Abstract
Houshiheisan is composed of wind-dispelling (chrysanthemun flower, divaricate saposhnikovia root, Manchurian wild ginger, cassia twig, Szechwan lovage rhizome, and platycodon root) and deficiency-nourishing (ginseng, Chinese angelica, large-head atractylodes rhizome, Indian bread, and zingiber) drugs. In this study, we assumed these drugs have protective effects against cerebral ischemia, on neurovascular units. Houshiheisan was intragastrically administered in a rat model of focal cerebral ischemia. Hematoxylin-eosin staining, transmission electron microscopy, immunofluorescence staining, and western blot assays showed that Houshiheisan reduced pathological injury to the ischemic penumbra, protected neurovascular units, visibly up-regulated neuronal nuclear antigen expression, and down-regulated amyloid precursor protein and amyloid-β 42 expression. Wind-dispelling and deficiency-nourishing drugs maintained NeuN expression to varying degrees, but did not affect amyloid precursor protein or amyloid-β 42 expression in the ischemic penumbra. Our results suggest that the compound prescription Houshiheisan effectively suppresses abnormal amyloid precursor protein accumulation, reduces amyloid substance deposition, maintains stabilization of the internal environment of neurovascular units, and minimizes injury to neurovascular units in the ischemic penumbra.
Keywords: nerve regeneration, brain injury, cerebral ischemia, Houshiheisan, wind-dispelling drugs, deficiency-nourishing drugs, neurovascular units, amyloid precursor protein, β-amyloid, neuronal nuclear antigen, NSFC grant, neural regeneration
Introduction
Apart from ultra-early thrombolytic therapy, there are no effective therapies for the treatment of ischemic cerebrovascular disease. Various neuroprotective agents have been developed, but their clinical outcomes are limited (Gao et al., 2008). Failure of clinical application of neuroprotective agents is attributed to the complicated pathological changes present in the ischemic brain, and also to drugs acting on single targets (Hu et al., 2009). Based on the physiopathological complexity of stroke, the National Institute of Neurological Disorders and Stroke proposed a conceptual model for stroke therapy, the “neurovascular unit” (Macrez et al., 2011), with interconnecting and interacting components (neurons, glial cells, and vascular endothelial cells) (Shi et al., 2012; Boltze et al., 2012; Pillai et al., 2013; Wong et al., 2013). Protecting neurovascular units provides a novel opportunity for stroke treatment (Del, 2010; Zlokovlc, 2011; Dirnagl, 2012; Busse et al., 2013). Abnormal amyloid-β deposition is strongly associated with injury to neurovascular units. Following brain injury, the cytoskeleton is damaged and axonal transport of amyloid precursor protein blocked (Baranova et al., 2007; Green et al., 2011). Amyloid precursor protein hydrolyzed through the amyloid production pathway induces abnormal deposition of its metabolite, amyloid-β, in the brain, leading to cell apoptosis, metabolic disturbance of free radicals, and mitochondrial injury, thereby influencing energy metabolism and activating the inflammatory cascade (Malito et al., 2008; Giuffrida et al., 2009; Koilke et al., 2012).
Houshiheisan, produced by Zhongjing Zhang for the treatment of ischemic stroke, has proved valuable in clinical practice (Zhao et al., 2003; Zhang et al., 2010). Houshiheisan is composed of wind-dispelling (chrysanthemun flower, divaricate saposhnikovia root, Manchurian wild ginger, cassia twig, Szechwan lovage rhizome, and platycodon root) and deficiency-nourishing (ginseng, Chinese angelica, large-head atractylodes rhizome, Indian bread, and zingiber) drugs, and has key anti-inflammatory, anti-apoptotic, and anti-oxidant effects on improving energy metabolism (Zhao et al., 2006; Zhang et al., 2008). In this study, we examined pathological alterations in neurovascular units, and amyloid-β and amyloid precursor protein expression, in the ischemic penumbra of rats subjected to cerebral ischemia, to determine if Houshiheisan reduces abnormal amyloid-β deposition or protects neurovascular units. Moreover, we examined the effects of the wind-dispelling and deficiency-nourishing drugs of Houshiheisan on inhibiting abnormal amyloid-β deposition and maintaining neurovascular unit function.
Materials and Methods
Animals
A total of 72 healthy, pathogen-free, male Sprague-Dawley rats, weighing 320–350 g, were provided by the Vital River Laboratory Animal Technology Co., Ltd., Beijing, China (license No. SCXK (Jing) 2004-0005).
Grouping
All 72 Sprague-Dawley rats were randomly assigned to five groups: sham surgery (n = 12), model (cerebral ischemia; n = 15), wind-dispelling drug (cerebral ischemia + wind-dispelling drugs; n = 15), deficiency-nourishing drug (cerebral ischemia + deficiency-nourishing drugs; n = 15), and Houshiheisan (cerebral ischemia + Houshiheisan; n = 15).
Traditional Chinese medicine
Wind-dispelling drugs are composed of chrysanthemum flower (40 g), divaricate saposhnikovia root (10 g), cassia twig (3 g), Szechwan lovage rhizome (3 g), Manchurian wild ginger (3 g), and platycodon root (8 g). Deficiency-nourishing drugs are composed of crude large-head atractylodes rhizome (10 g), Indian bread (3 g), zingiber (3 g), Chinese angelica (3 g), and ginseng (3 g). Houshiheisan is composed of chrysanthemum flower (40 g), divaricate saposhnikovia root (10 g), cassia twig (3 g), Szechwan lovage rhizome (3 g), Manchurian wild ginger (3 g), platycodon root (8 g), crude large-head atractylodes rhizome (10 g), Indian bread (3 g), zingiber (3 g), Chinese angelica (3 g), and ginseng (3 g). All Chinese medicinal materials were purchased from Beijing Tongrentang Pharmacy, China.
Preparation of medicines
Wind-dispelling drugs: medicinal materials were triturated, immersed in 200 mL of distilled water for 30 minutes, and treated by ultrasound in a Double-frequency Numerical Control Ultrasonic Cleaner (Model KQ-500VDE; Kunshan, China) at 50°C for 2 hours. A total of 86 mL of filtrate was collected.
Deficiency-nourishing drugs: medicinal materials were triturated, immersed in 60 mL of distilled water for 30 minutes, and treated with ultrasound at 50°C for 2 hours. A total of 40 mL of filtrate was collected.
Houshiheisan: medicinal materials were triturated, immersed in 300 mL of distilled water for 30 minutes, and treated with ultrasound at 50°C for 2 hours. A total of 90 mL of filtrate was collected.
Establishment of a cerebral ischemia model using the suture method
Rats were anesthetized and fixed in the supine position. Proximal ends of the common and external carotid arteries were ligated. The distal end of the internal carotid artery was occluded using a bulldog clamp. An incision was made at the junction of the external and internal carotid arteries. A button-head nylon thread of 0.265 mm diameter was inserted and a depth of 18 mm labeled. The middle cerebral artery blood supply was blocked. Artery stumps were tightly tied and the skin sutured. Rats in the sham surgery group underwent exposure of the internal and external carotid artery branches only. Once conscious, rats that could not completely extend the left forepaw and rotated to the left were considered successful models.
Administration method
The 72 rats were randomly divided into five groups: sham surgery, model, wind-dispelling drug, deficiency-nourishing drug, and Houshiheisan groups. Rats in the sham surgery and model groups were gavaged with physiological saline (of the same volume as treatment groups; 1 mL/100 g). In accordance with body surface area, equivalent doses in the wind-dispelling drug, deficiency-nourishing drug, and Houshiheisan groups were respectively 7.7, 2.59, and 10.5 g/kg. Each treatment group was gavaged with medicines or saline for 3 days. On the 4th day, 20 minutes after administration, operations were performed. Each treatment group was administered once every 12 hours after surgery. Brain tissues were obtained at 24 and 72 hours after ischemia for analysis.
Score of neurological function
In accordance with Zea Longa's method (Longa et al., 1989), behavior was assessed at 24, 48, and 72 hours after surgery. Exclusion criteria: rats scored 0, could not walk, or suffered from coma. Score criteria: 0, no obvious symptom; 1, cannot completely extend the left forepaw; 2, rotate to the left; 3, fall to the left during walking; 4, only walk during stimulation.
Hematoxylin-eosin staining
Seventy-two hours after ischemia, three rats from each group were obtained, anesthetized, perfused with 500 mL of physiological saline. Brain tissue was immersed in 4% paraformaldehyde, dehydrated, embedded in paraffin, sliced into sections, and stained with hematoxylin and eosin. Pathological alterations were observed using a light microscope (Nikon, Tokyo, Japan).
Ultrastructure observation using a transmission electron microscope
Seventy-two hours after ischemia, one rat was obtained from each group, fixed in 2% paraformaldehyde and 2% glutaraldehyde, and then 3% glutaraldehyde for 2 hours. Samples were placed in 0.1 mol/L phosphate buffer at 4°C and ultrathin sections cut. Ultrastructural alterations in neurovascular units were observed using a transmission electron microscope (Hitachi, Tokyo, Japan).
Western blot assay
Phenylmethyl sulfonylfluoride (100 mmol/L) was added to protein extract reagent. Ischemic cortex was lysed in protein extract reagent (1:9), homogenized on ice, and then centrifuged at 10,000 r/min for 20 minutes. Supernatant was stored at −80°C. Protein concentrations were measured using the bicinchoninic acid assay. Protein samples were electrophoresed on sodium dodecyl sulfate polyacrylamide gel for 2 hours, and transferred onto membrane for 1 hour. Membranes were blocked with rotation in 5% skimmed milk powder for 1 hour, and then incubated in primary antibodies, including rabbit anti-rat amyloid precursor protein (1:20,000; 5524-1, Epitomics, Burlingame, CA, USA), rabbit anti-rat amyloid-β 42 (1:1,000; ab10148, Abcam, Cambridge, UK), and rabbit anti-rat GAPDH antibody (1:20,000; CW0101, Tiangen, Beijing, China) at 4°C for 20 hours. Samples were incubated in goat anti-rabbit secondary antibody (IgG, 1:20,000; ANR02-1, Beijing, China) for 1 hour at room temperature. Membranes were washed four times for 5 minutes each time, incubated in enhanced chemiluminescence for 5 minutes, exposed with Kodak film (Comwin, Beijing, China) in the dark room, visualized, and fixed. After air-drying, X-ray film was scanned using a YLN-2000 gel image analysis system (Yalien, Beijing, China). ImageJ software (National Institutes of Health, Santa Clara, CA, USA) was used to process images, and Image Quant TL software (Amersham Biosciences, Piscataway, NJ, USA) to read integrated absorbance values. Relative amounts of target proteins were calculated by determining the integrated absorbance ratio of target proteins/the internal reference GAPDH.
Detection of NeuN immunofluorescence
Two-step immunofluorescence staining was used. Sections were immersed in 0.01 mol/L citrate buffer solution, heated for retrieval for 20 minutes, and then kept at room temperature. After washing with PBS, each section was treated with 50 µL primary antibody mouse anti-NeuU monoclonal antibody (1:100; #MAB377, Millipore, MA, USA) at 4°C for 40 hours and then 37°C for 1 hour, before washing with PBS. Each section was incubated with 50 µL secondary antibody Alexa Flur488 goat anti-mouse IgG (1:200; Tiangen) at 37°C for 2 hours, and washed with PBS and distilled water. Sections were mounted using Dapi-Fluoromount-G at 4°C. A light microscope (Nikon) with filter and excitation lights was used to observe the stained sections. Five fields of ischemic (right) cortex in each group were selected. NIS-Elements Basic Research Image Collection Analysis system (Nikon) was used to analyze fluorescence intensity (integrated absorbance value) of NeuN expression and the number of NeuN-positive cells in each visual field.
Statistical analysis
Measurement data were expressed as mean ± SD, and analyzed using SPSS 11.5 software (SPSS, Chicago, IL, USA). Mean difference among multiple groups was compared using one-way analysis of variance. Paired comparison of intergroup data was performed using least significant difference test. A value of P < 0.05 was considered statistically significant.
Results
Quantitative analysis of experimental animals
Twenty-four hours after model induction, two rats died in each group, except the sham surgery group. After 48 hours, one rat died in the model and wind-dispelling drug groups. At 72 hours, one rat died in the deficiency-nourishing drug and Houshiheisan groups. A total of 60 rats were included in the final analysis, with 12 rats in each group.
Houshiheisan effects on neurological deficits in rats after cerebral ischemia
In accordance with Longa's method (Longa et al.,1989), behavior was examined. Rats with cerebral ischemia had apparent neurological deficits; specifically, the left forepaw did not completely extend, and the rats circled or fell to the left during walking. At 24 and 72 hours after ischemia, neurological deficit scores were lower in the Houshiheisan group than in the model group (P < 0.05). At 48 hours after ischemia, Houshiheisan and wind-dispelling drugs significantly decreased neurological deficit scores (P < 0.01; Table 1).
Table 1.
Influence of wind-dispelling and deficiency-nourishing drugs on neurological deficit scores in rats with cerebral ischemia
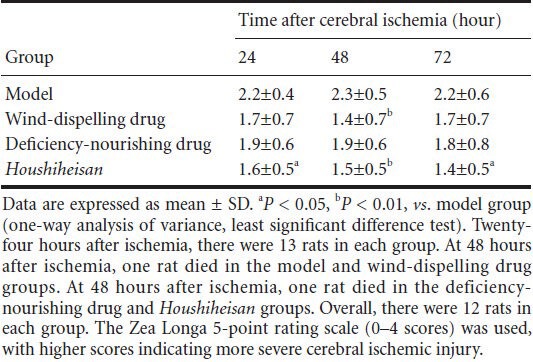
Houshiheisan effects on pathological changes in rat brain tissue 72 hours after cerebral ischemia
In the sham surgery group at 72 hours, hematoxylin-eosin staining showed no abnormal neurons, blood vessels, or glial cells, and nerve fibers were distributed regularly in the medial caudate nucleus. However, in the model group 72 hours after cerebral ischemia, many necrotic tissues were identified in the right infarct foci. Neuronal pyknosis was present, the blood vessel interspace increased, and disordered and some disrupted nerve fibers were present in the medial caudate nucleus and frontal and parietal cortices of the ischemic border zone. In the Houshiheisan group, ischemic degenerated necrotic tissues were reduced. The number of nerve cells was visibly increased in the ischemic penumbra, and the blood vessel interspace slightly increased. There was infiltration of a small number of inflammatory cells, but the blood vessel wall was intact. In the wind-dispelling drug group, some neuronal loss and obvious glial cell proliferation were detected, but with reduced angioedema. In the deficiency-nourishing drug group, neuronal loss was visibly reduced, as well as reduced angioedema and neuronal swelling in the ischemic border zone (Figure 1).
Figure 1.
Ischemic pathological changes in rats 72 hours after cerebral ischemia (hematoxylin-eosin staining, × 1,000; scale bars: 20 μm).
(A) Sham surgery group: normal neurons, blood vessels, gliocytes, and nerve fibers. (B) Model group: neuronal damage, angioedema, and disor-dered nerve fibers. (C) Wind-dispelling drug group: neuronal loss, gliocyte proliferation, and vascular edema. (D) Deficiency-nourishing drug group: decreased neuronal loss and reduced vascular edema. (E) Houshiheisan group: reduced necrosis. Arrows indicate neurons.
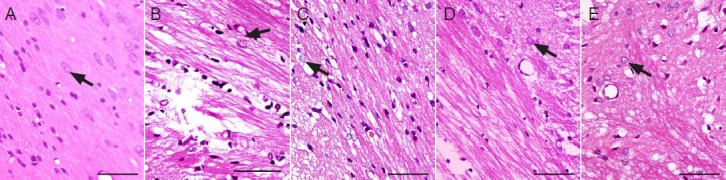
Houshiheisan effects on the ultrastructure of neurovascular units
In the sham surgery group at 72 hours, transmission electron microscopy revealed intact nerve cell membranes, and uniform electron density of the cytoplasm and organelles. Mitochondrial structure was distinct, and endothelial cells intact with continuous basement membranes and apparent tight junctions. Astrocytes were normal. In the model group at 72 hours after ischemia, nerve cell edema, expanded organelles, and damaged mitochondrial cristae were visible in the frontal and parietal cortices. Astrocytes showed marginated chromatin, increased cytoplasmic electron density, slightly extended organelles, and obvious edema. Surrounding endothelial cells exhibited severe edema, open tight junctions, and increased permeability. In the Houshiheisan group, neuronal pyknosis or apoptosis was diminished. Edema was reduced, with continuous basement membranes, and obvious tight junctions surrounding endothelial cells. Swelling of astrocyte foot processes was reduced, showing a protective effect of Houshiheisan on neurovascular units. In the wind-dispelling drug group, nerve cells showed endoplasmic reticulum expansion and slight edema. Capillary endothelial cells and astrocytes had regular structures. Endothelial cell tight junctions were open. In the deficiency-nourishing drug group, neuronal nuclear membranes were intact. Astrocyte foot processes and capillaries were visibly swelled (Figure 2).
Figure 2.
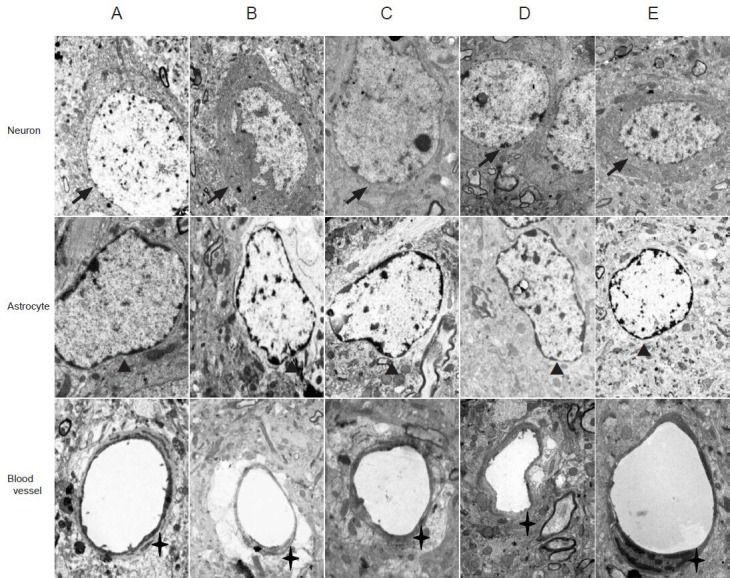
Ischemic ultrastructural changes in rats 72 hours after cerebral ischemia (transmission electron microscope, × 10,000).
(A) Sham surgery group: various organelles (e.g., rough endoplasmic reticulum, mitochondria, lysosomes, and microtubules) are detected evenly in the neuronal cytoplasm. Small pieces of chromatin are present in neuronal nuclei, and astrocytes have elliptical nuclei with uniform chromatin. Junc-tions between endothelial cells are successive. (B) Model group: neuronal edema is evident and various organelles damaged. Chromatin margination is detected, and damaged junctions between endothelial cells. (C) Wind-dispelling drug group: reduced neuronal edema, normal astrocyte structure, and endothelial cells with reduced edema. (D) Deficiency-nourishing drug group: normal nuclear membranes. Neuronal, astrocytic, and endothelial cell edema observed. (E) Houshiheisan group: integrated neuronal structure, and reduced edema of neurons, astrocytes, and endothelial cells. Arrows: Neurons; arrowheads: astrocytes; crosses: blood vessels.
Houshiheisan effects on neurons in the rat cortical ischemic penumbra after cerebral ischemia
Seventy-two hours after ischemia, immunofluorescence staining detected neuronal nuclear antigen (NeuN) expression. Compared with the sham surgery group, the numbers of NeuN-positive neurons and immunological competence were significantly lower in the model group (P < 0.01). Compared with the model group, the numbers of NeuN-positive neurons and immunological competence were significantly higher in the Houshiheisan, wind-dispelling drug, and deficiency-nourishing drug groups (P < 0.05, P < 0.01; Table 2, Figure 3).
Table 2.
Influence of wind-dispelling and deficiency-nourishing drugs on NeuN expression 72 hours after cerebral ischemia in rats (immunofluorescence staining)
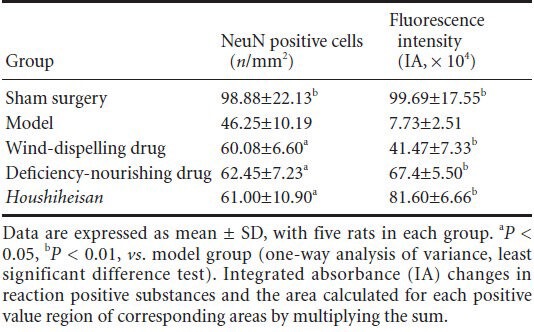
Figure 3.
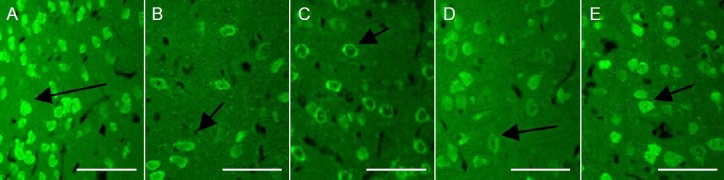
Effects of Houshiheisan on NeuN expression in the ischemic penumbra of rat brain cortex 72 hours after cerebral ischemia (immunofluorescence staining, × 400; scale bars: 50 μm).
Wind-dispelling drugs, deficiency-nourishing drugs, and Houshiheisan increased cortical NeuN expression after cerebral ischemia. (A) Sham sur-gery group; (B) model group; (C) wind-dispelling drug group; (D) deficiency-nourishing drug group; (E) Houshiheisan group. The number of NeuN-positive neurons was significantly higher in the ischemic penumbra in the Houshiheisan, wind-dispelling drug and deficiency-nourishing drug groups compared with the model group. Arrows indicate NeuN-positive cells. NeuN: Neuronal nuclear antigen.
Houshiheisan effects on abnormal amyloid precursor protein and amyloid-β 42 accumulation in rat cerebral cortex after cerebral ischemia
Western blot assays showed that compared with the model group, amyloid-β 42 protein expression was significantly lower in the Houshiheisan group 24 hours after ischemia (P < 0.05). Amyloid precursor protein expression was also significantly lower in the Houshiheisan group 72 hours after ischemia (P < 0.05; Table 3, Figure 4). Wind-dispelling and deficiency-nourishing drugs alone did not influence abnormal amyloid precursor protein and amyloid-β 42 expression.
Table 3.
Influence of wind-dispelling and deficiency-nourishing drugs on amyloid precursor protein (APP) and amyloid-β 42 (Aβ42) expression in rats 24 and 72 hours after cerebral ischemia
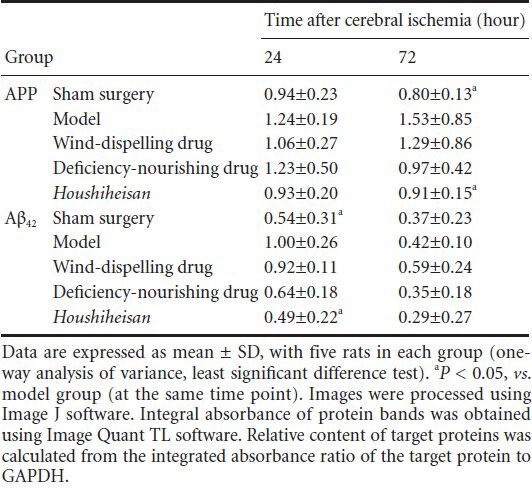
Figure 4.
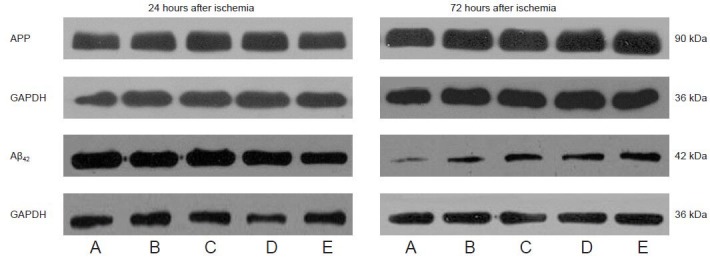
Houshiheisan effects on amyloid precursor protein (APP) and amyloid-β 42 (Aβ42) protein expression in rat brain cortex 24 and 72 hours after cerebral ischemia (western blot assay).
(A) Sham surgery group; (B) model group; (C) wind-dispelling drug group; (D) deficiency-nourishing drug group; (E) Houshiheisan group. Houshiheisan significantly reduced APP protein expression at 72 hours, and Aβ42 protein expression at 24 hours after ischemia.
Discussion
The ischemic penumbra is a region between the infarct core and undamaged region (Sharp et al., 2000). The blood supply of the frontal and parietal cortices is provided by the anterior and middle cerebral arteries (Chen et al., 2002). In a model of cerebral ischemia, ischemia of the frontal and parietal cortices is mild, i.e., the ischemic penumbra is present (Chen et al., 2002). Our ultrastructural observations revealed neuronal pyknosis and apoptosis, evident swelling of astrocyte foot processes, swelling of surrounding blood vessels, and nerve fiber demyelination in the ischemic penumbral cortex of rats, indicating that various neurovascular unit components are injured 72 hours after ischemia. The ischemic penumbra is a dynamically changing region, and after restoration of blood flow can become either normal or infarcted tissue (Heiss, 2011). Neurons in the penumbral region cannot maintain normal physiological function, but do maintain the ability to synthesize proteins. We examined the degree of neuronal degeneration in the ischemic penumbral cortex by NeuN expression. Decreased NeuN expression in the ischemic penumbral cortex indicates degenerating neurons. Minimizing neuronal degeneration in the ischemic penumbral region may protect neurovascular units.
Injury to neurovascular units is strongly associated with abnormal amyloid-β deposition. Amyloid-β is an amyloid precursor protein fragment, obtained after shearing. Under normal conditions, amyloid precursor protein is hydrolyzed through the non-amyloid production pathway (Nalivaeva et al., 2013). After brain injury, axonal transport of amyloid precursor protein is interrupted, and amyloid precursor protein abnormally aggregates. Amyloid precursor protein is then hydrolyzed through the amyloid production pathway, resulting in amyloid-β accumulation (Nalivaeva et al., 2013). Amyloid-β exists in amyloid-β 42 and amyloid-β 40 forms, and exhibits neuronal toxicity. Compared with amyloid-β 40, the two additional amino acids of amyloid-β 42 not only increase amyloid-β hydrophobicity but also allow easier amyloid-β aggregation. Our results show abnormal amyloid-β 42 deposition in the ischemic cortex 24 hours after ischemia, consistent with a previous study (Qi et al., 2006). Amyloid-β 42 directly activates the complement system, producing neurotoxic effects through the classical complement pathway by forming a membrane attack complex that destroys synaptic structures, induces free radical production and oxidative stress by lipid peroxidation, and finally results in neuronal degeneration (Kienlen et al., 2000; Walter, 2006). Amyloid-β 42 acts on vascular endothelial cells causing vasoconstriction and decreased cerebral blood flow, and activates the cerebral ischemic cascade. Accumulated amyloid-β in turn acts on amyloid-β in amyloid precursor protein, accelerating amyloid precursor protein aggregation (Walsh et al., 2005). Cerebral ischemia leads to axonal injury, axonal transport dysfunction, and abnormal amyloid precursor protein accumulation (Guan et al. 2011; Xing et al., 2012). Neuronal degeneration and synaptic loss can be induced by inhibiting establishment of collateral circulation, reducing blood flow in the penumbral region, and increasing the infarct area (Malito et al., 2008). In this study, amyloid precursor protein expression was noticeably up-regulated 72 hours after cerebral ischemia. Thus, suppressing abnormal amyloid precursor protein and amyloid-β 42 accumulation is of significance to protecting neurovascular units.
Whether Houshiheisan suppressed abnormal amyloid precursor protein expression or reduced abnormal amyloid-β deposition is a focus of this study. Our western blot assay results demonstrated Houshiheisan down-regulates amyloid-β 42 protein expression in rat cortex at 24 hours, and reduces amyloid precursor protein expression at 72 hours. Previous studies have shown that cerebral ischemic injury can induce biochemical and metabolic disorders, and Houshiheisan improves energy metabolism, prevents oxidation, reduces inflammatory cascades, inhibits neural cell apoptosis, and promotes recovery of neurological function (Zhao et al., 2006; Zhang et al., 2008). Therefore, this study assumed that Houshiheisan inhibits amyloid-β 42 neurotoxicity by preventing oxidation, scavenging oxygen free radicals, and reducing inflammatory cascades. Alternatively, Houshiheisan may reduce amyloid-β 42 production by suppressing abnormal amyloid precursor protein accumulation and controlling the hydrolytic pathway of amyloid precursor protein.
Compatibility of the Houshiheisan prescription is a serious consideration. To deeply understand the subtleties of Zhongjing Zhang's method for stroke, we examined his prescription to determine the effect of specific medicinal components. In accordance with classification of traditional Chinese medicine in Medicine Origin, chrysanthemum flower, divaricate saposhnikovia root, Manchurian wild ginger, cassia twig, Szechwan lovage rhizome, and platycodon root are wind-dispelling drugs. Ginseng, Chinese angelica, large-head atractylodes rhizome, Indian bread, and zingiber are deficiency-nourishing drugs. There is no dispute about using deficiency-nourishing drugs for stroke treatment in China, but the application of wind-dispelling drugs for stroke has gradually decreased from the Tang and Song Dynasties. However, long-period medical practice has verified that reasonable selection of wind-dispelling drugs is a key method to elevate therapeutic effects of stroke treatment in the clinic (Wang et al., 2006). Pharmacological studies suggest that divaricate saposhnikovia root, Manchurian wild ginger, cassia twig, and Szechwan lovage rhizome can expand coronary arteries, decrease blood pressure and blood lipids, improve microcirculation, prevent thrombosis (Yang, 2012), and also increase the body's tolerance to ischemia and hypoxia (Gong, 2000). A recent study demonstrated that ligustrazine of Szechwan lovage rhizome suppresses the waterfall effect of inflammatory cell infiltration after cerebral ischemia (Lei et al., 2000).
Our previous study found that the wind-dispelling drugs of Houshiheisan inhibit caspase-3 activation, while the deficiency-nourishing drugs up-regulate DNA repair protein poly (ADP-ribose) polymerase expression. Houshiheisan also minimized oxidative DNA injury after cerebral ischemia via different pathways (Zhang et al., 2013). In this study, wind-dispelling or deficiency-nourishing drugs alone did not alter abnormal amyloid precursor protein or amyloid-β 42 expression, but combined application of wind-dispelling and deficiency-nourishing drugs suppressed abnormal amyloid precursor protein and amyloid-β 42 expression. Our results show that wind-dispelling and deficiency-nourishing drugs complement each other, inhibiting abnormal amyloid precursor protein accumulation, diminishing amyloid substance deposition, and maintaining stabilization of the internal environment of neurovascular units by intervening with amyloid-β-induced toxicity.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 30973782, 81373526; the Natural Science Foundation of Beijing, No. 7102014, 7122018; the Beijing Municipal Higher Learning Institution Talent Teaching Plan “Young and Middle-aged Talented People Training” Project, No. PXM2011014226.
Conflicts of interest: None declared.
Copyedited by James R, Wysong S, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse mouse model of transient focal cerebral ischemia. J Neurosci. 2007;23:6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boltze J, Kleinschnitz C, Reymann KG, Reiser G, Wagner DC, Kranz A, Michalski D. Neurovascular pathophysiology in ischemia, dementia and the ageing brain-current trends in basic, translational and clinical research. Exp Transl Stroke Med. 2012;1:1–14. doi: 10.1186/2040-7378-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busse O, Rother J, Hamann GF, Hamann GF, Hupp T, Jansen O, Meixensberger J, Neumann-Haefelin T, Schackert G, Ringelstein EB. Interdisciplinary neurovascular network: a new structure for treatment of stroke and other cerebrovascular diseases in Germany. Nervenarzt. 2012;10:1228–1232. doi: 10.1007/s00115-013-3848-y. [DOI] [PubMed] [Google Scholar]
- 4.Chen YX, Lin XT, Zhao G. Relationship between amyloid precursor protein mRNA expression in ischemic penumbra and infarct size of rats. Zhongguo Shenjing Kexue Zazhi. 2002;1:454–457. [Google Scholar]
- 5.Del Zoppo GJ. The neurovascular unit in the setting of stroke. J Intern Med. 2002;2:156–171. doi: 10.1111/j.1365-2796.2009.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dirnagl U. Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann N Y Acad Sci. 2012;1268:21–25. doi: 10.1111/j.1749-6632.2012.06691.x. [DOI] [PubMed] [Google Scholar]
- 7.Gao M, Liu R, Du GH. Ischemic stroke treatment targeting new strategy-neurovascular unit. Zhongguo Linchuang Yaolixue yu Zhiliaoxue Zazhi. 2008;7:813–821. [Google Scholar]
- 8.Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, Bruno V, Molinaro G, Pappalardo G, Messina A, Palmigiano A, Garozzo D, Nicoletti F, Rizzarelli E, Copani A. Beta-amyloid monomers are neuroprotective. J Neurosci. 2009;34:10582–10587. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong DH. Treatment of 41 Cases of acute-cerebral infarction mainly with medicines of wind nature. Hunan Zhongyi Daobao. 2000;20:18–19. [Google Scholar]
- 10.Green KN, Peers C. Amyloid beta peptides mediate hypoxic augmentation of Ca(2+) channels. J Neurochem. 3:953–956. doi: 10.1046/j.1471-4159.2001.00338.x. [DOI] [PubMed] [Google Scholar]
- 11.Guan SY, Tang WG, Zhu WY. Study on the effect of amyloid precursor protein in the treatment of cerebral infarction with gastrodin. Linchuang Tantao. 2011;3:142–143. [Google Scholar]
- 12.Heiss WD. The ischemic penumbra: correlates in imaging and implications for treatment of ischemic stroke. The Johann Jacob Wepfer award 2011. Cerebrovasc Dis. 2011;4:307–320. doi: 10.1159/000330462. [DOI] [PubMed] [Google Scholar]
- 13.Hu S, Zhao H, Wang DX, Zhang YB. The Research of neurovascular unit. Shenjing Sunshang yu Gongneng Chongjian. 2009;6:441–443. [Google Scholar]
- 14.Kienlen-Campard P, Tasiaux B, Octave JN. The processing and biological function of the human amyloid precursor protein (APP): lessons from different cellular models. Exp Gerontol. 2000;6:843–850. doi: 10.1016/s0531-5565(00)00170-4. [DOI] [PubMed] [Google Scholar]
- 15.Koike MA, Lin AJ, Pham J, Nguyen E, Yeh JJ, Rahimian R, Tromberg BJ, Choi B, Green KN, LaFerla FM. APP knockout mice experience acute mortality as the result of ischemia. PLoS One. 2012;8:1–8. doi: 10.1371/journal.pone.0042665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei WL, Liu Y, Yuan QF, Yao ZB. Protective effect of ligustrazine on cerebral ischemia. Zhonghua Shenjingke Zazhi. 2000;2:100. [Google Scholar]
- 17.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;1:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 18.Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, Vivien D. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;5:471–480. doi: 10.1016/S1474-4422(11)70066-7. [DOI] [PubMed] [Google Scholar]
- 19.Malito E, Hulse RE, Tang WJ. Amyloid β-degrading cryptidases: insulin degrading enzyme, presequence peptidase, and neprilysin. Cell Mol Life Sci. 2008;16:2574–2585. doi: 10.1007/s00018-008-8112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nalivaeva NN, Turner AJ. The amyloid precursor protein: a biochemical enigma in brain development, function and disease. FEBS Lett. 2013;13:2046–2054. doi: 10.1016/j.febslet.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Pillai DR, Shanbhag NC, Dittmar MS, Bogdahn U, Schlachetzki F. Neurovascular protection by targeting early blood-brain barrier disruption with neurotrophic factors after ischemia-reperfusion in rats. J Cereb Blood Flow Metab. 2013;4:557–566. doi: 10.1038/jcbfm.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi JP, Wang DD, Wang DS, Tang Y, Zhang Y, Wang Y, Wang WZ, Chen YX, Dong YY. The expression of β-amyloid protein in hippocampus of patients with cerebral infarction. Zhonghua Shenjingke Zazhi. 2006;2:118–121. [Google Scholar]
- 23.Sharp FR, Lu A, Tang Y, Millhorn DE. Multiple molecular penumbras after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;7:1011–1032. doi: 10.1097/00004647-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Shi QH, Xiang J, Zhu XY, Cai DF. Protective effects of Chinese herbal medicine Naoshuantong on neurovascular unit in rats with cerebral ischemia/reperfusion injure. Zhong Xi Yi Jie He Xue Bao. 2012;10:1135–1139. doi: 10.3736/jcim20121010. [DOI] [PubMed] [Google Scholar]
- 25.Walsh DM, Townsend M, Podlisny MB, Shankar GM, Fadeeva JV, EL Agnaf O, Hartley DM, Selkoe DJ. Certain inhibitors of synthetic amyloid β-peptide (Aβ) fibrillogenesis block oligomerization of natural Aβ and thereby rescue long-term potentiation. J Neurosci. 2005;10:2455–2462. doi: 10.1523/JNEUROSCI.4391-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walter J. Control of amyloid-β-peptide generation by subcellular trafficking of the β-amyloid precursor protein and β-secretase. Neurodegener Dis. 2006;4-5:247–254. doi: 10.1159/000095263. [DOI] [PubMed] [Google Scholar]
- 27.Wang JP, Dui LL, Zhang YJ. Interactions of amyloid precursor protein (APP) and its biological significance. Shengming Kexue. 2009;2:253–258. [Google Scholar]
- 28.Wang LP, Bai X, Yang SJ. The Dispelling Wind-drug for the Treatment of Ischemic Stroke. Zhongxuyi Jiehe Xinnao Xueguan Bing Zazhi. 2006;2:146–148. [Google Scholar]
- 29.Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC. The blood-brain barrier: an engineering perspective. Front Neuroeng. 2013;7:1–7. doi: 10.3389/fneng.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing ZH, Zhang QX, Zhao H. Effects of buyang huanwu decoction combined with liuwei dihuang wan on app expression and reconstruction of neuron synapses in permanent cerebral ischemia model in rats. Zhongguo Shiyan Fangjixue Zazhi. 2012;20:161–165. [Google Scholar]
- 31.Yang KQ. To investigate the wind drug application in the brain. Guangming Zhongyi. 2012;6:1081–1083. [Google Scholar]
- 32.Zhang QX, Zhao H. The effects of houshiheisan on activeness of Na + -K + -ATP and nos in cerebral tissue of middle cerebral artery occlusion model in rat. Zhongguo Shiyan Fangjixue Zazhi. 2008;7:31–33. [Google Scholar]
- 33.Zhang QX, Zhao H, Wang L. Effect of Houshihei Powder combining with enriched environment on neurocyte lesion in rats with cerebral ischemia. Beijing Zhongyiyao Daxue Xuebao. 2010;3:166–170. [Google Scholar]
- 34.Zhang QX, Zhao H, Zhang C, Wang L, Li R, Zhang Q, Wang HZ. Influences of wind-dispelling and tonfying Chinese medicine on expressions of Caspase-3 and PARP in rat with cerebral ischemia. Beijing Zhongyiyao Daxue Xuebao. 2013;4:246–249. [Google Scholar]
- 35.Zhao H, Zhang QX, Mu Y. Influence of Houshiheisan on hemorheological parameters and metabolism of free radicals in brain of rats with ischemia. Zhongguo Shiyan Fangjixue Zazhi. 2006;12:38–40. [Google Scholar]
- 36.Zhao ZX, Peng J. “Golden Chamber” stroke treatment from the wind Theory. Hunan Zhongyiyao Xueyuan Xuebao. 2003;2:26–28. [Google Scholar]
- 37.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]


