Abstract
Astrocytes are intimately involved in the formation and development of retinal vessels. Astrocyte dysfunction is a major cause of blood-retinal barrier injury and other retinal vascular diseases. In this study, the development of the retinal vascular system and the formation of the blood-retinal barrier in mice were investigated using immunofluorescence staining, gelatin-ink perfusion, and transmission electron microscopy. The results showed that the retinal vascular system of mice develops from the optic disc after birth, and radiates out gradually to cover the entire retina, taking the papilla optica as the center. First, the superficial vasculature is formed on the inner retinal layer; then, the vasculature extends into the inner and outer edges of the retinal inner nuclear layer, forming the deep vasculature that is parallel to the superficial vasculature. The blood-retinal barrier is mainly composed of endothelium, basal lamina and the end-feet of astrocytes, which become mature during mouse development. Initially, the naive endothelial cells were immature with few organelles and many microvilli. The basal lamina was uniform in thickness, and the glial end-feet surrounded the outer basal lamina incompletely. In the end, the blood-retinal barrier matures with smooth endothelia connected through tight junctions, relatively thin and even basal lamina, and relatively thin glial cell end-feet. These findings indicate that the development of the vasculature in the retina follows the rules of “center to periphery” and “superficial layer to deep layers”. Its development and maturation are spatially and temporally consistent with the functional performance of retinal neurons and photosensitivity. The blood-retinal barrier gradually becomes mature via the process of interactions between astrocytes and blood vessel cells.
Keywords: nerve regeneration, retina, growth, development, blood vessels, blood-retinal barrier, astrocytes, immunofluorescence, ultrastructure, mouse, collagen IV, glial fibrillary acidic protein, NSFC grant, neural regeneration
Introduction
The retinal vasculature in most non-primate mammals develops postnatally and is located in a thin and flat space; therefore, the retina becomes a good model for the study of the generation and development of vasculature. The retinal vasculature of adult mice can be divided into two layers: the superficial vessels, which are located in the ganglion cell layer, and the deep vessels, which are located on the inner and outer edges of the inner nuclear layer. This specific distribution of vasculature ensures the supply of blood to the whole retina (Dorrell and Friedlander, 2006; Uemura et al., 2006; Fruttiger, 2007). The anatomy and function of the retinal vasculature have been well studied, and the process of development of the retinal vasculature in rodents has been widely reported (Starr et al., 2003; Sabanayagam et al., 2009; Diaz et al., 2010; Kumase et al., 2010; Cheung et al., 2011, 2012; Lutty et al., 2011; Rangasamy et al., 2011; Xu and Le, 2011; Cheung et al., 2012; Dou et al., 2012). Two concepts, vasculogenesis and angiogenesis, are used to describe the mechanisms underlying the development of the vasculature (Hughes et al., 2000; Fruttiger, 2002; Tomanek, 2005; Ratajska and Czarnowska, 2006). In the retinas of non-primate mammals, both angiogenesis and vasculogenesis are reported to participate in vascularization (Chan-Ling et al., 1990; Jiang et al., 1995; Lutty and McLeod, 2003).
The other important issue relating to the retinal vasculature is the formation of the blood-retinal barrier. The blood-retinal barrier can be divided into two distinct regions: the inner blood-retinal barrier near the vitreous body and the outer blood-retinal barrier near the choroids (Choi and Kim, 2008; Lee et al., 2011; Toda et al., 2011). The inner blood-retinal barrier plays essential roles in protecting neural tissues from harmful materials and maintaining the neural functions of the retina. The inner blood-retinal barrier is composed of the specialized microvessels of the retina and their surrounding pericyte and astrocyte end-feet (Choi and Kim, 2008; Lee et al., 2011; Toda et al., 2011). The mechanisms underlying blood-retinal barrier formation are complex. It is probably formed by the coordinated induction of endothelial cells, pericytes and astrocytes, because those cells can secrete cytokines inducing them to bind tightly to form the blood-retinal barrier (Choi and Kim, 2008; Lee et al., 2011; Toda et al., 2011). The tight connections between endothelial cells in the blood-retinal barrier can be strengthened further by the pericytes and astrocytes around them (Kim et al., 2009). It is reported that the activation and dysfunction of astrocytes are key contributors to the blood-retinal barrier injury in many retinal vascular diseases (Zheng et al., 2008; Shen et al., 2010).
Studies addressing the generation of the vasculature and blood-retinal barrier are of important biological significance, especially in regard to therapies for eye diseases. Most pathological progression of retinal diseases resulting in blindness is related to abnormal changes of the optic vasculature. For example, in diabetic retinopathy, the structure of the retinal vasculature is changed, resulting in edema and bleeding of the retina that leads to a reduction of vision (Frank, 2004; Kim et al., 2009; Thornit et al., 2010; Huang et al., 2011). Therefore, a better understanding of the development of the retinal vasculature could help elucidate the pathological alterations of the vasculature and blood-retinal barrier. However, many details need to be investigated further. In the present study, by observing the generation and development of the retinal vasculature and blood-retinal barrier in mice, we tried to elucidate the correlations between them during development to better understand the pathological basis of disease and suggest therapeutic approaches.
Materials and Methods
Animals
One-hundred and sixty-four healthy male and female C57BL/6J mice at various ages (P0, P3, P7, P9, P14, P30, P60; P = days postnatal, P0 = the first 24 hours after birth) were randomly provided by the Experimental Animal Center of Henan University, China (license No. SCXK (Yu) 2009-0001). All experiments were carried out in accordance with the guidelines and approval of the Animal Welfare and Use Committees of Henan University to ensure animal welfare during experiments. Adult male and female mice were housed in standard breeding cages with a 12-hour light/dark cycle. Postnatal offspring were produced from timed pregnancies at embryonic day 19. The animals were housed in a periodically sanitized environment at 22–25°C with a relative humidity of 60–70%. The animals were allowed to access food and water freely.
Preparation of whole-mount retinas and retinal sections for immunofluorescence staining
Mice at different ages (P0, P3, P7, P9, P14, P30, P60) were used to prepare whole-mount retinas and retinal sections, with at least 12 mice of each age used. Mice were anesthetized with 1% pentobarbital sodium at a dosage of 20 mg/kg, and 4% w/v paraformaldehyde (in 0.1 mol/L PB) was perfused through the left ventricle. Then, the eyeballs were taken out, and the ocular anterior segment (cornea and crystalline lens) was cut off; the retina was carefully ablated under dissection microscope (BX61, Olympus, Tokyo, Japan) and a “+” pattern was cut into the four edges of the retina (above, below, left, and right) for flattening, before the sample was continually fixed in 4% paraformaldehyde at 4°C for 2–3 hours. Subsequently, the retinas were washed 3 times (15 minutes each) in 0.01 mol/L PBS (pH 7.4), before being incubated in primary antibodies at 4°C for 2 days. The primary antibodies were rabbit anti-mouse glial fibrillary acidic protein (1:2; ZA-0117; Beijing Zhongshan Golden Brideg Biotechnology Co., Ltd., Beijing, China) and rabbit anti-mouse collagen IV (1:200, AB756P; Chemicon, Temecula, CA, USA). After washing 3 times in 0.01 mol/L PBS for 15 minutes each time, the samples were incubated in secondary antibody, Alex Fluro 568 goat anti-rabbit IgG (1:600, A11011; Invitrogen) or Alex Fluro 488 donkey anti-rabbit IgG (1:300, A21206; Invitrogen) at 4°C overnight. After washing 3 times in PBS for 15 minutes each time, the retinal sample was mounted on clean glass slides with the ganglion cell layer facing upwards. Samples were coverslipped using mounting media. Then, the samples were examined under a fluorescence microscope (BX61, Olympus). For section preparation, fixed retinas were immersed successively in 10, 20, and 30% sucrose solution (in 0.1 mol/L PB) overnight; then, 20-μm-thickness sections were made using acryostat microtome for collagen IV immunofluorescence labeling as described above. The retinal vasculature (stained by the anti-collagen IV antibody) and retinal astrocytes (stained by the anti-glial fibrillary acidic protein antibody) were observed under a fluorescence microscope (BX61, Olympus).
Gelatin-ink perfusion to label the different layers of vasculature
Twelve adult mice aged 2 months were anesthetized peritoneally using 1% pentobarbital sodium at 20 mg/kg as above. Paraformaldehyde (4%) was perfused through the left ventricle for 5 minutes; then, 6.5% gelatin-ink at 60°C was perfused throughout until the body turned black. After tightening of the cardiac vessels, the mice were put into an ice box for about 2 hours for gelatin condensation. Subsequently, the eyeballs were taken out, and the retinas were ablated and cut with “+” patterns for flattening on clean glass slides. Cover slipping was carried out using 65% glycerin in 0.01 mol/L PBS. The samples were examined under a fluorescence microscope (BX61, Olympus) to observe the different layers of vasculature in the retinas. Superficial and deep layers of vasculature perfused with gelatin-ink were observed by adjusting the focus.
Preparation of electron microscopy samples
Twelve mice at P0, P3, P14, and P30 were used for electron microscopic examinations. These animals were anesthetized peritoneally using 1% pentobarbital sodium as above, and 4% paraformaldehyde and 1% glutaraldehyde (in 0.1 mol/L PB) were used for perfusion through the left ventricle. The eye-balls were then taken out, and the retinas were ablated and cut radially into 2 mm × 2 mm tissue pieces using a double-edged razor blade. The samples were then fixed in 4% glutaraldehyde for 2–4 hours and washed 3 times in 0.01 mol/L PBS for 15 minutes each time. After fixing in 1% osmic acid for 30 minutes and washing in 0.01 mol/L PBS, the samples were dehydrated successively in a series of acetones of increasing concentration. After embedding in Epon 812 resin, the samples were polymerized at 37°C for 3 hours, 45°C for 6 hours, and 63°C for 24 hours; then, the samples were cut into 70 nm ultra-thin slices and stained with uranyl acetate and lead citrate for transmission electron microscopy (H-7500, Hitachi, Tokyo, Japan) to observe the ultrastructure of capillary vessels and the blood-retinal barrier in different layers of the retinas.
Results
Development of retinal vascular system in mice
Collagen IV is a protein located in the vascular basal lamina and can be used to label the vasculature in the retina. It is generally accepted that the retinal vascular system of mice develops postnatally from the optic disc and radiates out to cover the entire retina. In our study, as early as P0, the vascular generation that started from the optic disc formed the superficial vasculature on the ganglion cell layer (Figure 1A). Then, the blood vessels at the optic disc grew and branched repeatedly, and the vascular buds, which are believed to be key germinal apparatuses for vessel generation, were produced in the tips of vessels (Figure 1A, B). The vessels grew from the vascular buds and were sent forth into the superficial network, and several stem vessels interconnected with each other around the optic disc. Later, the radial-like stem vessels around the optic disc grew and differentiated further into arteries and veins. Arteries and veins could be identified according to their own characteristics. For instance, the arteries often gave rise to several large vessels that grew into the retina, and a capillary-free zone appeared around the main artery; however, the veins connected with the capillaries directly. Many capillaries were located between arteries and veins. The vascular network continued to radiate out into the periphery of the retina to provide further coverage. The capillaries in the retina connected with each other via hive-like structures (Figure 1A–D). In the meantime, the diameters of arteries and veins became broader, and they radiated out to the retinal periphery (Figure 1C–E). The vessels in the peripheral area were thinner, so that there was only a capillary network in the periphery. At P10, the retinal superficial vasculature had already reached the periphery and covered the entire retina. At the same time, some vessels sprouted and penetrated the deep retina. At P14, deep networks had formed on both edges of the inner nuclear layer, the inner edge near the inner plexiform layer and the outer edge near the outer plexiform layer (Figure 2A–C). At first, the deep vasculature entered the inner edge of the inner nuclear layer and then invaded the outer edge of the inner nuclear layer (Figure 2A–C). Figure 2D–F) provide an overview of the deep vasculature with different focuses. Figure 2G–I) show the superficial vasculature and deep vasculature with different focuses.
Figure 1.
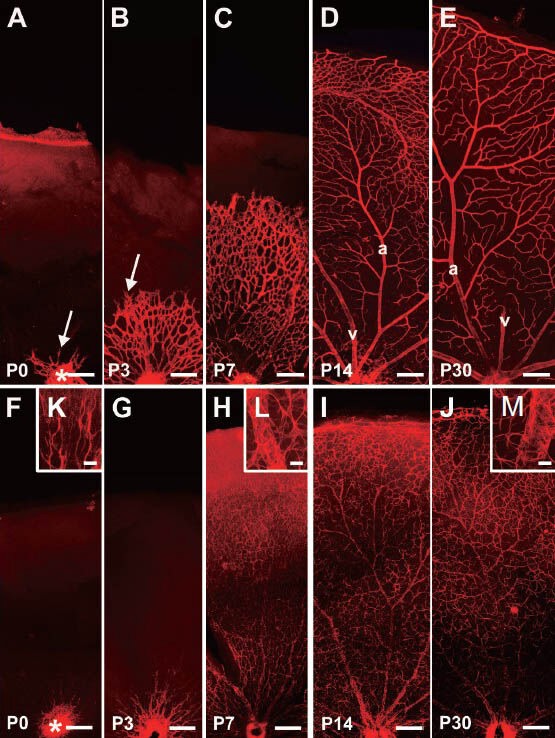
The retinal superficial vasculature system (red) and astrocytic network (red) of mice at different ages by Alex Fluro 568 immunofluorescentstaining.
(A–E) The retinal superficial vasculature system with collagen IV antibody immunofluorescence staining. The mouse retinal superficial vasculature radiates out from the optic disc (*)(A, B), eventually forming the mature vasculature with differing branching orders of arterioles and veins that cover the entire retina (C–E). The vascular buds, which are believed to be key germinal apparatuses for vasculogenesis, are marked with arrows. From P7, arteries and veins could be recognized. Usually, the arteries give rise to several large vessels into the retina; however, veins connected with the capillaries directly. In the photos, the arteries and veins are marked “a” and “v”, respectively. (F–M) The development of astrocytes at various ages with anti-glial fibrillary acidic protein antibody (GFAP) immunofluorescence staining. The immature retinal astrocytes appear from the optic disc (*) (F, G, K), eventually forming the astrocytic network in the entire retina (H–J). The processes of astrocytes contact each other to form the glial network, and many of them envelop vessels to participate in the formation of the blood-retinal barrier (H–J, L, M). Scale bars: A–J: 200 μm; K–M: 25 μm. P: Postnatal days.
Figure 2.
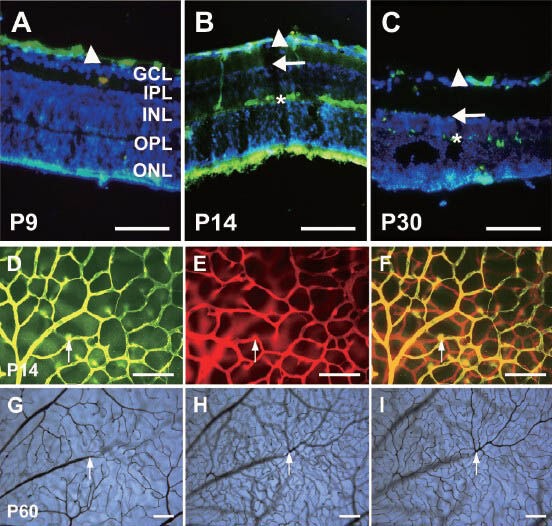
The vasculature in various layers of whole-mount retinas and retinal cross-sections by Alex Fluor 568 (red) and 488 (green) immunofluorescence staining and gelatin-ink (black) perfusion.
(A–C) The vascular distribution in retinal cross-sections with collagen IV immunofluorescence staining (green) and DAPI counterstaining cell nucleus (blue). The superficial vessels (arrowheads) were fully formed at P9, and were located in the inner surface of the ganglion cell layer. The deep vasculature formed at the inner edge (arrows) of the inner nuclear layer at first, and then invaded the outer edge (asterisks) of the inner nuclear layer at P14. The outer nuclear layer (ONL), outer plexiform layer (OPL) and inner plexiform layer (IPL) are marked as well. (D–F) The vascular distribution in a whole-mount retina at P14 (collagen IV immunofluorescence staining). The superficial vasculature at the inner surface of the ganglion cell layer (green) has been transformed into a green color using Photoshop software, and is shown in (D). The deep vasculature at the outer edge of the inner nuclear layer (red) is shown in (E). (F) is the merge of the images shown in D and E. The arrows indicate the branch into the deep vasculature. (G–I) The retinal vasculature in various layers visualized with ink staining at P60. The superficial vasculature (G) and deep vasculature at the inner edge (H) and outer edge (I) of the inner nuclear layer were visualized using different microscope focuses. The arrows point to a main vessel in the superficial vasculature, which branches into the deep vessels. Scale bars: 100 μm. P: Postnatal days.
Astroglial cells and vasculature development
It is known that astroglial cells and the retinal vasculature develop together closely. In this study, using glial fibrillary acidic protein immunofluorescence labeling, the developing astroglial cells in the retina could be investigated. Interestingly, the astroglial cells also began to develop from the optic disc synchronously as the vasculature did, and they also radiated along the inner retinal surface to cover the entire retina (Figure 1F–J). The cell processes contacted and intertwined each other to form a network. Importantly, one or two processes from astrocytes extended toward the vessels, and the end-feet of glial process contacted and enveloped the vasculature. Figure 1F–J shows the distribution of retinal astrocytes in mice at different ages after birth. At P1, glial fibrillary acidic protein-positive cells mainly appeared at the zone near the optic disc with two processes (Figure 1F, K). At P7, glial fibrillary acidic protein-positive cells had typical star shapes with several processes, and the processes intertwined to make contact with each other to form a network-like structure (Figure 1H, L). The network formed by astrocytes was dense in the optic disc and gradually became sparse in the peripheral retina (Figure 1F–J). By adulthood, the glial fibrillary acidic protein-labeled astrocytic long processes intertwined to form network structures (Figure 1J). Another remarkable structure was the vessel-like structure composed of astroglial end-feet (Figure 1H–J, L, M). Their distribution and development were consistent with the collagen IV-positive vasculature, suggesting that they participated in the formation of the blood-retinal barrier (Figure 1A–J).
Blood-retinal barrier structure under light microscopy
To observe the composition of the blood-retinal barrier, triple labeling was used to visualize the retinal vasculature. Gelatin-ink could be perfused into blood vessels for adhesion onto the vessel endothelium; this method could effectively indicate the vascular lumen and endothelium (Figure 3A). Collagen IV is secreted by blood vascular endothelial cells and forms a part of the basal lamina (Bai et al., 2009; Wang et al., 2011); therefore, collagen IV can be used as a marker to visualize the vascular basal lamina (Figure 3B). Glial fibrillary acidic protein is expressed specifically in astroglial cells, and glial fibrillary acidic protein immunostaining could be used to visualize the morphology and distribution of the cellular processes of astrocytes (Figure 3C). Using these three labeling methods, the blood-retinal barrier was found as early as P3 and it was shown to be composed of at least three components: endothelial cells, basal lamina, and the end-feet of astroglial cells (Figure 3A–C). This is similar to the composition of the blood-brain barrier, which is composed of endothelial cells, basal lamina, and the end-feet of pericytes (Prat et al., 2001; Choi and Kim, 2008; Hosoya et al., 2010; Toda et al., 2011; Ek et al., 2012; Saunders et al., 2012; Mizee et al., 2013).
Figure 3.
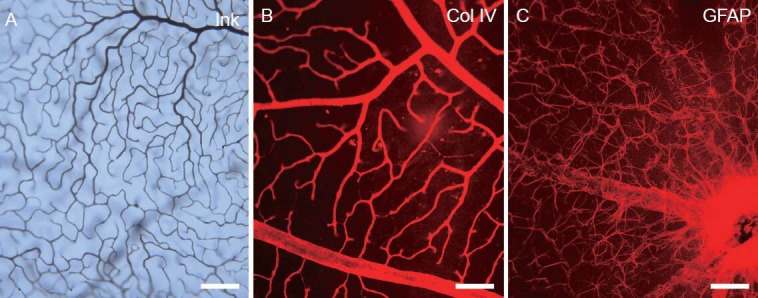
The structure of the blood-retinal barrier of adult mice under light microscopy by gelatin-ink (black) perfusion, collagen IV and glial fibrillary acidic protein secondary antibody Alex Fluor 568 (red) immunofluorescence staining.
(A) The blood vessel lumen and endothelium is shown by gelatin-ink perfusion under a light microscope. (B) The basal lamina of the vessel is shown by anti-collagen (Col) IV immunofluorescence staining under a light microscope. (C) The astrocytic processes enveloping the retinal vessels are shown by anti-glial fibrillary acidic protein (GFAP) immunofluorescence staining under a light microscope. Scale bars: 100 μm.
Blood-retinal barrier ultrastructure under electron microscopy
The blood-retinal barrier is composed of the specialized microvessels, the surrounding pericytes, and the end-feet of astrocytes. In addition, tight junctions are always found among the endothelial cells of microvessels. The ultrastructure data in our study showed that the blood-retinal barrier is gradually formed via the process of interactions between astroglial and endothelial cells. At P0, no capillary vessels were observed in any layer of the mouse retina, and at P3, some preliminary capillary vessels were observed only in the ganglion cell layer. In our observations of blood-retinal barrier development, three components, namely the endothelium, basal lamina and the end-feet of astrocytes, appeared almost at the same time. Initially, endothelial cells formed close connections with each other and secreted matrix substances that would become part of the basal lamina. After that, the end-feet of astrocytes, which were thick and short, enclosed the blood vessel wall to form the premature blood-retinal barrier. The lumen of these preliminary capillary vessels was surrounded by a single layer of endothelia without fenestra in the cytoplasm, and the uneven thickness and obscure boundaries were seen in the basal lamina outside of the vessel wall (Figure 4A). Some thick end-feet or processes of astroglial cells were observed surrounding the outer basal lamina incompletely (Figure 4A). At P14, many microvilli of endothelial cells were seen toward the lumen of capillary vessels, and tight junctions were found between the endothelia (Figure 4B, white arrowhead). The basal lamina on the outside of the endothelium had obscure borders and an uneven thickness; furthermore, the basal lamina was surrounded by a thin layer of astroglial end-feet (Figure 4B). At P30 the blood-retinal barrier was relatively mature and typical. At this time, the capillary endothelial surface facing the lumen was smooth, and the endothelial cells contained many organelles, such as mitochondria and endocytic vesicles (black bold arrow in (Figure 4C, D). The basal lamina had an even thickness (Figure 4C, D). Pericytes were located outside of the endothelium by chance (not shown in figures), surrounded by a thin layer of astroglial end-feet or processes (Figure 4C, D). The ultrastructure of the mature blood-retinal barrier is similar to that of the mature blood-brain barrier (Choi and Kim, 2008). Typically, capillaries are the continuous type. The endothelia with various organelles, such as mitochondria, endoplasmic reticula, are connected via tight junctions. The basal lamina completely surrounds the capillary endothelia completely, and sometimes there are pericytes below the lamina. In addition, astrocytic end feet surround the lamina tightly.
Figure 4.
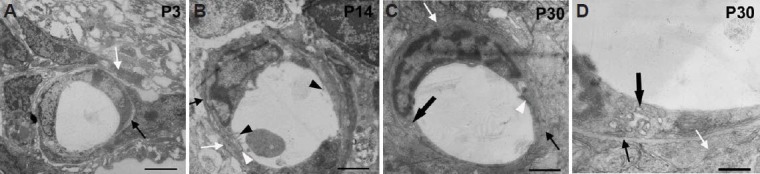
The ultrastructure of the blood-retinal barrier under electron microscopy during development in mouse.
(A) The blood-retinal barrier ultrastructure in the retinal nerve fiber layer at P3. The barrier is composed of endothelia, basal lamina and astrocytic end-feet. At this time, no tight junctions can be seen between the endothelia. The development of the basal lamina (black arrow) and the astrocytic end-feet (white arrow) is incomplete. (B) The blood-retinal barrier ultrastructure in the retinal inner nuclear layer at P14. The endothelium has many microvilli on the capillary lumen surface (black arrowheads). The basal lamina (black arrow) and astroglial end-feet (white arrow) are relatively more mature than those at P3. (C) The mature blood-retinal barrier ultrastructure in the retinal outer plexiform layer at P30. At this time, the endothelium is smooth, with tight junctions (white arrowheads) between cells, and the basal lamina is complete. (D) shows a higher magnification of the specimen in (C). Typical basal lamina (thin black arrow) and astroglial end-feet (white arrow) are marked. Inside the endothelial cell, there are many organelles, such as endocytic vesicles (bold black arrow). Scale bars: 3,300 nm in A, 1,700 nm in B, 1,000 nm in C, 333 nm in D. P: Postnatal days.
Discussion
Development of the vascular system harmonizes with cellular histogenesis
The retinal vasculature of most non-primate mammals develops postnatally. After birth, the retinal vascular network generates from the optic disc and radiates out into the inner ganglion cell layer. The retinal vasculature of adult rodents can be roughly divided into two layers: the superficial vasculature located on the inner ganglion cell layer; and the deep-layer vasculature located on both the outer and inner edges of the inner nuclear layer (Dorrell and Friedlander, 2006; Uemura et al., 2006; Fruttiger, 2007). The main function of the retinal vasculature is to provide oxygen and nutrients to the retina. In this study, the postnatal process of development of the retinal vasculature in mice was observed in detail. We found that superficial vasculature radiated from the optic disc, forming a vascular network on the ganglion cell layer. The superficial vasculature covered the entire retina at about P10 and began to grow into the deep layers. During this process, the area covered by the retinal superficial network increased, and so did the vascular density. The uniform vascular network gradually differentiated into main blood vessels with smaller branchings, and arteries and veins could be distinguished. At P14 the secondary vasculature at the inner and outer edges of the inner nuclear layer formed and continued to grow.
The generation and maturation of the retinal vasculature spreads from the center into the periphery, which is temporally and spatially consistent with the generation and differentiation of retinal cells (Young, 1985; Rapaport et al., 2004). Radioactive labeling studies have shown that the first cells to differentiate in the retinas of mice are those in the center, and the cells in the retinal periphery develop relatively late. Cell division ceased by P5–6 in the center and by P11 in the periphery of the retina (Young, 1985). Retinal cells in rat and mouse are generated in the following sequence (from early to late): retinal ganglion cells, horizontal cells, cone photoreceptors, amacrine cells, rod photoreceptors, bipolar cells, and Müller cells (Young, 1985; Cepko et al., 1996; Livesey and Cepko, 2001; Rapaport et al., 2004; Bassett and Wallace, 2012). The differentiation and maturation of retinal cells follows a temporal and spatial sequence of “center followed by periphery”, “superficial followed by deep layers”, and this is highly consistent with the developmental sequence of the retinal vasculature, which is also “center followed by periphery” and “superficial vasculature” (located on the ganglion cell layer) followed by deep-layer vasculature (located on the inner and outer edges of the inner nuclear layer, where amacrine cells and horizontal cells are located, respectively). This suggests that the differentiation and maturation of retinal cells may have a relationship with the provision of oxygen and nutrients by the retinal vasculature. Furthermore, the deep-layer retinal vasculature is formed at around P14 when mice first open their eyes (Koehler et al., 2011), suggesting that the formation of the deep-layer vasculature is related to the maturation of visual function.
Similarity between the blood-retinal barrier and the blood-brain barrier
The maturation of the vasculature in the central nervous system also includes the formation of selectively permeable barriers, such as the blood-brain barrier and the blood-retinal barrier. During brain development, the neural vasculature, even in the earliest stages, forms barriers to block the passage of proteins and large molecules (Lossinsky and Shivers, 2004; Ek et al., 2012; Saunders et al., 2012). In later stages, astroglial cells enable the blood-brain barrier to further develop, forming a mature structure composed of endothelial cells, endothelial basal lamina, and astroglial end-feet; furthermore, there are also neurons and microglia surrounding the blood vessels (Choi and Kim, 2008; Abbott and Friedman, 2012; Daneman, 2012). The blood-brain barrier maintains a stable environment within the brain by being selectively permeable to the entrance of blood substances and blocking the passage of high-molecular weight substances in adulthood (Huang et al., 2013; Mizee et al., 2013). The mature blood-retinal barrier is composed of continuous capillaries, surrounded by pericytes and astroglial end-feet (Zhang et al., 2005; Choi and Kim, 2008; Xu and Le, 2011). Researches have shown that neither the retinal vasculature nor the blood-retinal barrier can be formed without retinal astroglial cells (Prat et al., 2001; Fruttiger, 2002; West et al., 2005; Uemura et al., 2006; Kubota and Suda, 2009; Scott et al., 2010). In fact, retinal astroglial cells exist only in species with a vascularized retina, and these cells are only restricted to vascularized zones (Schnitzer, 1988; Fruttiger et al., 1996; Provis et al., 2000, 2001; Fruttiger, 2007). It was also found that the astroglial cells on rodent retina radiated from the optic disk to form a networked structure on the inner retinal surface and that the vasculature followed a similar developmental trend in accordance with the above-mentioned research results (Ling and Stone, 1988; Huxlin et al., 1992).
In this study, triple labeling was used together with light microscopy to observe the blood-retinal barrier structure in developing mice, and transmission electron microscopy was also applied to observe the development of the blood-retinal barrier at an ultrastructural level. Our results indicate that blood-retinal barrier is composed of vascular endothelial cells, basal lamina, pericytes (in some photos, the pericytes are not shown), and the processes of astroglial cells, similar to the structure of the blood-brain barrier. The ultrastructural observations indicate the following: the blood-retinal barrier did not mature until P14. For instance, at P3, the basal lamina was thin and incomplete, and astroglial end-feet were not close to each other. At P14, tight junctions were seen between endothelia, and the lumen-side of the vascular endothelium had some microvilli, suggesting that the endothelial cells had a very active metabolism, and that the barrier functions were developing further. At P30, a typical blood-retinal barrier could be found. The endothelium contained various organelles, such as endocytic vesicles and mitochondria. Furthermore, the microvessels were usually surrounded tightly by end-feet extending from astroglial cells. At this age, the vascular basal lamina became even in structure and thickness. Compared with the endothelial cells of the splenic sinusoid with large cellular gaps and an incomplete basal lamina (Frenzel et al., 1976; Bamroongwong et al., 1991), the multiple layers of astroglial end-feet, the complete and even basal lamina, and the closely connected endothelium of the blood-retinal barrier formed a natural barrier structure, selectively blocking the entrance of harmful substances in the blood into retinal tissues. This effectively maintained the stability of the inner retinal environment and the neuronal functions of the retina, playing an important role in the anti-infectious immunity of the eyes (Lobo et al., 2004; Xu et al., 2005; Zhang et al., 2005; Tomasek et al., 2006; Cunha-Vaz, 2007; Chen et al., 2011).
In summary, the development of the vasculature in retina follows the rules of “from center to periphery” and “from the superficial layer to the deep layers”. The development of astrocytes is highly consistent with the development of the vasculature. The development of the mouse retinal vascular system is also closely related to the development of retinal cells and the establishment of retinal visual functions. The mature blood-retinal barrier functions in anti-infectious immunity and maintaining the stability of the environment in the retina.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 30771140, 31070952 and U1204311.
Conflicts of interest: None declared.
Copyedited by McGowan D, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
References
- 1.Abbott NJ, Friedman A. Overview and introduction: the blood-brain barrier in health and disease. Epilepsia. 2012;53(Suppl 6):1–6. doi: 10.1111/j.1528-1167.2012.03696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai X, Dilworth DJ, Weng YC, Gould DB. Developmental distribution of collagen IV isoforms and relevance to ocular diseases. Matrix Biol. 2009;28:194–201. doi: 10.1016/j.matbio.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamroongwong S, Somana R, Rojananeungnit S, Chunhabundit P, Rattanachaikunsopon P. Scanning electron microscopic study of the splenic vascular casts in common tree shrew (Tupaia glis) Anat Embryol (Berl) 1991;184:301–304. doi: 10.1007/BF01673264. [DOI] [PubMed] [Google Scholar]
- 4.Bassett EA, Wallace VA. Cell fate determination in the vertebrate retina. Trends Neurosci. 2012;35:565–573. doi: 10.1016/j.tins.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci U S A. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan-Ling TL, Halasz P, Stone J. Development of retinal vasculature in the cat: processes and mechanisms. Curr Eye Res. 1990;9:459–478. doi: 10.3109/02713689008999612. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Zhang M, Jiang C, Guo W, Liu H, Wei S. Estrogen attenuates vegf-initiated blood-retina barrier breakdown in male rats. Horm Metab Res. 2011;43:614–618. doi: 10.1055/s-0031-1283149. [DOI] [PubMed] [Google Scholar]
- 8.Cheung CY, Ikram MK, Sabanayagam C, Wong TY. Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension. 2012;60:1094–1103. doi: 10.1161/HYPERTENSIONAHA.111.189142. [DOI] [PubMed] [Google Scholar]
- 9.Cheung CY, Zheng Y, Hsu W, Lee ML, Lau QP, Mitchell P, Wang JJ, Klein R, Wong TY. Retinal vascular tortuosity, blood pressure, and cardiovascular risk factors. Ophthalmology. 2011;118:812–818. doi: 10.1016/j.ophtha.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 10.Choi YK, Kim KW. Blood-neural barrier: its diversity and coordinated cell-to-cell communication. BMB Rep. 2008;41:345–352. doi: 10.5483/bmbrep.2008.41.5.345. [DOI] [PubMed] [Google Scholar]
- 11.Cunha-Vaz J. Characterization and relevance of different diabetic retinopathy phenotypes. Dev Ophthalmol. 2007;39:13–30. doi: 10.1159/000098497. [DOI] [PubMed] [Google Scholar]
- 12.Daneman R. The blood-brain barrier in health and disease. Ann Neurol. 2012;72:648–672. doi: 10.1002/ana.23648. [DOI] [PubMed] [Google Scholar]
- 13.Diaz F, Villena A, Vidal L, Moreno M, Garcia-Campos J, Perez DVI. Experimental model of ocular hypertension in the rat: study of the optic nerve capillaries and action of hypotensive drugs. Invest Ophthalmol Vis Sci. 2010;51:946–951. doi: 10.1167/iovs.09-3667. [DOI] [PubMed] [Google Scholar]
- 14.Dorrell MI, Friedlander M. Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog Retin Eye Res. 2006;25:277–295. doi: 10.1016/j.preteyeres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Dou GR, Wang L, Wang YS, Han H. Notch signaling in ocular vasculature development and diseases. Mol Med. 2012;18:47–55. doi: 10.2119/molmed.2011.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ek CJ, Dziegielewska KM, Habgood MD, Saunders NR. Barriers in the developing brain and Neurotoxicology. Neurotoxicology. 2012;33:586–604. doi: 10.1016/j.neuro.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 18.Frenzel H, Hucker H, Kremer B, Richter IE. Scanning electron microscopic studies of the sinusoids of the liver and spleen in rats. Anat Anz. 1976;139:337–347. [PubMed] [Google Scholar]
- 19.Fruttiger M. Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Invest Ophthalmol Vis Sci. 2002;43:522–527. [PubMed] [Google Scholar]
- 20.Fruttiger M. Development of the retinal vasculature. Angiogenesis. 2007;10:77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- 21.Fruttiger M, Calver AR, Kruger WH, Mudhar HS, Michalovich D, Takakura N, Nishikawa S, Richardson WD. PDGF mediates a neuron-astrocyte interaction in the developing retina. Neuron. 1996;17:1117–1131. doi: 10.1016/s0896-6273(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 22.Hosoya K, Yamamoto A, Akanuma S, Tachikawa M. Lipophilicity and transporter influence on blood-retinal barrier permeability: a comparison with blood-brain barrier permeability. Pharm Res. 2010;27:2715–2724. doi: 10.1007/s11095-010-0272-x. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, Duh EJ, Vinores SA. TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci. 2011;52:1336–1344. doi: 10.1167/iovs.10-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Li Y, Tang Y, Tang G, Yang GY, Wang Y. CXCR4 antagonist AMD3100 protects blood-brain barrier integrity and reduces inflammatory response after focal ischemia in mice. Stroke. 2013;44:190–197. doi: 10.1161/STROKEAHA.112.670299. [DOI] [PubMed] [Google Scholar]
- 25.Hughes S, Yang H, Chan-Ling T. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 2000;41:1217–1228. [PubMed] [Google Scholar]
- 26.Huxlin KR, Sefton AJ, Furby JH. The origin and development of retinal astrocytes in the mouse. J Neurocytol. 1992;21:530–544. doi: 10.1007/BF01186955. [DOI] [PubMed] [Google Scholar]
- 27.Jiang B, Bezhadian MA, Caldwell RB. Astrocytes modulate retinal vasculogenesis: effects on endothelial cell differentiation. Glia. 1995;15:1–10. doi: 10.1002/glia.440150102. [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Kim JH, Yu YS, Cho CS, Kim KW. Blockade of angiotensin II attenuates VEGF-mediated blood-retinal barrier breakdown in diabetic retinopathy. J Cereb Blood Flow Metab. 2009;29:621–628. doi: 10.1038/jcbfm.2008.154. [DOI] [PubMed] [Google Scholar]
- 29.Kim JH, Kim JH, Yu YS, Kim DH, Kim KW. Recruitment of pericytes and astrocytes is closely related to the formation of tight junction in developing retinal vessels. J Neurosci Res. 2009;87:653–659. doi: 10.1002/jnr.21884. [DOI] [PubMed] [Google Scholar]
- 30.Koehler CL, Akimov NP, Renteria RC. Receptive field center size decreases and firing properties mature in ON and OFF retinal ganglion cells after eye opening in the mouse. J Neurophysiol. 2011;106:895–904. doi: 10.1152/jn.01046.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubota Y, Suda T. Feedback mechanism between blood vessels and astrocytes in retinal vascular development. Trends Cardiovasc Med. 2009;19:38–43. doi: 10.1016/j.tcm.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Kumase F, Morizane Y, Mohri S, Takasu I, Ohtsuka A, Ohtsuki H. Glycocalyx degradation in retinal and choroidal capillary endothelium in rats with diabetes and hypertension. Acta Med Okayama. 2010;64:277–283. doi: 10.18926/AMO/40502. [DOI] [PubMed] [Google Scholar]
- 33.Lee IS, Lee JE, Kim HJ, Song JW, Choi SH. Immediate break-down of blood retinal barrier by infusion of triolein emulsion observed by fluorescein angiography. Curr Eye Res. 2011;36:358–363. doi: 10.3109/02713683.2010.548894. [DOI] [PubMed] [Google Scholar]
- 34.Ling TL, Stone J. The development of astrocytes in the cat retina: evidence of migration from the optic nerve. Brain Res Dev Brain Res. 1988;44:73–85. doi: 10.1016/0165-3806(88)90119-8. [DOI] [PubMed] [Google Scholar]
- 35.Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- 36.Lobo CL, Bernardes RC, Figueira JP, de Abreu JR, Cunha-Vaz JG. Three-year follow-up study of blood-retinal barrier and retinal thickness alterations in patients with type 2 diabetes mellitus and mild nonproliferative diabetic retinopathy. Arch Ophthalmol. 2004;122:211–217. doi: 10.1001/archopht.122.2.211. [DOI] [PubMed] [Google Scholar]
- 37.Lossinsky AS, Shivers RR. Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Reveiw. Histol Histopathol. 2004;19:535–564. doi: 10.14670/HH-19.535. [DOI] [PubMed] [Google Scholar]
- 38.Lutty GA, McLeod DS. Retinal vascular development and oxygen-induced retinopathy: a role for adenosine. Prog Retin Eye Res. 2003;22:95–111. doi: 10.1016/s1350-9462(02)00058-7. [DOI] [PubMed] [Google Scholar]
- 39.Lutty GA, McLeod DS, Bhutto I, Wiegand SJ. Effect of VEGF trap on normal retinal vascular development and oxygen-induced retinopathy in the dog. Invest Ophthalmol Vis Sci. 2011;52:4039–4047. doi: 10.1167/iovs.10-6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizee MR, Wooldrik D, Lakeman KA, van Het HB, Drexhage JA, Geerts D, Bugiani M, Aronica E, Mebius RE, Prat A, de Vries HE, Reijerkerk A. Retinoic acid induces blood-brain barrier development. J Neurosci. 2013;33:1660–1671. doi: 10.1523/JNEUROSCI.1338-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prat A, Biernacki K, Wosik K, Antel JP. Glial cell influence on the human blood-brain barrier. Glia. 2001;36:145–155. doi: 10.1002/glia.1104. [DOI] [PubMed] [Google Scholar]
- 42.Provis JM. Development of the primate retinal vasculature. Prog Retin Eye Res. 2001;20:799–821. doi: 10.1016/s1350-9462(01)00012-x. [DOI] [PubMed] [Google Scholar]
- 43.Provis JM, Sandercoe T, Hendrickson AE. Astrocytes and blood vessels define the foveal rim during primate retinal development. Invest Ophthalmol Vis Sci. 2000;41:2827–2836. [PubMed] [Google Scholar]
- 44.Rangasamy S, Srinivasan R, Maestas J, McGuire PG, Das A. A potential role for angiopoietin 2 in the regulation of the blood-retinal barrier in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:3784–3791. doi: 10.1167/iovs.10-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rapaport DH, Wong LL, Wood ED, Yasumura D, LaVail MM. Timing and topography of cell genesis in the rat retina. J Comp Neurol. 2004;474:304–324. doi: 10.1002/cne.20134. [DOI] [PubMed] [Google Scholar]
- 46.Ratajska A, Czarnowska E. Vasculogenesis of the embryonic heart: contribution of nucleated red blood cells to early vascular structures. Cardiovasc Hematol Disord Drug Targets. 2006;6:219–225. doi: 10.2174/187152906778249527. [DOI] [PubMed] [Google Scholar]
- 47.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 48.Sabanayagam C, Tai ES, Shankar A, Lee J, Sun C, Wong TY. Retinal arteriolar narrowing increases the likelihood of chronic kidney disease in hypertension. J Hypertens. 2009;27:2209–2217. doi: 10.1097/HJH.0b013e328330141d. [DOI] [PubMed] [Google Scholar]
- 49.Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Front Pharmacol. 2012;3:46. doi: 10.3389/fphar.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schnitzer J. Astrocytes in the guinea pig, horse, and monkey retina: their occurrence coincides with the presence of blood vessels. Glia. 1988;1:74–89. doi: 10.1002/glia.440010109. [DOI] [PubMed] [Google Scholar]
- 51.Scott A, Powner MB, Gandhi P, Clarkin C, Gutmann DH, Johnson RS, Ferrara N, Fruttiger M. Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. PLoS One. 2010;5:e11863. doi: 10.1371/journal.pone.0011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen W, Li S, Chung SH, Gillies MC. Retinal vascular changes after glial disruption in rats. J Neurosci Res. 2010;88:1485–1499. doi: 10.1002/jnr.22317. [DOI] [PubMed] [Google Scholar]
- 53.Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2003;74:70–76. doi: 10.1136/jnnp.74.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thornit DN, Vinten CM, Sander B, Lund-Andersen H, la Cour M. Blood-retinal barrier glycerol permeability in diabetic macular edema and healthy eyes: estimations from macular volume changes after peroral glycerol. Invest Ophthalmol Vis Sci. 2010;51:2827–2834. doi: 10.1167/iovs.09-4172. [DOI] [PubMed] [Google Scholar]
- 55.Toda R, Kawazu K, Oyabu M, Miyazaki T, Kiuchi Y. Comparison of drug permeabilities across the blood-retinal barrier, blood-aqueous humor barrier, and blood-brain barrier. J Pharm Sci. 2011;100:3904–3911. doi: 10.1002/jps.22610. [DOI] [PubMed] [Google Scholar]
- 56.Tomanek RJ. Formation of the coronary vasculature during development. Angiogenesis. 2005;8:273–284. doi: 10.1007/s10456-005-9014-9. [DOI] [PubMed] [Google Scholar]
- 57.Tomasek JJ, Haaksma CJ, Schwartz RJ, Vuong DT, Zhang SX, Ash JD, Ma JX, Al-Ubaidi MR. Deletion of smooth muscle alpha-actin alters blood-retina barrier permeability and retinal function. Invest Ophthalmol Vis Sci. 2006;47:2693–2700. doi: 10.1167/iovs.05-1297. [DOI] [PubMed] [Google Scholar]
- 58.Uemura A, Kusuhara S, Katsuta H, Nishikawa S. Angiogenesis in the mouse retina: a model system for experimental manipulation. Exp Cell Res. 2006;312:676–683. doi: 10.1016/j.yexcr.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 59.Wang S, Park S, Fei P, Sorenson CM. Bim is responsible for the inherent sensitivity of the developing retinal vasculature to hyperoxia. Dev Biol. 2011;349:296–309. doi: 10.1016/j.ydbio.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.West H, Richardson WD, Fruttiger M. Stabilization of the retinal vascular network by reciprocal feedback between blood vessels and astrocytes. Development. 2005;132:1855–1862. doi: 10.1242/dev.01732. [DOI] [PubMed] [Google Scholar]
- 61.Xu H, Dawson R, Crane IJ, Liversidge J. Leukocyte diapedesis in vivo induces transient loss of tight junction protein at the blood-retina barrier. Invest Ophthalmol Vis Sci. 2005;46:2487–2494. doi: 10.1167/iovs.04-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu HZ, Le YZ. Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Invest Ophthalmol Vis Sci. 2011;52:2160–2164. doi: 10.1167/iovs.10-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- 64.Zhang SX, Ma JX, Sima J, Chen Y, Hu MS, Ottlecz A, Lambrou GN. Genetic difference in susceptibility to the blood-retina barrier breakdown in diabetes and oxygen-induced retinopathy. Am J Pathol. 2005;166:313–321. doi: 10.1016/S0002-9440(10)62255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng X, Li PH, Song SF. Expression of glial fibrillary acidic protein in retina of rats in acute experimental autoimmune encephalomyelitis. Sichuan Da Xue Xue Bao Yi Xue Ban. 2008;39:719–722. [PubMed] [Google Scholar]


