Abstract
This study investigated the effects of small interfering RNA (siRNA)-mediated silencing of chemokine receptor 4 (CXCR4) on the invasion capacity of human neuroblastoma cell line SH-SY5Y in vitro. Three siRNAs targeting CXCR4 were chemically synthesized and individually transfected into SH-SY5Y cells. Expression of CXCR4 mRNA and protein was significantly suppressed in transfected cells by all three sequence-specific siRNAs compared with control groups. Furthermore, the invasion capacity of SH-SY5Y cells was significantly decreased following transfection with CXCR4-specific siRNA compared with the control groups. These data demonstrate that down-regulation of CXCR4 can inhibit in vitro invasion of neuroblastoma.
Keywords: nerve regeneration, chemokine receptor 4, small interfering RNA, neuroblastoma, invasion, Transwell chamber, liposome, NSFC grant, neural regeneration
Introduction
Neuroblastoma is one of the most common solid tumors in children. It is highly malignant and metastasis occurs even in the early stages of the disease. Lung and brain metastases are very common and bone marrow metastases occur in 60% of cases; these are the main causes of death in patients with neuroblastoma (Ara and DeClerck, 2006). A theoretical model has been proposed in which tumor cells form organ-specific metastasis through the specific interaction of chemokine receptor 4 (CXCR4) and its ligand, CXCL12 (Muller et al., 2001). CXCR4 participates in tumor occurrence, growth and metastasis, and CXCR4 expression is upregulated during neuroblastoma metastasis (Hao et al., 2005). In this study, RNA interference technology was used to investigate the effect of silenced CXCR4 on the metastatic capacity of the human neuroblastoma cell line SH-SY5Y in vitro.
Materials and methods
Preparation of recombinant CXCR4 siRNA plasmids
Three CXCR4 sequence-specific siRNAs incorporating BamHI and HindIII restriction enzyme sites for cloning purposes were designed and synthesized according to the human CXCR4 cDNA sequence (GenBank accession No. NM-003467; Table 1). Formation of double-stranded DNA by annealing of siRNAs was confirmed by agarose gel electrophoresis. The pSilencer™ neo expression vector was digested with BamHI and HindIII, and the resulting 4.5-kb fragments were separated by agarose gel electrophoresis. The siRNA fragments were ligated into the vector using T4 DNA ligase and then transformed into competent XL1-blue Escherichia coli by heat shock. Cells were then cultured on Lysogeny broth agar containing ampicillin to screen for monoclonal colonies. DNA was extracted by alkaline lysis and clones containing the correct insert were identified by diagnostic restriction enzyme digestion.
Table 1.
CXCR4 siRNA sequences
Culture and recombinant plasmid transfection of SH-SY5Y neuroblastoma cells
One day prior to transfection, SH-SY5Y cells were treated with 0.25% pancreatin, diluted with complete medium (serum supplemented) and inoculated into six-well plates (1 × 106 cells/well). Cells were cultured to 60–80% confluence, washed with serum-free Dulbecco's modified Eagle's medium (DMEM) three times and then 1.5 mL Opti-MEM (Gibco, Cat. No. 31985) serum-free medium was added to each well. Cells were transfected in the following groups and incubated for 30 minutes: (1) siRNA groups (1, 2 and 3): mixture containing 25 μg Silencer/siRNA plasmid (1, 2 or 3) (final concentration of siRNA: 50 nmol/L), 240 μL Opti-MEM serum-free medium and 10 μL Lipofectamine 2000 (Invitrogen, Cat. # 11668); (2) blank control group: untreated neuroblastoma SH-SY5Y cells; (3) empty vector control group: empty plasmid + liposome control group, mixture containing 25 μg empty plasmid, 240 μL Opti-MEM serum-free medium and 10 μL Lipofectamine 2000. After 6 hours of culture, the supernatant was removed and replaced with complete medium and culture was continued for a further 72 hours. Efficient transfection was confirmed by visualization of green fluorescent protein using fluorescence microscopy.
Semi-quantitative RT-PCR analysis of CXCR4 mRNA levels in siRNA-transfected SH-SY5Y cells
Cells were harvested 72 hours after transfection and total RNA was extracted using the Trizol one-step method. The RNA concentration was estimated by ultraviolet spectrophotometry, and RNA integrity was assessed by 1% agarose gel electrophoresis. Forward and reverse PCR primers (synthesized by Sangon Biotech, Shanghai, China) were designed using Primer Premier 5.0 software for the amplification of CXCR4 (546-bp product) and β-actin (500-bp product) (primer sequences are listed in Table 2).
Table 2.
RT-PCR primers

The following conditions were used for PCR amplification: 94°C for 3 minutes; 30 cycles of 94°C for 40 seconds, 56°C for 30 seconds and 72°C for 60 seconds, with a final extension step at 72°C for 10 minutes. The correct sizes of amplification products were confirmed by 1% agarose gel electrophoresis. The integrated absorbance of the bands was analyzed using a gel imaging system and the ratio of CXCR4/β-actin was calculated to represent the relative level of CXCR4 mRNA.
Immunocytochemical detection of CXCR4 protein in siRNA-transfected SH-SY5Y cells
Cells in the logarithmic growth phase were harvested 72 hours after transfection and mounted onto microscope slides (polylysine treated and high-pressure sterilized), rinsed with PBS and fixed in acetone at −4°C for 30 minutes. Immunocytochemical staining was performed using the PV-6000 method. Five randomly selected fields were analyzed using ImagesPlus software and the average gray value was calculated.
Western blot assay of CXCR4 protein levels in siRNA-transfected SH-SY5Y cells
Cells were harvested 72 hours after siRNA transfection and lysed by addition of radioimmune precipitation assay lysis buffer for extraction of total cellular proteins. Protein concentrations were measured using a bicinchoninic acid kit. Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes, blocked, and incubated with rabbit-anti-human CXCR4 monoclonal antibody (1:200) overnight at 4°C. Membranes were incubated with a horseradish peroxidase-conjugated secondary detection antibody (1:500) for 2 hours. Proteins were visualized by enhanced chemiluminescence and analyzed using a gel imaging system to scan the band gray value. CXCR4 (43 kDa) protein was quantified with reference to the β-tubulin (55 kDa) internal control.
Chemotaxis assay to assess metastatic capacity of SH-SY5Y neuroblastoma cells
Transwell chamber polycarbonate membranes were layered with extracellular matrix matrigel (approximately 40 μg/well), incubated at 37°C for 5 hours to enable polymerization and dried at room temperature overnight. Transfected cells (100 μL; cell density, 2.5 × 105/mL) were added to the lower transwell chamber. High-glucose DMEM (600 μL) containing 10% fetal bovine serum and stromal cell-derived factor 1 (CXCL12, 100 ng/mL) was added to the upper chamber. Transwell chemotaxis plates were incubated in 5% CO2 at 37°C for 48 hours. Cells on the polycarbonate membrane in the upper chamber were then removed, fixed with 4% paraformaldehyde for 30 minutes and stained with hematoxylin. The total number of cells that had migrated to the upper chamber was calculated by examination under an inverted microscope. The mean number of cells in five fields of view was counted in each well.
Statistical analysis
Data were expressed as mean ± SD. Results from different groups were statistically assessed with t-tests using SPSS 10.0 software. A value of P < 0.05 was considered statistically significant.
Results
CXCR4 siRNA recombinant plasmid construction and verification
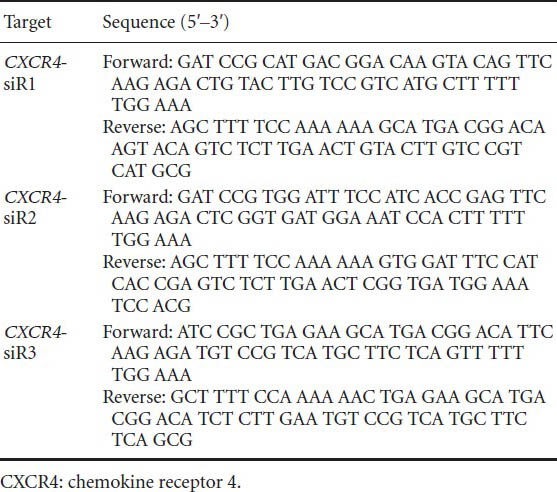
Restriction enzyme digestion indicated correct incorporation of target siRNA fragments in each clone (Figure 1). This was confirmed by sequence analysis (performed by Tianjin Saier Purcell Biotechnology in China).
Figure 1.
CXCR4 siRNA recombinant plasmid construction and verification.
Diagnostic restriction enzyme digestion indicated correct incorporation of target siRNA fragments (4.5 kb, arrow) in each clone (siRNA 1, 2 and 3). P1–3: Product 1–3; C1–4: CXCR4 siRNAs.
CXCR4 siRNA plasmid transfection efficiency of neuroblastoma SH-SY5Y cells
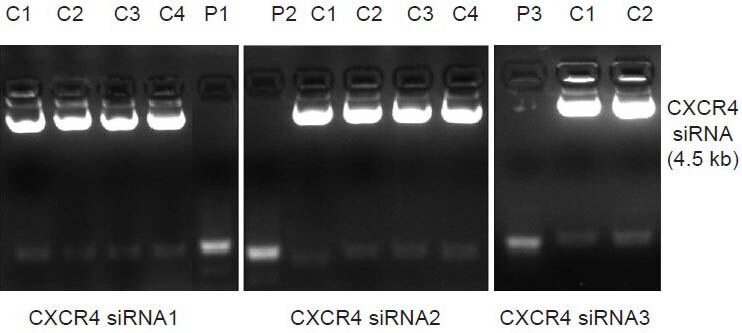
Efficient transfection was confirmed by co-expression of a green fluorescent protein reporter gene visualized by fluorescence microscopy. Transfection efficiency was 70% (Figure 2).
Figure 2.
Efficiency of SH-SY5Y transfection with CXCR4 siRNA plasmid.
Confirmation of efficient transfection of SH-SY5Y cells by visualization of green fluorescent protein reporter gene co-expression 72 hours after transfection. (A) Light microscopy image, × 100; (B) fluorescence microscopy image, wavelength 488 nm, × 100.
CXCR4 mRNA levels in siRNA-transfected SH-SY5Y cells
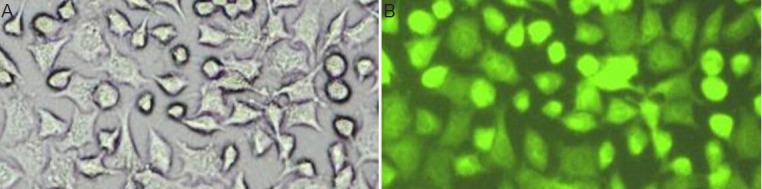
The relative absorbance values of CXCR4 RT-PCR assays in the test groups (siR1, siR2 and siR3) were 0.32 ± 0.09, 0.35 ± 0.13 and 0.33 ± 0.11, respectively. These values were significantly lower than 0.62 ± 0.10 for the empty vector control group and 0.58 ± 0.13 for the blank control group (P < 0. 05; Figure 3A).
Figure 3.
Detection of CXCR4 mRNA (A) and protein (B) in siRNA-transfected SH-SY5Y cells by RT-PCR and western blot assay.
(A)The relative absorbance of CXCR4 mRNA in the test groups (siR1, siR2 and siR3) was significantly lower than that in the empty vector control group and blank control group. (B) Compared with the empty vector control group and blank control group, the expression of CXCR4 protein was significantly decreased following transfection with CXCR4-specific siRNAs.
Immunocytochemical detection of CXCR4 protein in siRNA-transfected SH-SY5Y cells
CXCR4 protein in cytoplasm and cell membranes was detected as brown/yellow particles. The mean absorbance values in each group were: blank control, 92.20 ± 3.89; empty vector control, 94.20 ± 4.19; siR1, 75.98 ± 4.81; siR2, 75.52 ± 3.95; siR3 76.35 ± 6.51. Compared with the empty vector control and blank control groups, the expression of CXCR4 protein was significantly decreased following transfection with CXCR4-specific siRNAs (P < 0.01; Figure 4).
Figure 4.
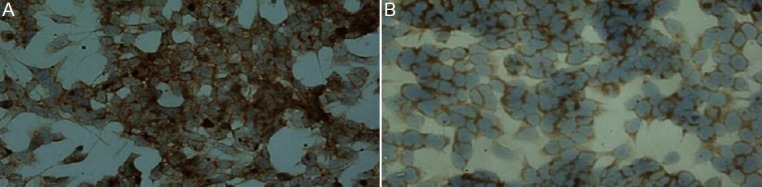
Immunocytochemical detection of CXCR4 protein in siRNA-transfected SH-SY5Y cells (light microscopy).
CXCR4 protein in cytoplasm and cell membranes was detected as brown/yellow particles. (A, × 200) CXCR4 in the negative control group is strongly expressed on the cell membrane and in the cytoplasm. (B, × 200) After transfection, CXCR4 in the siR1 transfection group is weakly expressed on the cell membrane and in the cytoplasm.
CXCR4 protein levels in siRNA-transfected SH-SY5Y cells
The relative gray values of CXCR4 protein analyzed by western blot assay were: blank control, 0.4832 ± 0.0012; empty vector control, 0.4231 ± 0.0023; siR1, 0.1103 ± 0.0023; siR2, 0.1203 ± 0.0015; siR3 0.1308 ± 0.0018. Compared with the empty vector control and blank control groups, the level of CXCR4 protein was significantly decreased following transfection with CXCR4-specific siRNAs (P < 0.01; Figure 3B).
Chemotaxis assay of metastatic capacity of SH-SY5Y neuroblastoma cells
Extracellular matrix invasion capacity of each group was assessed in chemotaxis assays as the number of cells passing from the lower to the upper chamber. The results for each group were: blank control, 53.11 ± 3.72; empty vector control, 49.53 ± 2.78; siR1, 25.48 ± 2.81; siR2, 30.89 ± 2.77; siR3 18.83 ± 1.79. Compared with the empty vector control and blank control groups, cell invasion capacity was significantly decreased following transfection with CXCR4-specific siRNAs (P < 0.05; Figure 5).
Figure 5.
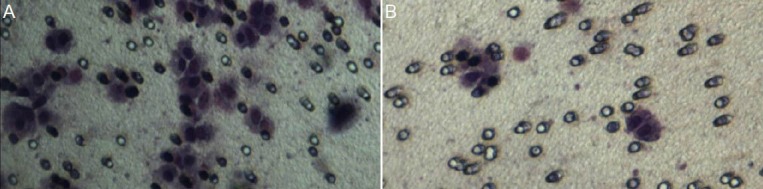
Chemotaxis assay of SH-SY5Y neuroblastoma cells (inverted microscopy, × 100).
The extracellular matrix invasion capacity of each group was assessed as the number of cells passing from the lower to the upper chamber in chemotaxis assays. Compared with the empty vector control group and blank control group (A), cell invasion capacity was significantly decreased following transfection with CXCR4-specific siRNA (B).
Discussion
Chemokines, originally named for their ability to induce leukocyte chemotaxis, are a superfamily of inducible pro-inflammatory cytokines. They are single-stranded proteins (8–10 kDa) and are produced by a variety of cells. CXCR4 (chemokine receptor 4) is a highly conserved G protein-coupled seven-transmembrane receptor consisting of 352 amino acids. In this study, RNA interference technology was adopted to investigate the effect of silenced CXCR4 expression on the in vitro invasion capacity of the human neuroblastoma cell line SH-SY5Y.
The interaction between chemokine CXCL12 and its specific receptor, CXCR4, known as the CXCR4-CXCL12 axis, involves a specific high-affinity interaction between these two closely related molecules for the transfer of intercellular information and induction of cell migration. Chemokines play an important role in tumor-initiated organ metastasis with “homing theory” proposed as the main mechanism underlying this process (Cui et al., 1999). A theoretical model for organ-specific tumor metastasis is based on the binding of a specific tumor-associated chemokine receptor and its ligand (Jiang and Wu, 2007). Tumor models in animals have shown that expression of CXCL12 and CXCR4 was upregulated in neuroblastoma metastases (Hao et al., 2005). Therefore, investigation of the CXCR4-CXCL12 axis in tumor invasion and metastasis represents a new focus for research into the mechanism of metastasis in neuroblastoma.
The CXCR4-CXCL12 axis induces the chemotactic migration of tumor cells and plays an important role in driving tumor invasion and organ-specific metastasis. This involves rearrangement of the intracellular skeleton and enhancement of target cell adhesion capacity. Furthermore, the CXCR4-CXCL12 axis is involved in the occurrence, development and prognosis of malignant tumors through the induction and guidance of leukocyte infiltration into tumor tissues, regulation of angiogenesis and direct activation and regulation of tumor cell functions (Vandercappellen et al., 2008; Yoshitake et al., 2008). CXCR4-CXCL12-mediated tumor cell proliferation and invasion occur through a signal transduction mechanism. Binding of CXCR4 and CXCL12 leads to G-protein structural changes and activation of the mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2 and SAPK/JNK pathways. MEK-1 is the upstream signal transduction pathway that activates extracellular signal-regulated kinase 1/2, mitogen-activated protein kinase and phosphoinositide-3 kinase pathways resulting in nuclear factor-κB activation. Activated CXCR4 also activates the Akt pathway, and extracellular signal-regulated kinase 1/2 and Akt activation leads to resistance to cell apoptosis and promotes proliferation (Balkwill, 2004). Binding of CXCL12 to CXCR4 also promotes hematopoiesis through a multi-signaling pathway that induces vascular endothelial growth factor secretion, resulting in angiogenesis and tumor cell growth and invasion (Salvucci et al., 2002). Following tumor cell stimulation by CXCL12, high-level F-actin polymerization and redistribution to the front of the cell with significant pseudopod formation is visible. This is necessary for malignant cell invasion and metastasis.
In this study, efficient transfection of the SH-SY5Y human neuroblastoma cell line with siRNA-encoding plasmids carrying a green fluorescent protein reporter gene was confirmed by fluorescence microscopy visualization of green fluorescent protein co-expression. Expression of CXCR4 mRNA was detected by semi-quantitative RT-PCR 72 hours after transfection and CXCR4 protein was detected by immunocytochemistry and western blot assays. The chemotactic invasion capacity of transfected cells was assessed in extracellular matrix gel transwell chemotaxis assays. Data analysis revealed significantly decreased levels of CXCR4 mRNA and protein as well as reduced cell invasion and metastasis capacity following transfection with CXCR4-specific siRNAs compared with the empty vector control and blank control groups. Therefore, these data confirm siRNA-mediated down-regulation of CXCR4 expression and indicate that this molecule is involved in metastasis of neuroblastoma. Furthermore, it was observed that the high in vitro invasion capacity of the SH-SY5Y neuroblastoma cell line correlates with high levels of CXCR4, thus implicating the CXCR4-CXCL12 axis in neuroblastoma invasion and metastasis. CXCL12 is highly expressed in bone marrow, a common site for neuroblastoma metastasis. Therefore, we speculate that the CXCR4-CXCL12 axis is pivotal to the development of bone marrow-specific metastasis in neuroblastoma. This hypothesis requires further investigation and may provide new targets for the treatment of neuroblastoma.
Although the results of this study demonstrate a relationship between CXCR4 expression and the invasion capacity of SH-SY5Y cells in vitro, it should be noted that the relationship between CXCR4 expression and invasion and metastasis in neuroblastoma is currently in dispute. Furthermore, non-functional CXCR4 expression has been identified in the tissues of patients with neuroblastoma (Airoldi et al., 2006). Results obtained by Meier et al. (2007) are in conflict with those obtained in this study. However, this may be attributed to differences in cell lines investigated.
In conclusion, specific siRNA-mediated down-regulation of CXCR4 expression results in reduced invasion capacity in SH-SY5Y cells. However, the complex network of intracellular chemokines and the signaling pathways involved remain to be identified. Such information will provide a greater understanding of the mechanism of metastasis in neuroblastoma and inform the selection of novel targets for therapy.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81272986 and the Natural Science Foundation of Shandong Province, No. ZR2011HZ002.
Conflicts of interest: None declared.
Copyedited by Allen J, Pack M, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Airoldi I, Raffaghello L, Piovan E, Cocco C, Carlini B, Amadori A, Corrias MV, Pistoia V. CXCL12 does not attract CXCR4 + human metastatic neuroblastoma cells: clinical implications. Clin Cancer Res. 2006;12:77–82. doi: 10.1158/1078-0432.CCR-05-1376. [DOI] [PubMed] [Google Scholar]
- 2.Ara T, DeClerck YA. Mechanisms of invasion and metastasis in human neuroblastoma. Cancer Metast Rev. 2006;25:645–657. doi: 10.1007/s10555-006-9028-9. [DOI] [PubMed] [Google Scholar]
- 3.Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004:171–179. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Cui J, Wahl RL, Shen T, Fisher SJ, Recker E, Ginsburg D, Long MW. Bone marrow cell trafficking following intravenous administration. Brit J Haematol. 1999;107:895–902. doi: 10.1046/j.1365-2141.1999.01779.x. [DOI] [PubMed] [Google Scholar]
- 5.Hao X, Dong Q, Lu H, Lv Z, Sun L, Jiang B. Establishment of neuroblastoma's model in mice. Zhonghua Xiaoer Waike Zazhi. 2005;26:372–375. [Google Scholar]
- 6.Jiang Y, Wu X. Correlations of chemokine CXCL12 and its receptor to tumor metastasis. Aizheng. 2007;26:220–224. [PubMed] [Google Scholar]
- 7.Meier R, Mühlethaler-Mottet A, Flahaut M, Coulon A, Fusco C, Louache F, Auderset K, Bourloud KB, Daudigeos E, Ruegg C. The chemokine receptor CXCR4 strongly promotes neuroblastoma primary tumour and metastatic growth, but not invasion. PLoS One. 2007;2:e1016. doi: 10.1371/journal.pone.0001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 9.Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99:2703–2711. doi: 10.1182/blood.v99.8.2703. [DOI] [PubMed] [Google Scholar]
- 10.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 11.Yoshitake N, Fukui H, Yamagishi H, Sekikawa A, Fujii S, Tomita S, Ichikawa K, Imura J, Hiraishi H, Fujimori T. Expression of SDF-1 alpha and nuclear CXCR4 predicts lymph node metastasis in colorectal cancer. Brit J Cancer. 2008;98:1682–1689. doi: 10.1038/sj.bjc.6604363. [DOI] [PMC free article] [PubMed] [Google Scholar]


