Abstract
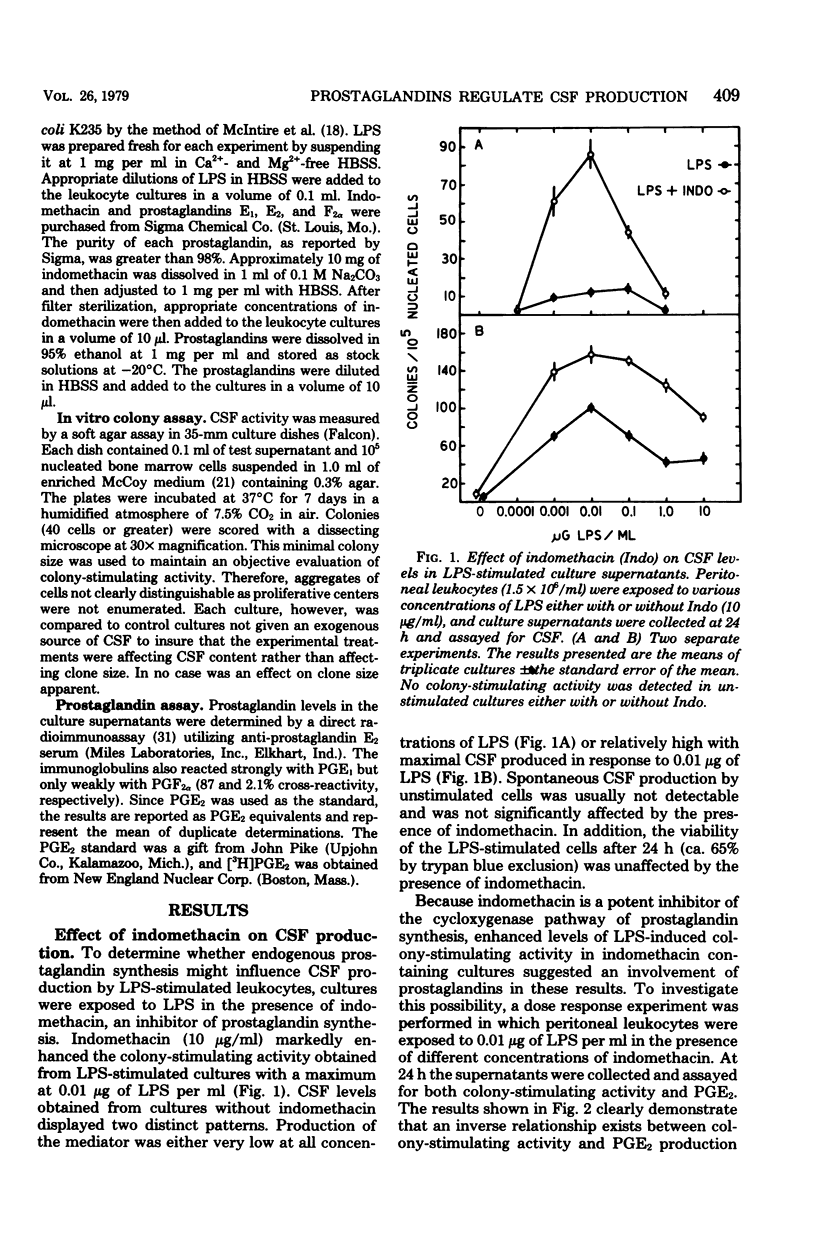
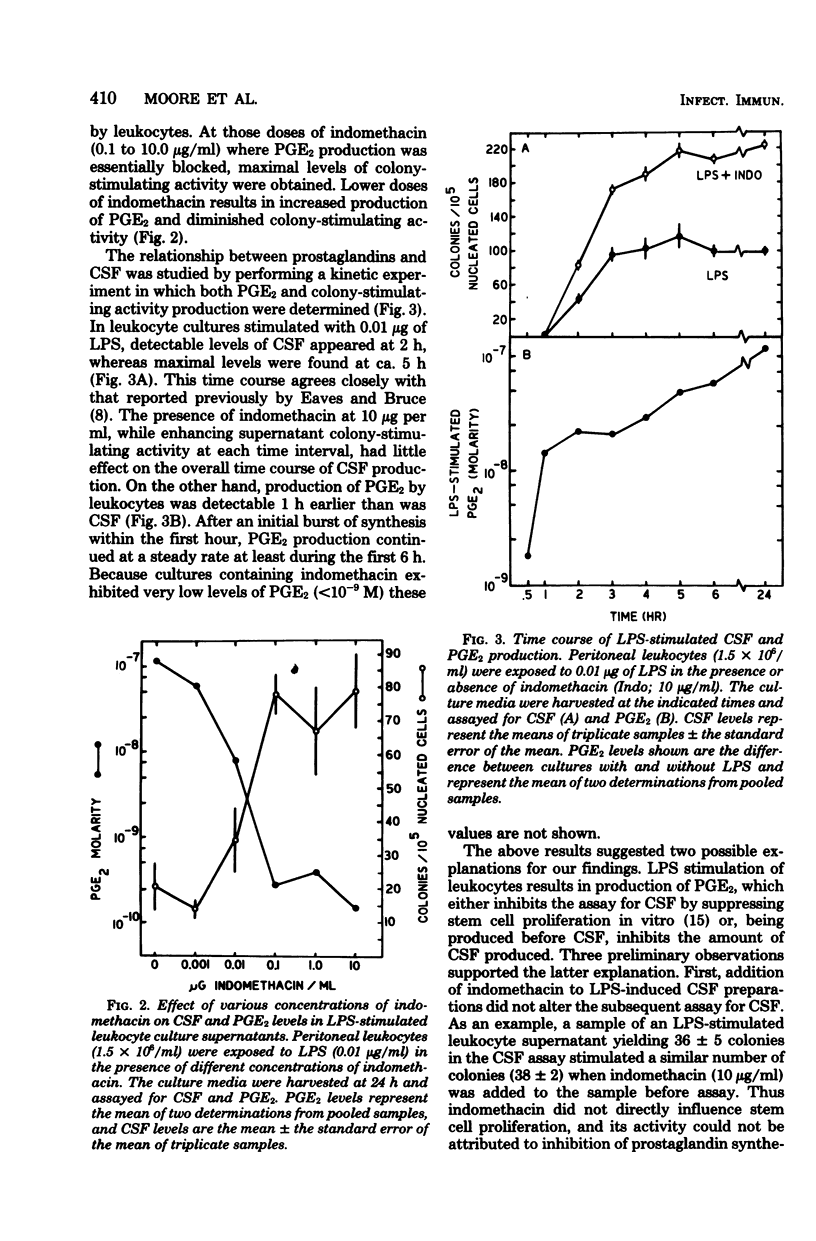
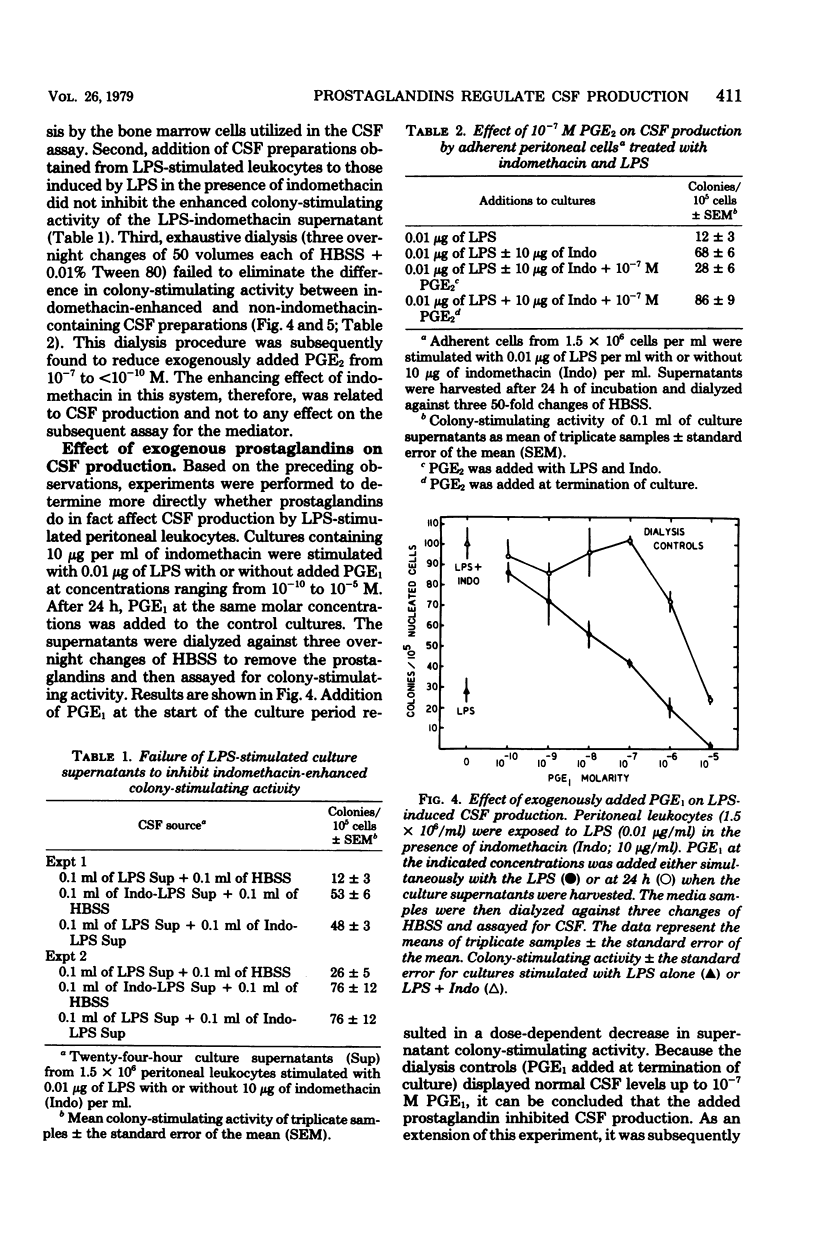
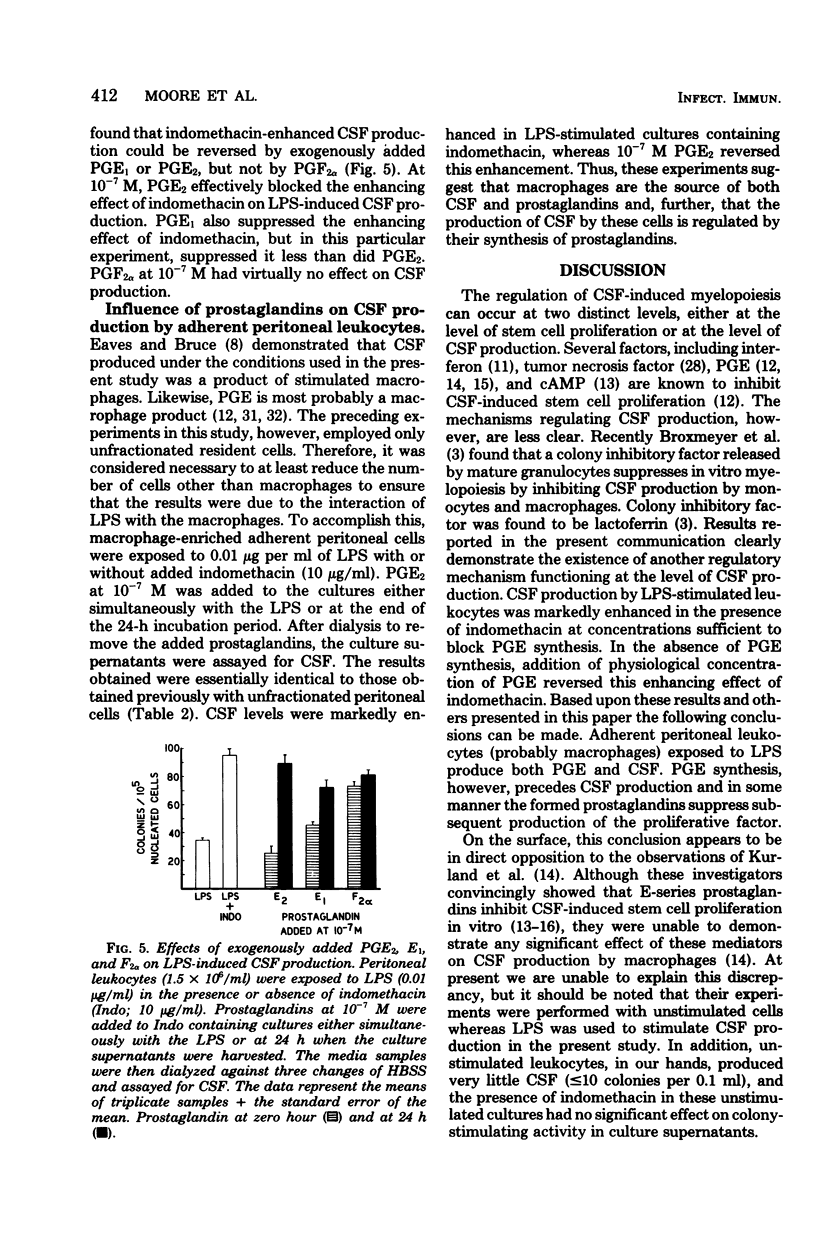
The production of colony-stimulating factor by lipopolysaccharide-stimulated murine peritoneal leukocytes and their adherent subpopulations (greater than 80% macrophages) was markedly enhanced by indomethacin at concentrations sufficient to block prostaglandin E synthesis. Addition of physiological concentrations of E-series prostaglandins reversed this enhancing effect of indomethacin in a dose-dependent manner. These results indicate that colony-stimulating factor production by stimulated leukocytes is regulated by E-series prostaglandins and suggest that prostaglandins function to limit myelopoiesis by inhibiting colony-stimulating factor production and concomitantly the induction of cell proliferation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Bray M. A., Gordon D., Morley J. Prostaglandins as regulators in cellular immunity. Prostaglandins Med. 1978 Sep;1(3):183–199. doi: 10.1016/0161-4630(78)90105-2. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Smithyman A., Eger R. R., Meyers P. A., de Sousa M. Identification of lactoferrin as the granulocyte-derived inhibitor of colony-stimulating activity production. J Exp Med. 1978 Oct 1;148(4):1052–1067. doi: 10.1084/jem.148.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A. W., Metcalf D., Watt S. M. Regulation of hemopoietic cell differentiation and proliferation. J Supramol Struct. 1978;8(4):489–500. doi: 10.1002/jss.400080411. [DOI] [PubMed] [Google Scholar]
- Butler R. C., Abdelnoor A. M., Nowotny A. Bone marrow colony-stimulating factor and tumor resistance-enhancing activity of postendotoxin mouse sera. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2893–2896. doi: 10.1073/pnas.75.6.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. J., Rothman B., Golde D. W. Effect of endotoxin on the production of colony-stimulating factor by human monocytes and macrophages. J Cell Physiol. 1974 Oct;84(2):193–196. doi: 10.1002/jcp.1040840205. [DOI] [PubMed] [Google Scholar]
- DeRubertis F. R., Zenser T. V., Adler W. H., Hudson T. Role of cyclic adenosine 3',5'-monophosphate in lymphocyte mitogenesis. J Immunol. 1974 Jul;113(1):151–161. [PubMed] [Google Scholar]
- Eaves A. C., Bruce W. R. In vitro production of colony-stimulating activity. I. Exposure of mouse peritoneal cells to endotoxin. Cell Tissue Kinet. 1974 Jan;7(1):19–30. doi: 10.1111/j.1365-2184.1974.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Bankhurst A. D., Peake G. T., Saiki J. H., Williams R. C., Jr Prostaglandin-producing suppressor cells in Hodgkin's disease. N Engl J Med. 1977 Nov 3;297(18):963–968. doi: 10.1056/NEJM197711032971802. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. S., Broxmeyer H. E., Moore M. A. Limitation of excessive myelopoiesis by the intrinsic modulation of macrophage-derived prostaglandin E. Science. 1978 Feb 3;199(4328):552–555. doi: 10.1126/science.304600. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978 Mar 1;147(3):952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland J. I., Broxmeyer H. E., Pelus L. M., Bockman R. S., Moore M. A. Role for monocyte-macrophage-derived colony-stimulating factor and prostaglandin E in the positive and negative feedback control of myeloid stem cell proliferation. Blood. 1978 Aug;52(2):388–407. [PubMed] [Google Scholar]
- Kurland J. I., Hadden J. W., Moore M. A. Role of cyclic nucleotides in the proliferation of committed granulocyte-macrophage progenitor cells. Cancer Res. 1977 Dec;37(12):4534–4538. [PubMed] [Google Scholar]
- Kurland J., Moore M. A. Modulation of hemopoiesis by prostaglandins. Exp Hematol. 1977 Sep;5(5):357–373. [PubMed] [Google Scholar]
- McIntire F. C., Sievert H. W., Barlow G. H., Finley R. A., Lee A. Y. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967 Aug;6(8):2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Acute antigen-induced elevation of serum colony stimulating factor (CFS) levels. Immunology. 1971 Sep;21(3):427–436. [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Chan S. H., Gunz F. W., Vincent P., Ravich R. B. Colony-stimulating factor and inhibitor levels in acute granulocytic leukemia. Blood. 1971 Aug;38(2):143–152. [PubMed] [Google Scholar]
- Pike B. L., Robinson W. A. Human bone marrow colony growth in agar-gel. J Cell Physiol. 1970 Aug;76(1):77–84. doi: 10.1002/jcp.1040760111. [DOI] [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The induction of clones of normal mast cells by a substance from conditioned medium. Exp Cell Res. 1966 Oct;43(3):553–563. doi: 10.1016/0014-4827(66)90026-7. [DOI] [PubMed] [Google Scholar]
- Ralph P., Broxmeyer H. E., Nakoinz I. Immunostimulators induce granulocyte/macrophage colony-stimulating activity and block proliferation in a monocyte tumor cell line. J Exp Med. 1977 Aug 1;146(2):611–616. doi: 10.1084/jem.146.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin E., Bauminger S., Globerson A. Effect of prostaglandins on phagocytosis of sheep erythrocytes by mouse peritoneal macrophages. J Reticuloendothel Soc. 1978 Apr;23(4):237–242. [PubMed] [Google Scholar]
- Samuelsson B., Goldyne M., Granström E., Hamberg M., Hammarström S., Malmsten C. Prostaglandins and thromboxanes. Annu Rev Biochem. 1978;47:997–1029. doi: 10.1146/annurev.bi.47.070178.005025. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Pavlidis N. A., Stylos W. A., Chirigos M. A. Regulation of macrophage tumoricidal function: a role for prostaglandins of the E series. Science. 1978 Oct 20;202(4365):320–321. doi: 10.1126/science.694537. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Parker C. W. Human lymphocytic metabolism. Effects of cyclic and noncyclic nucleotides on stimulation by phytohemagglutinin. J Clin Invest. 1971 Feb;50(2):442–448. doi: 10.1172/JCI106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Hamilton J., Reich E. Macrophage plasminogen activator: modulation of enzyme production by anti-inflammatory steroids, mitotic inhibitors, and cyclic nucleotides. Cell. 1976 Jun;8(2):271–281. doi: 10.1016/0092-8674(76)90011-8. [DOI] [PubMed] [Google Scholar]
- Wahl L. M., Olsen C. E., Sandberg A. L., Mergenhagen S. E. Prostaglandin regulation of macrophage collagenase production. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4955–4958. doi: 10.1073/pnas.74.11.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Rosenstreich D. L., Glode L. M., Sandberg A. L., Mergenhagen S. E. Defective prostaglandin synthesis by C3H/HeJ mouse macrophages stimulated with endotoxin preparations. Infect Immun. 1979 Jan;23(1):8–13. doi: 10.1128/iai.23.1.8-13.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb D. R., Nowowiejski I. The role of prostaglandins in the control of the primary 19S immune response to sRBC. Cell Immunol. 1977 Sep;33(1):1–10. doi: 10.1016/0008-8749(77)90129-0. [DOI] [PubMed] [Google Scholar]
- Webb D. R. The effects of prostaglandins on cAMP levels in subpopulations of mouse lymphocytes. Prostaglandins Med. 1978 Dec;1(6):441–453. doi: 10.1016/0161-4630(78)90115-5. [DOI] [PubMed] [Google Scholar]
- Yoneda T., Mundy G. R. Prostaglandins are necessary for osteoclast-activating factor production by activated peripheral blood leukocytes. J Exp Med. 1979 Jan 1;149(1):279–283. doi: 10.1084/jem.149.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimecki M., Webb D. R. The regulation of the immune response to T-independent antigens by prostaglandins and B cells. J Immunol. 1976 Dec;117(6):2158–2164. [PubMed] [Google Scholar]
- van 't Hull E., Schellekens H., Löwenberg B., de Vries M. J. Influence of interferon preparations on the proliferative capacity of human and mouse bone marrow cells in vitro. Cancer Res. 1978 Apr;38(4):911–914. [PubMed] [Google Scholar]



