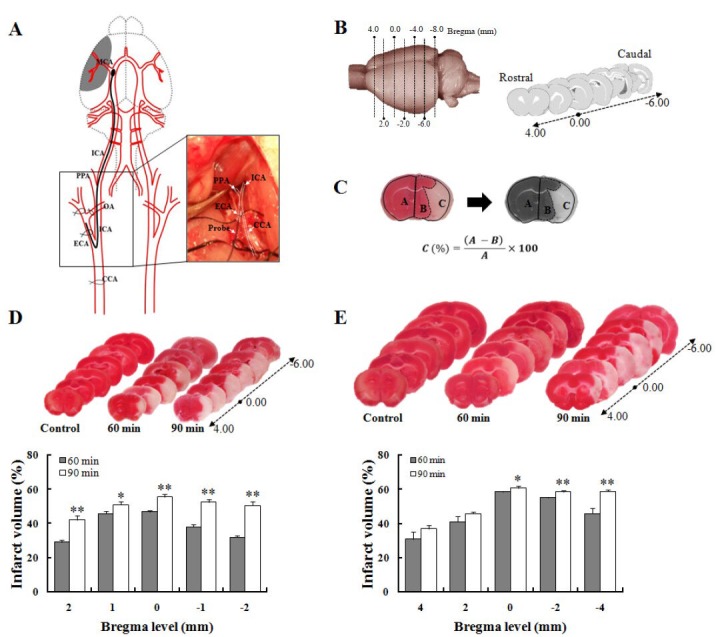
Schematic representation of surgical procedure and quantification of infarct volume in subjected rodents.
(A) Structural diagram of MCAO microsurgery. Photo image of right square box was captured during procedure. (B) Standard lines for rat and mouse brain slices. (C) Calculation formula of TTC-defined infarct volume from rat and mouse brains. In the formula, A: Contralateral region; B: ipsilateral intact region; C: infarct lesion. TTC-stained brain slice features in (D) mice and (E) rats (upper panel), and quantitative results of infarct volume (lower) following 60 minutes (rats: n = 19, mice: n = 16) and 90 minutes (rats: n = 26, mice: n = 18) after MCAO surgery. TTC-stained red color indicates normal region, and white color is an infarct lesion. A Student's t-test was used for statistical analysis. Data are shown as mean ± SD. *P < 0.05, **P < 0.01, vs. 60 minutes. CCA: Common carotid artery; ECA: external carotid artery; ICA: internal carotid artery; OA: occipital artery; PPA: pterygopalatine artery; MCA: middle cerebral artery; MCAO: occlusion of the middle cerebral artery.
In summary, to develop standard and high-quality rodent models of stroke, several points should be taken in MCAO: (1) 0.40–0.45 mm outer diameter 4-0 monofilament nylon (for rats) and 0.15–0.18 mm outer diameter of 6-0 monofilament nylon (for mice) by heating or poly-L-lysine coating. (2) Thread insertion length: 18–20 mm (for rats) and 9–11 mm (for mice). (3) Operation period: maximum of 15 minutes. (4) Occlusion period: 60 minutes. (5) MCA occlusion allows CCA reperfusion for rats or bilateral CCA occlusion for mice.
Acknowledgments:
We would like to express our sincere thanks to former members of the Laboratory of “Biological Clock & Aging” for their help during the course of these studies.
Footnotes
Funding: The study was supported by the 2013 Inje University Research Grant.
Conflicts of interest: None declared.
Copyedited by Xie ZH, Ji Z, Li CH, Song LP, Zhao M
References
- 1.Armstead WM, Ganguly K, Kiessling JW, Riley J, Chen XH, Smith DH, Stein SC, Higazi AA, Cines DB, Bdeir K, Zaitsev S, Muzykantov VR. Signaling, delivery and age as emerging issues in the benefit/risk ratio outcome of tPA For treatment of CNS ischemic disorders. J Neurochem. 2010;113:303–312. doi: 10.1111/j.1471-4159.2010.06613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986a;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 3.Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- 4.Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology,and therapy of focal cerebral ischemia. Pharmacol Biochem Behav. 2007;87:179–197. doi: 10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Shin J, Lee M, Hong Y, Lee SK, Lee Y, Lkhagvasuren T, Kim DW, Yang YA, Chang KT, Hong Y. Melatonin combined with exercise cannot alleviate cerebral injury in a rat model of focal cerebral ischemia/reperfusion injury. Neural Regen Res. 2012;7:993–999. doi: 10.3969/j.issn.1673-5374.2012.13.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F, McCullough LD. Middle cerebral artery occlusion model in rodents: methods and potential pitfalls. J Biomed Biotechnol. 2011 doi: 10.1155/2011/464701. doi:10.1155/2011/464701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 8.Park S, Shin J, Hong Y, Kim S, Lee S, Park K, Lkhagvasuren T, Lee SR, Chang KT, Hong Y. Forced exercise enhances functional recovery after focal cerebral ischemia in spontaneously hypertensive rats. Brain Sci. 2012;2:483–503. doi: 10.3390/brainsci2040483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]


