For decades, numerous investigations have only focused on axon regeneration to restore function after traumatic spinal cord injury (SCI), as interrupted neuronal pathways have to be reconnected for sensorimotor and autonomic recovery to occur. Experimental approaches have ranged from drug delivery and cell transplantation to genetic manipulations. Certainly, it would be an extraordinary achievement for injured axons to regenerate over long distances, to form synapses with target neurons, and to result in dramatic functional improvement. However, these efforts have been rewarded with limited success to date suggesting that axon regeneration alone may be insufficient to repair compromised functions. Upon exogenous stimulation, sensory afferent fibers and at least some brainstem-derived supraspinal axons are able to regrow across a lesion site, whereas corticospinal tract (CST) axons do not or are less responsive. Yet, even terminals of the longest regenerated sensory axons are usually far from the original target. To reestablish neuronal pathways, introduction of a new host or graft-derived neuron may therefore be necessary to relay supraspinal signal transmission to target neurons.
Indeed, neuronal relays are widely present in the normal central nervous system. In ascending sensory pathways, for instance, primary large-diameter mechanoreceptive Aβ fibers enter the ipsilateral dorsal column of the spinal cord and project directly to the medulla. In contrast, small-diameter C and Aδ fibers conveying nociceptive and thermoreceptive data synapse onto neuronal cells in the substantia gelatinosa of the dorsal horn and second-order neurons spread primary sensory information to the brain (Fyffe, 1992). Following SCI, the local elevation of neural growth factors contributes to adaptive intraspinal plasticity, in which relays occur spontaneously to reorganize neuronal circuitry. Using animal models of lateral spinal cord hemisection, several studies have demonstrated that locomotor recovery can be mediated by remodeling of bulbospinal and propriospinal connections; de novo propriospinal relay circuits transmitting neuronal signals bypass the lesion site and reestablish supraspinal motor control (Courtine et al., 2008). As a cellular machinery to rebuild injured pathways, neuronal relays do not only develop spontaneously in studies of axon regeneration, but can also be introduced in neuronal cell-based implantations. Depending on the experimental strategy, a relay can be attributed to host interneurons or grafted neurons.
In experimental interventions without neuronal cell grafts, spinal interneurons may relay supraspinal information to target neurons. If injured supraspinal axons are induced to bridge a lesion, their terminals can form synaptic connections with interneurons that link to motor or autonomic neurons in the distal spinal cord. Due to a higher regenerative capacity, propriospinal neurons above the injury level may regrow axons across the lesion, which may project directly to efferent neurons or pass the information via another interneuron below the lesion (Figure 1A). In incomplete SCI, sustained spared axons undergo spontaneous sprouting to rebuild neuronal circuitry. Together with axon regeneration and relay formation, this plasticity can give rise to dramatic motor functional recovery (Courtine et al., 2008). To facilitate axon growth, a peripheral nerve was grafted into a rat spinal cord hemisection and chondrionitinase ABC (ChABC) was simultaneously administered to attenuate inhibitory extracellular matrix components. Improved forelimb motor function correlated with significantly larger number of axons regenerated into the host spinal cord caudal to the injury (Houle et al., 2006). Subsequent retrograde tracing confirmed that most of the regenerated neurons have propriospinal profiles, and fewer were found in the brainstem. When similar therapeutic strategies were used to restore injured respiratory pathways in animals with high cervical spinal cord hemisection, regeneration of serotonergic (5-HT+) axons and other bulbospinal fibers over extended distances led to remarkable recovery of diaphragmatic function (Alilain et al., 2011). In a model of complete spinal cord transection, grafting peripheral nerve plus an acid fibroblast growth factor and ChABC induced robust regeneration of bulbospinal catecholaminergic (tyrosine hydroxylase-positive, TH+)/5-HT+ and propriospinal axons across the injury site, relevant to urinary functional improvement (Lee et al., 2013). This indicates that central neuronal regeneration can rebuild descending pathways of bladder control for the partial recovery of micturition function. Combined with biomaterials, investigators transplanted Schwann cells overexpressing glial cell line-derived neurotrophic factors into the lesion gap of a spinal cord hemisection; consequently, descending propriospinal axons regenerated through and beyond the filled lesion into the distal spinal cord, resulting in partial recovery of motor function (Deng et al., 2013). By means of stimulating propriospinal neurons, recording of extracellular field potentials in the distal spinal cord elicited action potential, providing direct evidence of an interneuronal relay of supraspinal signals. Collectively, it is necessary for supraspinal or propriospinal axons to regrow beyond the lesion site into the spinal cord parenchyma to form a relay.
Figure 1.
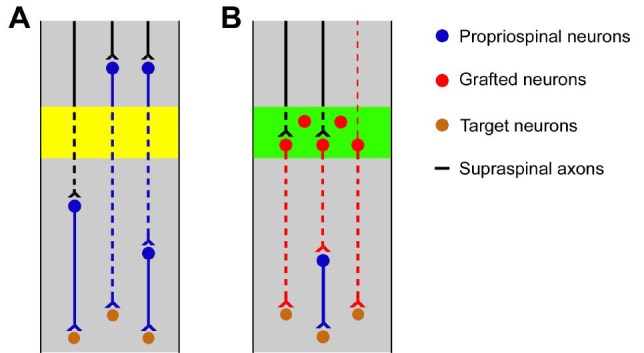
Schematic illustration of neuronal relays in the restoration of complete spinal cord injury.
(A) Under therapeutic interventions without neuronal cell grafting, some supraspinal axons may regenerate across the lesion (yellow area) of injured spinal cords and input to target neurons via an interneuronal relay. In parallel, most propriospinal neurons are prone to regrow axons across the injury and relay brain signal directly to motor neurons or mediated by a spinal interneuron. (B) Neuron-based grafts provide a substrate to relay supraspinal signal transmission passing the lesion of injured spinal cords. Early stage neurons transplanted to the lesion site (green area) can form connectivities with regenerated supraspinal axons in the implant. Their axons may project directly to the target neurons in the distal spinal cord or via a host interneuron. Graft-derived neurons may outgrow dendrites rostrally and convey information from higher centers to spinal neurons.
Grafts can relay signal when early stage neurons or stem cells are transplanted into the lesion site of an adult injured spinal cord. Compared to contusive or compressive injury, axon regeneration is even more refractory in severe SCI such as complete transection. In this situation, the main extrinsic reason of regenerative difficulty is the harsh local environment of the lesion site, including glial scar and fibroblast barrier, detrimental for axon growth. These inhibitory elements have rendered most therapeutic efforts unsuccessful. In recent decades, numerous studies have examined cell-grafting strategies to repair SCI. As an encouraging approach, neuron-based transplantation to a spinal cord lesion site can fill neural tissue deficits so that disrupted supraspinal pathways may be reestablished across the injury. Unlike adult CNS neurons with a poor capacity to regenerate, the developmental stage of neurons transplanted in the lesioned mature nervous system can specify sufficient information to permit extensive axonal regrowth. It has been shown that early stage neurons/cells grafted to the injured spinal cord differentiate into neurons, extend axons over long distances, and improve functional recovery. Most of these studies reported a relay mechanism to underlie the functional restoration. Information from higher centers can be transmitted by grafted neuronal cells with different connections: grafted neuronal cells may receive regenerated supraspinal axon input and may project directly to target neurons caudal to the graft; implanted cells may relay supraspinal signals via an interneuron to the distal target; grafted neurons may extend dendrites rostrally for host neurons or fibers to synapse on, and may project axons to caudal spinal neurons (Figure 1B). Reier and colleagues extensively investigated the transplantation of fetal spinal cord tissue in the adult injured spinal cord. They demonstrated that rat embryonic central nerve cells grafted into contusive or incomplete spinal cord lesions exhibit a high rate of survival and reliable differentiation; neurite outgrowth extends from well-integrated grafts to the surrounding host tissue (Reier et al., 1992). The pioneering work provided important guidance and hints for subsequent studies. Taking advantage of transgenic techniques, we can now visualize the destination of a graft by implanting embryonic tissue expressing visible reporter genes. Neuronal restricted precursors (NRP) isolated from alkaline phosphatase (AP) transgenic fetal rat spinal cord were transplanted into a unilateral dorsal column lesion in the spinal cord; a relay formed by grafted neuronal cells was revealed to ascend across the lesion site to the intended sensory target nuclei (Bonner et al., 2011). To address graft survival in severe SCI, Lu and colleagues embedded embryonic neural stem cells (NSCs), dissected from green fluorescent protein (GFP) transgenic rats, into fibrin matrices containing growth factors, and grafted the cells into the lesion site of completely transected adult spinal cords. The results indicated that differentiated neurons can extend numerous axons over remarkable distance and form abundant synapses with host neurons, leading to electrophysiologically active relays across the lesion (Lu et al., 2012). Using the same approaches, we implanted embryonic brainstem-derived NSCs into the completely transected spinal cord and found numerous differentiated 5-HT+ or TH+ neurons in the graft. These neurons projected axons across the lesion and topographically innervated to caudal autonomic nuclei; supraspinal vasomotor pathways regenerated into the graft, suggesting possible reestablishment of higher level control. As a result, cardiovascular function partially recovered in measures of basal hemodynamics and autonomic dysreflexia (Hou et al., 2013). For clinical relevance, xenografting with human cells was explored in SCI animals. Human NSCs or induced pluripotent stem cells (hiPSCs) implanted to a spinal cord lesion in immunodeficient mice can differentiate into human neurons and form synaptic connectivity with host neurons (Cummings et al., 2005, Nori et al., 2011). Likewise, 566RSC human stem cells transplanted into the lesioned spinal cord of athymic nude rats showed similar results (Lu et al., 2012). Together, the grafting of early-stage neurons or stem cells is a meaningful relay strategy to restore neuronal signal transmission in the severely injured spinal cord.
With either host interneurons or grafted neurons, a relay can occur as a “point to point” connection, in which one supraspinal neuron synapses only onto one neuron and conveys information to one target neuron. Alternatively, one relay neuron may pass multiple supraspinal signals to multiple targets. In consideration of profound neural networks in the spinal cord, multiple connections are likely to be a dominant means of information conduction. The majority of relay interneurons might be GABAergic or glycinergic with regard to inhibitory characteristics of most propriospinal neurons, whereas excitatory neurotransmitters have to be included in order to induce corresponding electrophysiological activities. To examine relay neurons involved in an entire novel neuronal pathway, one may employ transsynaptic neural tracing or electrophysiological recording techniques to obtain morphological and physiological evidence. In addition, expression of the immediate early gene c-fos has widely been used to identify neurons that respond to an acute stimulus. It can therefore be utilized to examine the connectivity between relay and start/target neurons (Bonner et al., 2011).
Relay strategies change the pattern of neuronal circuitry, thus an important question is: can relayed signals stimulate the same functional responses as the original neuronal input? Recent work has shown considerable plasticity of 5-HT+ and TH+ axons in the injured spinal cord of zebra fish; most of the regrowing axons fail to directly reinnervate the original caudal motoneurons, however the full swimming ability recovers (Kuscha et al., 2012). This observation provides valuable insights that it might indeed be unnecessary to restore identical neuronal connections for functional improvement. Such findings are also true in primates. For a long time, it was assumed that only a direct monosynaptic corticospinal pathway controls fine voluntary movements in humans and monkeys. However, reestablished disynaptic propriospinal projections have been shown to mediate dexterous finger movements in rhesus monkeys with complete CST transection (Sasaki et al., 2004). Hence, relayed neuronal pathways enable functional recovery in higher mammals.
For both host and graft-derived neuronal relays, current limitations are scar formation and tissue cavitation restricting host axonal regeneration into an implant or regrowth of axons from grafted neurons to the rostral and caudal cord. As a response to the injury, meningeal fibroblasts proliferate and invade the lesion site, forming a physical barrier for axon growth. It separates the portions of spinal cord rostral and caudal to the injury and prevents signal transmission. Although Lu and colleagues demonstrated functional relay mechanism of recovery (Lu et al., 2012), a recent replication reported long-distance axon outgrowth but non-significant locomotor improvement (Sharp et al., 2014). In this study, the occurrence of partitions and cavities were revealed in the middle of grafts in some cases. There was no fusion of rostral and caudal parts of the graft to create a continuous bridge. It appeared that axons were not capable of crossing the barrier to establish a relay. This might be one main reason of unsuccessful duplication of functional recovery. Thus, an urgent need is to further refine transplantation techniques to overcome these difficulties.
In summary, neuronal relays are an essential mechanism of SCI repair. Host interneuronal relays are one means of recovery in incomplete SCI, but damaged neuronal circuitry in severe SCI might need to be reconnected via grafted neurons. With further advancements in neural transplantation, relay strategies combined with axon regeneration might be the most promising prospect to restore SCI.
Acknowledgments:
The author gratefully thanks Dr. Armin Blesch for critically reading the manuscript.
Footnotes
Funding: This work was supported by the Craig H. Neilsen Foundation (280072).
References
- 1.Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475:196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011;31:4675–4686. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng LX, Deng P, Ruan Y, Xu ZC, Liu NK, Wen X, Smith GM, Xu XM. A novel growth-promoting pathway formed by GDNF-overexpressing Schwann cells promotes propriospinal axonal regeneration, synapse formation, and partial recovery of function after spinal cord injury. J Neurosci. 2013;33:5655–5667. doi: 10.1523/JNEUROSCI.2973-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fyffe REW. Laminar organization of primary afferent terminations in the mammalian spinal cord. In: Scott S. A, editor. Sensory neurons: diversity, development, and plasticity. New York: Oxford University Press; 1992. pp. 131–139. [Google Scholar]
- 7.Hou S, Tom VJ, Graham L, Lu P, Blesch A. Partial restoration of cardiovascular function by embryonic neural stem cell grafts after complete spinal cord transection. J Neurosci. 2013;33:17138–17149. doi: 10.1523/JNEUROSCI.2851-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuscha V, Barreiro-Iglesias A, Becker CG, Becker T. Plasticity of tyrosine hydroxylase and serotonergic systems in the regenerating spinal cord of adult zebrafish. J Comp Neurol. 2012;520:933–951. doi: 10.1002/cne.22739. [DOI] [PubMed] [Google Scholar]
- 10.Lee YS, Lin CY, Jiang HH, Depaul M, Lin VW, Silver J. Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J Neurosci. 2013;33:10591–10606. doi: 10.1523/JNEUROSCI.1116-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y, Fujiyoshi K, Koike M, Uchiyama Y, Ikeda E, Toyama Y, Yamanaka S, Nakamura M, Okano H. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci U S A. 2011;108:16825–16830. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reier PJ, Stokes BT, Thompson FJ, Anderson DK. Fetal cell grafts into resection and contusion/compression injuries of the rat and cat spinal cord. Exp Neurol. 1992;115:177–188. doi: 10.1016/0014-4886(92)90245-l. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki S, Isa T, Pettersson LG, Alstermark B, Naito K, Yoshimura K, Seki K, Ohki Y. Dexterous finger movements in primate without monosynaptic corticomotoneuronal excitation. J Neurophysiol. 2004;92:3142–3147. doi: 10.1152/jn.00342.2004. [DOI] [PubMed] [Google Scholar]
- 15.Sharp KG, Yee KM, Steward O. A re-assessment of long distance growth and connectivity of neural stem cells after severe spinal cord injury. Exp Neurol. 2014 doi: 10.1016/j.expneurol.2014.04.008. doi:10.1016.j.expneurol.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


