Abstract
Current research on bone marrow stem cell transplantation and autologous or xenogenic nerve transplantation for peripheral nerve regeneration has mainly focused on the repair of peripheral nerve defects in rodents. In this study, we established a standardized experimental model of radial nerve defects in primates and evaluated the effect of repair on peripheral nerve injury. We repaired 2.5-cm lesions in the radial nerve of rhesus monkeys by transplantation of autografts, acellular allografts, or acellular allografts seeded with autologous bone marrow stem cells. Five months after surgery, regenerated nerve tissue was assessed for function, electrophysiology, and histomorphometry. Postoperative functional recovery was evaluated by the wrist-extension test. Compared with the simple autografts, the acellular allografts and allografts seeded with bone marrow stem cells facilitated remarkable recovery of the wrist-extension functions in the rhesus monkeys. This functional improvement was coupled with radial nerve distal axon growth, a higher percentage of neuron survival, increased nerve fiber density and diameter, increased myelin sheath thickness, and increased nerve conduction velocities and peak amplitudes of compound motor action potentials. Furthermore, the quality of nerve regeneration in the bone marrow stem cells-laden allografts group was comparable to that achieved with autografts. The wrist-extension test is a simple behavioral method for objective quantification of peripheral nerve regeneration.
Keywords: nerve regeneration, peripheral nerve injury, rhesus monkeys, bone marrow stem cells, allogeneic nerve, transplantation, wrist-extension test, electrophysiology, neurological function, NSFC grant, neural regeneration
Introduction
A wide variety of tissue-engineered nerve grafts have recently been developed that exhibit robust potential for the repair of nerve damage. However, current research on the effects of bone marrow stem cells (BMSC) on peripheral nerve regeneration have mainly focused on the repair of nerve defects in rodent models (Kelly et al., 2007; Tos et al., 2008; Sinis et al., 2011) and have rarely involved peripheral nerve defects in primates. Compared with primates, rodents have a higher capacity for nerve regeneration. As such, rodent models do not accurately reflect the functional recovery of peripheral nerve injury in humans. Therefore, it is necessary to develop a simple and effective animal model that is as close to humans as possible. Because the forelimb of monkeys is very similar to human upper limbs in terms of anatomy and function, we expect peripheral nerve regeneration research to trend towards the use of forelimb nerves and primates as an appropriate animal model.
Levi et al. (1997) used a brachioradialis nerve defect model in monkeys to evaluate nerve regeneration with repair channels containing Schwann cells. However, the presence of the deformity increased the difficulty of determining whether the flexion capacity of the elbow had been recovered. The monkey brachioradialis nerve defect model was not a simple model to assess behavioral recovery because the brachioradialis muscle is innervated by other branches of the radial nerve that can also flex the elbow. In addition, we have used an ulna nerve defect model in monkeys in a previous study, but we found observation of neurofunctional recovery through behavioral analysis of the little and ring finger flexion after difficult surgery (Hu et al., 2007). Radial nerve injury proximal to the elbow level results in a loss of wrist extension due to motor denervation of the extensor carpi radialis longus and extensor carpi radialis brevis muscles. We hypothesized that the radial nerve of monkeys represents a safe and valid model for experimental nerve regeneration. In the present study, the effects of radial nerve repair in nonhuman primates were analyzed.
Materials and Methods
Animals
Ten rhesus monkeys (3-year-old males, weighing 3.2–4.0 kg) were obtained from the South China Primate Research and Development Center, China. The monkeys were tuberculosis- and Herpes B-negative. All animal treatment and procedure protocols were approved by the Experimental Animal Administration Committee of Sun Yat-sen University in China that appropriately minimized the number of animals used and their suffering.
Cultivation of monkey BMSC
Monkey BMSC were isolated as reported previously (Wang et al., 2008). Briefly, 3 mL of bone marrow was obtained from the tuberositas tibiae of one monkey after anesthetization with a mixture of ketamine (10 mg/kg, intramuscular (i.m.); King York Group Co., Ltd., Tianjin, China) and diazepam (2 mg/kg, i.m.; Jiuxu Pharmacerutical Co., Ltd., Zhejiang Province, China). Bone marrow was then treated with heparin, washed with DMEM/F12 medium (Gibco, Carlsbad, CA, USA), loaded onto Ficoll at a density of 1.077 μg/mL (GE Healthcare, Little Chalfont, UK), and centrifuged for 25 minutes at 2,000 r/min at 4°C. The white layer (mononuclear cells) was aspirated and washed twice with PBS (HyClone, Logan, UT, USA). The cells were then resuspended and cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (Gibco), 2.0 mmol/L L-glutamine (Sigma, St Louis, MO, USA), and 1% penicillin/streptomycin (HyClone). Culture medium was replaced every 3 days.
Preparation of acellular allogenic nerves
During each surgery, the monkeys were kept anesthetized with a mixture of ketamine (10 mg/kg, i.m.) and diazepam (2 mg/kg, i.m.). Briefly, the right common peroneal nerve was exposed in each animal with an incision that extended from the lower interface along the biceps femoris distally towards to the popliteal fossa. A 7-cm (minimum) segment of the common peroneal nerve was then excised. The nerve was immediately placed in DMEM/F12 medium. Fatty and connective tissues were then removed from the nerve epineurium and the nerve tissue was cut into 3-cm segments. All subsequent steps were conducted according to previously published protocols (Sondell et al., 1998; Wang et al., 2008), and all washing steps were performed at room temperature. Briefly, the nerve tissue was agitated in deionized distilled water (ddH2O) for 7 hours and then soaked in 46 mmol/L TritonX-100 (Sigma) in ddH2O overnight. After washing in PBS three times for 10 minutes each, the nerve tissue was agitated in a solution of 96 mmol/L sodium deoxycholate (Sigma) in ddH2O for 24 hours. The above washing steps were repeated before performing three final washes in PBS for 6 hours. Each segment was then trimmed into 2.5-cm nerve tissues and stored in 10 mmol/L PBS at 4°C until use.
In vitro construction of tissue-engineered nerves
The autologous BMSC were lifted with 0.25% trypsin (HyClone), and resuspended at a concentration of 2 × 107 cells/mL in DMEM/F12 medium containing 10% fetal bovine serum. Ten 2.5-cm peroneal nerve sections prepared from individual monkeys were randomly selected for use as BMSC-laden grafts. A total of 2 × 106 BMSC in 100 μL were injected into each nerve section using a micro-injector under a SXP-10 microscope at 10 × magnification (Shanghai Medical Instruments Co., Ltd., Shanghai, China). The microinjector was first inserted through the entire length of the nerve section, and then cells were injected in equal volumes at four evenly spaced points as the injector was withdrawn. Another set of nerve segments prepared from individual monkeys was used as an acellular control group by injecting 100 μL of DMEM/F12 medium into the nerve grafts containing no BMSC. The nerve grafts were then incubated in DMEM/F12 medium containing 10% fetal bovine serum in a humidified atmosphere with 5% CO2 at 37°C for 48 hours until use in the in vivo experiments.
Nerve transplantation surgical procedures
The ten monkeys were further used to study peripheral nerve regeneration. Both forearms of each animal were used (20 forearms in total). Two forearms were used as the negative control that received no nerve graft, and the other 18 forearms were equally and randomly divided into three groups: the BMSC-laden group (repair with an acellular allogeneic nerve combined with autologous BMSC); the autograft group (repair with an autologous nerve); and the acellular group (repair with an acellular allogeneic nerve). The animals were anesthetized with a mixture of ketamine (10 mg/kg, i.m.) and diazepam (2 mg/kg, i.m.). The radial nerve was exposed bilaterally using an incision that extended along the lateral margin of the distal arm towards the antecubital fossa. The brachioradialis muscle was then retracted laterally, and the biceps brachii and brachialis muscles were retracted medially to expose the radial nerve. The branch between the radial nerve and the extensor carpi radialis longus muscle was identified by its anatomic location with the aid of a nerve stimulator (6805-A, Shantou Medical Equipment Co., Ltd., Shantou, Guangdong Province, China). Positive identification was verified by extension of the wrist joint upon stimulation. The nerve was then severed at the level distally towards its origin. After sufficient exploration of the proximal and distal portion of the radial nerve, the origin of the brachioradialis of the radial nerve and the lowest origin of the triceps brachii branch of the radial nerve were identified by their anatomic locations with the aid of a nerve stimulator. The diameter of the radial nerve at the level of these two points and the distance between these two points (DBBR-TR) were measured. The deep branch of the radial nerve was also identified with the aid of a nerve stimulator. The parent radial nerve was distally transected at the level 1.0-cm proximally towards the origin of the deep branch of the radial nerve. A 2.5-cm segment of the parent radial nerve was then excised, and one of the three types of grafts was implanted into that defect site in the BMSC-laden or acellular group. The nerve grafts were stitched to the cut ends of the radial nerves with 8-0 nylon sutures (Figure 1) under a SXP-10 microscope at 10 × magnification. All monkeys then had 2.0-mm diameter K-wires installed between their ulna and humerus to prevent excessive elbow flexion. On the third day after the operation, each monkey was injected with Ceftriaxone sodium (40 mg/kg per day, i.m.). The K-wires were removed after 5 weeks.
Figure 1.
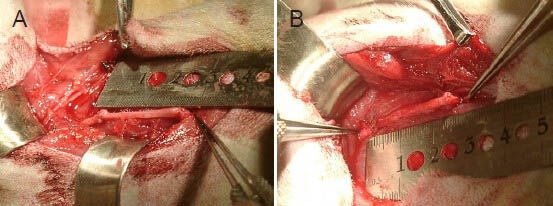
Intraoperative photograph of the radial nerve repaired with a nerve graft.
(A) A radial nerve repaired with a 2.5-cm allogeneic nerve containing autologous bone marrow stem cells. (B) A radial nerve repaired with a 2.5-cm autograft.
Functional evaluation of neurological recovery
A wrist-extension test was developed here as a behavioral method for functional evaluation of neurological recovery in this animal model 5 months after the surgery. Each animal was allowed to maximally extend its wrist from a fixed position to reach for food above its head. The wrist movement during this process was recorded by photos, and the maximum wrist extension angle (the angle between the ulna and the metacarpal) was measured. The experiment was repeated at least 10 times, and the average maximum wrist extension angle was determined for each experimental group.
Histomorphometry
Sections were taken from the nerve graft 5.0 mm proximal to the distal suture site and from the host nerve 5.0 mm distal to the distal connection site for toluidine blue staining. Briefly, the nerve segments were immersed in 2.5% Na-cacodylate-buffered glutaraldehyde for 2 hours, postfixed in 2% Na-cacodylate-buffered osmium tetroxide for 2 hours, serially dehydrated in gradient concentrations of ethanol, and infiltrated and embedded in Epon 812 (Ted Pella, CA, USA). Cross-sections at 1 μm thickness were cut and stained with toluidine blue to evaluate the extent of nerve regeneration. The slides were analyzed with an inverted phase contrast microscope (IX71, Olympus, Tokyo, Japan) using a computer-assisted system (Image-Pro Plus 4.5, Media Cybernetics, Rockville, MD, USA). To evaluate the nerves, the percentage of living neural tissue, the nerve fiber density (the number of nerve fibers/10,000 μm2), the nerve fiber diameter, and the myelin depth (half of the difference between the fiber diameter and the axon diameter) were determined. Four representative high-power fields (400 × magnification) were evaluated for each section. For each sample, three transverse sections were randomly selected and the mean values of these parameters were calculated.
Electrophysiology
Five months after surgery, electrophysiological tests were performed on all animals using a Keypoint electromyograph (Medtronic, Minneapolis, MN, USA) prior to the removal of the nerve grafts and extensor digitorum muscles. After the monkeys were anesthetized, the radial nerve was re-exposed following the procedures described above and dissected free of the surrounding tissues. The area of nerve repair was insulated with a piece of rubber dam. A stimulating electrode was placed under the radial nerve proximal to the graft, and a recording electrode was placed in the extensor digitorum. The distance between the stimulating electrode and the muscle recording electrode was 4.5 cm. Digitized data were collected and stored on a personal computer, and the onset latency and peak amplitude of compound motor action potentials, as well as the nerve conduction velocity were calculated from these data.
Statistical analysis
Data obtained from the functional evaluation of neurological recovery, histomorphometric analyses, and electrophysiological studies are expressed as mean ± SD. Statistical comparisons were performed as one-way analysis of variance tests with the Student-Newman-Keuls multiple comparisons method. Statistical analyses were performed in SPSS 11.0 software (SPSS, Chicago, IL, USA). P values less than 0.05 were considered statistically significant.
Results
Surgical records during the radial nerve injury model
For the total radial nerve model: (1) The mean blood loss was 4.7 mL, the mean operative time was 62.9 minutes, and the mean incision length was 6.5 cm. (2) During and after surgery, the animals generally remained in good condition as no evident infections or ulcers were found. (3) The mean diameter of the brachioradialis branches at their origin from the radial nerve was 0.21 cm. The mean diameter of the triceps brachii branch at the lowest origin point from the radial nerve was 0.26 cm. (4) The mean distance between the origin of the brachioradialis branches of the radial nerve and the lowest origin point of the triceps brachii of the radial nerve was 5.64 cm (2.9–6.8 cm).
Functional recovery of the radial nerves after transplantation
Although the animals demonstrated wrist-extension dysfunction after the operation, they maintained normal feeding using the flexion of their fingers and wrist. The animals that received acellular allografts showed muscle atrophy on the dorsal side of the forearm at 5 months post-surgery, while no muscle atrophy was observed in animals that received the BMSC-laden allografts or the autografts (Figure 2). All animals displayed a lack of wrist extension after the radial nerve surgery (Figure 3A). The wrist-extending capacity of the animals was partially restored by 5 months after nerve bridging (Figure 3B, C, D), although to varying extents. Moreover, the maximum wrist extension angle of the acellular group was significantly smaller than that in the BMSC-laden and autograft groups (P < 0.05), suggesting that BMSC-laden allografts can improve the functional recovery of the radial nerve.
Figure 2.
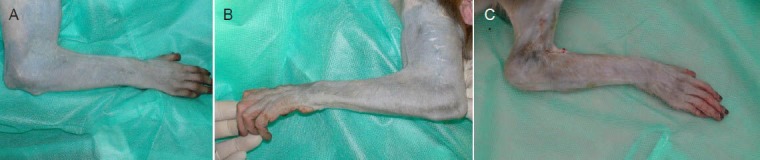
The appearance of the monkey forearms 5 months after surgery.
The animals that received acellular allografts showed muscle atrophy on the dorsal side of the forearm (C), while no muscle atrophy was observed in animals that received bone marrow stem cells-laden allografts (B) or autografts (A).
Figure 3.
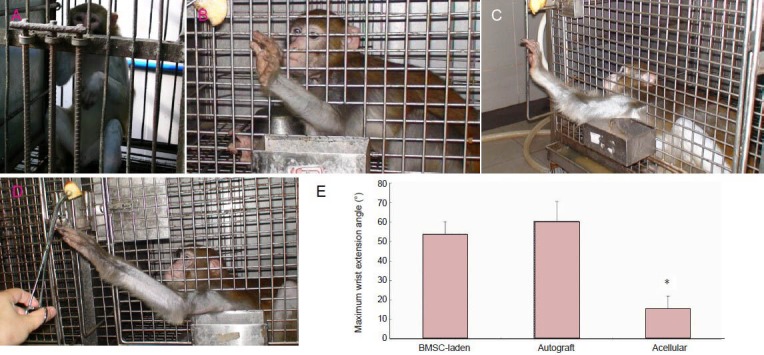
Behavioral assessment of the peripheral nerve 5 months after surgery.
All animals displayed a lack of wrist extension after the radial nerve surgery (A). Five months after the surgery, the monkeys implanted with the BMSC-laden allografts (C) showed a remarkable restoration of wrist-extension function, similar to that observed in the animals that received autografts (B). However, the animals that received acellular allografts exhibited reduced wrist-extension performance, with a smaller maximum wrist extension angle (D). (E) A smaller maximum wrist extension angle was seen in the acellular group compared with the BMSC-laden and autograft groups. *P < 0.05, vs. BMSC-laden and autograft groups (n = 6 forearms). Data are expressed as mean ± SD. One-way analysis of variance with the Student-Newman-Keuls multiple comparisons method was used for statistical testing. BMSC: Bone marrow stem cell.
Histomorphometric analysis of the radial nerves 5 months after transplantation
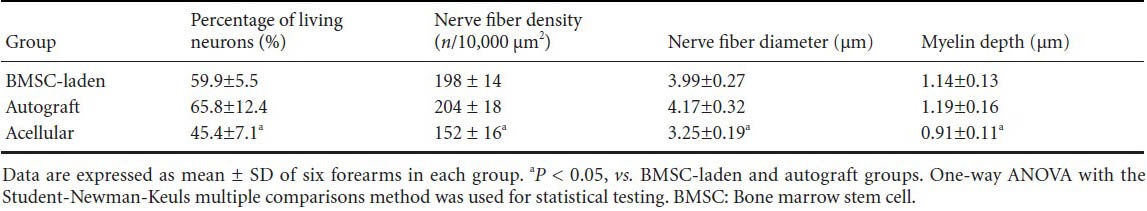
In agreement with the functional results, transplantation of BMSC-laden allografts and autografts possibly promoted remarkable axonal growth in the distal portion of the nerve grafts, manifested as an increased number of myelinated axons compared with the acellular allograft group (Figure 3). The percentage of living neural tissue, nerve fiber density, nerve fiber diameter, and myelin sheath thickness were significantly higher in the BMSC-laden and autograft groups than in the acellular group (P < 0.05). There were no statistically significant differences between the BMSC-laden and autograft groups (Table 1).
Table 1.
Histomorphometric data from the distal segment of the nerve grafts 5 months after nerve transplantation
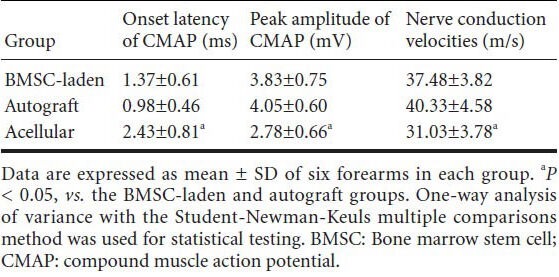
Electrophysiology of the radial nerves 5 months after transplantation
Five months after transplantation, the highest nerve conduction velocities and peak amplitudes were observed in the autograft group, which also showed the shortest latency of onset of compound muscle action potentials. The nerve conduction velocities, peak amplitude, and onset latency of compound muscle action potentials in the acellular group were significantly different than those in the BMSC-laden and autograft groups (P < 0.05; Table 2).
Table 2.
Electrophysiological analysis of the radial nerves 5 months after transplantation
Discussion
Traditional methods of assessing nerve recovery following peripheral nerve injury and repair, such as electrophysiology and histomorphometry, do not necessarily correlate with the return of motor and sensory functions (Dellon et al., 1989), despite being universally employed in neural regeneration experiments. Therefore, extrapolation of the electrophysiological parameters may lead to an inappropriate interpretation of the return of function (Kanaya et al., 1996).
The assessment of functional recovery is an important parameter for studies investigating peripheral nerve injury and repair. Behavioral methods are widely used as an effective measurement to grade or score quadrupedal rodent function after peripheral nerve injury (Ronchi et al., 2009, 2010; Bozkurt et al., 2011; Santos et al., 2012). However, such rodent models do not accurately reflect the functional recovery of peripheral nerve injury in humans. Rhesus monkeys, which are primates like humans, are more clinically relevant than rodents and more suitable for scaling up to humans. Therefore, it is necessary to develop a simple and effective peripheral nerve injury model in primates.
Levi et al. (1997) used a brachioradialis nerve model with repair channels to evaluate nerve regeneration in monkeys. Engaging the superficial head of the pronator teres above the elbow joint from the medial humeral epicondyle can cause compensatory hypertrophy of this muscle, resulting in elbow flexion coupled with forearm pronation, which might increase the number of false-positive results. Thus, the monkey brachioradialis nerve model is not a simple model for performing behavioral analysis.
The deep flexor of the ring and little fingers is dominated by the ulna nerve, but the superficial flexor of these two fingers is dominated by the median nerve. After the ulna nerve defect was made in the monkeys, the animals could still flex the proximal and distal interphalangeal joints of the thumb, index, and middle fingers, as well as the proximal interphalangeal joint of the ring and little fingers, which are dominated by the median nerve. Thus, the monkeys in this ulna nerve model can grasp food freely, even though they demonstrate dysfunctional flexion of the distal interphalangeal joint of the 4th and 5th fingers. We found that it was inconvenient to observe the functional recovery of the flexion of the distal interphalangeal joint of the ring and little fingers in the ulna nerve model (Hu et al., 2007). Therefore, the evaluation of functional recovery of nerve regeneration via behavior assessment in the monkey ulna nerve defect model is difficult.
To the best of our knowledge, the ulna nerve dominates the adductor pollicis and the deep head of the flexor pollicis brevis. After the defect was made in the median nerve, the animal still maintained the adduction and flexion function of the metacarpophalangeal joints of thumb, making the observation of the functional recovery in the median nerve monkey model also inconvenient. In addition, with dysfunctional opposition of the thumb and flexion of the thumb, index, and middle fingers, the animal may achieve poor functional recovery due to insufficient food intake.
Clinically, the radial nerve often shows better functional recovery than the ulna and median nerves after surgical repair (Xu et al., 2005). In the present study, our aim was to provide a standardized experimental model of radial nerve defects in primates. The mean distance between the origin of the brachioradialis branches of the radial nerve and the lowest origin point of the triceps brachii branch of the radial nerve (DBBR-TR) was 5.64 cm (2.9–6.8 cm) for all cases of radial nerve injury modeled in this study. It is impossible to make a 3.0-cm nerve defect without any injury to the brachioradialis and triceps brachii branches of the radial nerve in monkeys, where the DBBR-TR is less than 3.0 cm. Therefore, we chose 2.5-cm nerve defects of the radial nerve in monkeys for this study.
All animals (mean blood loss: 4.7 mL, mean operative time: 62.9 minutes, mean incision length: 6.5 cm) showed good wound healing without any infections or ulcers after surgery. Using their normal fingers and wrist flexion, the animals maintained normal feeding after surgery. These results demonstrated that the radial nerve injury model in monkeys is simple and safe for studies on peripheral nerve injury and repair.
Restoration of the dorsal forearm muscle in the rhesus monkeys occurred in both the BMSC-laden and autografts groups with no obvious atrophy. In contrast, visible atrophy appeared in the dorsal forearm muscles of monkeys in the acellular group. Five months after the surgery, nerve regeneration was assessed functionally, electrophysiologically, and histomorphometrically.
The selection of an appropriate behavioral evaluation method is crucial when measuring experimental nerve recovery. In the present study, we developed a wrist-extension test as an effective behavioral method for evaluating the functional recovery of nerve regeneration in this animal model. Each animal was forced to extend its wrist as far as possible from a fixed position in order to reach for food above its head. The wrist movement during this process was photographed, and the maximum wrist extension angle (the angle between the ulna and the metacarpal) was measured. The maximum wrist extension angle of the acellular group was significantly smaller than that of the BMSC-laden and autograft groups. The functional recovery of the radial nerve defect was clearly demonstrated by this wrist-extension test, which is very simple, non-invasive, and can be repeated as often as necessary in any research laboratory. Time-course studies to follow functional recovery after nerve repair are very easy to perform using this method. In addition, this wrist-extension test is very similar to the methods used clinically to assess nerve repair of the upper extremity.
In this study, the evaluation of nerve function using electrophysiology, histomorphometry, and behavior assessment should be better than simply using basic electrophysiology and histomorphometry testing. In conclusion, the wrist-extension test is a simple behavioral method for objective quantitative assessment of peripheral nerve regeneration in the radial nerve injury model in monkeys, and this model is safe and valid for studying experimental nerve regeneration.
Considering its wide availability, the radial nerve model in monkeys may provide a valuable example of a peripheral nerve injury model for other researchers.
Footnotes
Funding: This study was supported by the National High-Technology Research and Development Program of China (863 Program), No. 2006AA02A130; the National Natural Science Foundation of China, No. 81372041, 31070869, 30700847.
Conflicts of interest: None declared.
Copyedited by McCarty W, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
References
- 1.Bozkurt A, Scheffel J, Brook GA, Joosten EA, Suschek CV, O’Dey DM, Pallua N, Deumens R. Aspects of static and dynamic motor function in peripheral nerve regeneration: SSI and cat walk gait analysis. Behav Brain Res. 2011;219:55–62. doi: 10.1016/j.bbr.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Dellon AL, Mackinnon SE. Selection of the appropriate parameter to measure neural regeneration. Ann Plast Surg. 1989;23:197–202. doi: 10.1097/00000637-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Hu J, Zhu QT, Liu XL, Xu YB, Zhu JK. Repair of extended peripheral nerve lesions in rhesus monkeys using acellular allogenic nerve grafts implanted with autologous mesenchymal stem cells. Exp Neurol. 2007;204:658–666. doi: 10.1016/j.expneurol.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Kanaya F, Firrell JC, Breidenbach WC. Sciatic function index, nerve conduction tests, muscle contraction, and axon morphometry as indicators of regeneration. Plast Reconstr Surg. 1996;98:1264–1271. doi: 10.1097/00006534-199612000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Kelly EJ, Jacoby C, Terenghi G, Mennen U, Ljungberg C, Wiberg M. End-to-side nerve coaptation: a qualitative and quantitative assessment in the primate. J Plast Reconstr Aesthet Surg. 2007;60:1–12. doi: 10.1016/j.bjps.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 6.Levi AD, Sonntag VK, Dickman C, Mather J, Li RH, Cordoba SC, Bichard B, Berens M. The role of cultured Schwann cell grafts in the repair of gaps within the peripheral nervous system of primates. Exp Neurol. 1997;143:25–36. doi: 10.1006/exnr.1996.6344. [DOI] [PubMed] [Google Scholar]
- 7.Ronchi G, Nicolino S, Raimondo S, Tos P, Battiston B, Papalia I, Varejão AS, Giacobini-Robecchi MG, Perroteau I, Geuna S. Functional and morphological assessment of a standardized crush injury of the rat median nerve. J Neurosci Methods. 2009;179:51–57. doi: 10.1016/j.jneumeth.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Ronchi G, Raimondo S, Varejão AS, Tos P, Perroteau I, Geuna S. Standardized crush injury of the mouse median nerve. J Neurosci Methods. 2010;188:71–75. doi: 10.1016/j.jneumeth.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Santos AP, Suaid CA, Xavier M, Yamane F. Functional and morphometric differences between the early and delayed use of phototherapy in crushed median nerves of rats. Lasers Med Sci. 2012;27:479–486. doi: 10.1007/s10103-011-0972-4. [DOI] [PubMed] [Google Scholar]
- 10.Sinis N, Kraus A, Drakotos D, Doser M, Schlosshauer B, Müller HW, Skouras E, Bruck JC, Werdin F. Bioartificial reconstruction of peripheral nerves using the rat median nerve model. Ann Anat. 2011;193:341–346. doi: 10.1016/j.aanat.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Sondell M, Lundborg G, Kanje M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998;795:44–54. doi: 10.1016/s0006-8993(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 12.Tos P, Ronchi G, Nicolino S, Audisio C, Raimondo S, Fornaro M, Battiston B, Graziani A, Perroteau I, Geuna S. Employment of the mouse median nerve model for the experimental assessment of peripheral nerve regeneration. J Neurosci Methods. 2008;169:119–127. doi: 10.1016/j.jneumeth.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, Liu XL, Zhu JK, Jiang L, Hu J, Zhang Y, Yang LM, Wang HG, Yi JH. Bridging small-gap peripheral nerve defects using acellular nerve allograft implanted with autologous bone marrow stromal cells in primates. Brain Res. 2008;1188:44–53. doi: 10.1016/j.brainres.2007.09.098. [DOI] [PubMed] [Google Scholar]
- 14.Xu SD, Ge BF, Xu YK., 3rd . Beijing, China: People's Military Medical Press; 2005. Shiyong Guke Xue: Peripheral nerve injurie. [Google Scholar]


