Abstract
The purpose of this work was to investigate whether, by intranasal administration, the nerve growth factor bypasses the blood-brain barrier and turns over the spinal cord neurons and if such therapeutic approach could be of value in the treatment of spinal cord injury. Adult Sprague-Dawley rats with intact and injured spinal cord received daily intranasal nerve growth factor administration in both nostrils for 1 day or for 3 consecutive weeks. We found an increased content of nerve growth factor and enhanced expression of nerve growth factor receptor in the spinal cord 24 hours after a single intranasal administration of nerve growth factor in healthy rats, while daily treatment for 3 weeks in a model of spinal cord injury improved the deficits in locomotor behaviour and increased spinal content of both nerve growth factor and nerve growth factor receptors. These outcomes suggest that the intranasal nerve growth factor bypasses blood-brain barrier and affects spinal cord neurons in spinal cord injury. They also suggest exploiting the possible therapeutic role of intranasally delivered nerve growth factor for the neuroprotection of damaged spinal nerve cells.
Keywords: nerve regeneration, spinal cord injury, nerve growth factor, intranasal delivery, bloodbrain barrier, motor function, leptin, neuroprotection, rats, neural regeneration
Introduction
Nerve growth factor (NGF) is the member of the neurotrophic factor family of proteins (Huang and Reichardt, 2001). It is produced by and acts upon a number of developing, mature and damaged NGF-receptive neurons in the brain and the peripheral nervous system (Connor and Dragunow, 1998; Sofroniew et al., 2001; Aloe, 2004). NGF and NGF receptors (NGFRs) are also expressed in the spinal cord (Krenz and Weaver, 2000; Ferri et al., 2002; Brown et al., 2007; Davis-Lopez de Carrizosa et al., 2010; Liu et al., 2011), but whether they can act as neuroprotective agents on spinal cord neurons is not clear. The lack of knowledge is most likely due to the poor permeability of the blood-brain barrier (BBB) to NGF when this is injected intravenously (Pan et al., 1998) and to the possible development of hyperalgesia after intrathecal administration (Malik-Hall et al., 2005; Obata et al., 2005). Recent studies reported that NGF can be safely delivered into the brain by nasal (Chen et al., 1998; Koevary et al., 2003; Di Fausto et al., 2007) or ocular administration (Lambiase et al., 2005; Di Fausto et al., 2007; Lambiase et al., 2007; Lambiase et al., 2009), and be a potential therapeutic agent protecting injured brain and ocular cells (Levi-Montalcini, 1987; Connor and Dragunow, 1998; Aloe, 2004). Other studies have shown that molecules administered via nasal cavity can be transported not only to brain neurons (Chen et al., 1998; Koevary et al., 2003), but also to the spinal cord (Thorne et al., 1995; Illum, 2000; Liu et al., 2011). Based on these findings, the aim of the present work was to investigate whether intranasal NGF administration allows the neurotrophin to influence NGF and NGF receptor expression in spinal cord neurons and to affect locomotor behaviour after spinal hemisection in rats. In order to confirm the possibility of delivering proteins to the spinal cord by intranasal administration, we intranasally administered NGF or leptin, an adipokine with molecular weight similar to that of purified NGF, with a pattern of brain and spinal cord neuron receptor expression analogue to that of NGF receptors and with neuroprotective functions on the brain's cholinergic system (Di Marco et al., 2000; Harvey, 2007; Greco et al., 2010; Fernandez-Martos et al., 2012). Moreover, we used long-term intranasal delivery of NGF to rescue and neuro-protect spinal neurons after experimental spinal cord injury (SCI) in rats.
Materials and Methods
Animals
In order to perform the two experiments described below, forty-two 2-month-old male Sprague-Dawley rats, weighing 210 ± 12 g, were housed in polypropylene cages under standard light/dark conditions with food pellets and water at libitum. Animal care and handling were in compliance and conformity with National and International laws (EEC Council Directive 86/609, OJ L 358, 1, December 12, 1987).
Chemicals
NGF was purified in our laboratory from adult male mouse submaxillary glands, following the method described (Bocchini and Angeletti, 1969). Rat recombinant leptin (cat L 5037) was purchased from Sigma-Aldrich, Italy.
Experiment 1 (acute intranasal delivery in healthy animals)
In the first experiment, the spinal content of intranasally delivered proteins was measured. Healthy rats were randomized into three groups: one was treated with vehicle (controls), one treated with intranasal NGF (IN-NGF) and one with intranasal leptin (IN-Lep). Animals (n = 7 per experimental group) were intranasally administered 10 μL of either saline solution (Controls), or NGF (IN-NGF) or leptin (IN-Lep), 200 μg/mL each, dissolved in saline solution. Animals were sacrificed 24 hours after the treatments and the spinal cord was carefully removed, free of meninges, vessels, spinal roots and dorsal root ganglia. The spinal cord segments (T8–10) were snap-frozen by immersion in liquid nitrogen and immediately stored at −80°C, then utilized for tissue NGF quantification by ELISA. For hystochemical studies, two rats from each experimental group were used. Sections of the spinal cord were cut with a cryostat and used for histological analysis and immunohistochemical localization of NGF receptors and leptin.
Experiment 2 (long-term intranasal delivery in SCI)
In a further experiment, the effects of repeated IN-NGF on SCI were evaluated. A lesion of the spinal cord (n = 14) was performed in anesthetized rats (sodium pentobarbital, 50 mg/kg, intraperitoneally). Briefly, the spinal cord was exposed at the level of the vertebral segments T8–10. The T10 vertebral lamina was removed and a surgical lesion (hemisection) was done to the half portion of the spinal cord (Sharma). SCI rats were then randomized into two groups, one treated with daily vehicle (SCI; n = 7) and one with daily IN-NGF (SCI/NGF; n = 7) for 3 following weeks, with the same dosage used in the Experiment 1. A group of control rats (CT; n = 7) were not lesioned but daily treated with intranasal vehicle.
Locomotor behaviour
To evaluate the locomotor behaviour after 3 weeks of IN-NGF administration, rats (n = 7 for each experimental group) were starved for 12 hours and then set on a flat platform. The time required for the rats to get to a food source, distant 1 meter was recorded. Control and SCI and SCI/NGF rats were then sacrificed and the spinal cord was carefully removed as described above.
Sample collection
The spinal cord segments (T8–10) were snap-frozen by immersion in liquid nitrogen and immediately stored at −80°C. For histochemical studies, two rats from each experimental group were perfused with 4% paraformaldehyde in 0.1 mol/L phosphate buffer, pH 7.4, and their spinal cord was then removed and post-fixed overnight in the same buffer containing 20% sucrose.
NGF assay
The concentration of NGF in spinal cord tissue from both experiments was measured by a highly sensitive two-site ELISA (NGF Emax® ImmunoAssay System Cat. Nr.G7631, Promega Corporation, Madison, WI, USA), following the instructions provided by the manufacturer and the data are expressed as pg of NGF/mg of total tissue proteins.
Western blot analysis
Western blot analysis was performed on tissues from both experiments to assess NGF receptors and leptin content. Briefly, frozen spinal cord samples were homogenized by ultra-sonication in RIPA buffer (50 mmol/L Tris-HCl, pH7.4; 150 mmol/L NaCl; 5 mmol/L EDTA; 1% Triton X-100; 0.1% SDS; 0,5% sodium deoxycholate; 1mmol/L PMSF; 1 μg/mL leupeptin), centrifuged at 4°C for 20 minutes at 10,000 × g, then supernatants were stored at −20°C. Samples (30 μg of total protein) were dissolved in loading buffer (0.1 mol/L Tris-HCl buffer, pH 6.8, containing 0.2 mol/L dithiothreitol, DTT, 4% sodium-dodecyl-phosphate, SDS, 20% glycerol, and 0.1% bromophenol blue), separated by 8% or 12% SDS-PAGE, and electrophoretically transferred to polyvinylidene difluoride membranes overnight. The membranes were incubated for 1 hour at room temperature with blocking buffer constituted by 5% bovine serum albumin (for TrkA) or 5% non-fat dry milk (for leptin and GAPDH) in TBS-T (10 mmol/L Tris, pH 7.5, 100 mmol/L NaCl, and 0.1% Tween-20). Membranes were washed three times for 10 minutes each at room temperature in TBS-T followed by incubation overnight at 4°C with primary antibodies: rabbit polyclonal anti-high-affinity NGF receptor tyrosine kinase A (anti-TrkA, sc-118, Santa Cruz Biotechnology, Santa Cruz, CA, USA; working concentration: 1:1,000); rabbit polyclonal anti-leptin (sc-842, Santa Cruz Biotechnology; working concentration: 1:1,000); mouse monoclonal anti-GAPDH (sc-25778, Santa Cruz Biotechnology; working concentration: 1:5,000). After washing with TBS-T, membranes were incubated for 1 hour with horseradish peroxidase-conjugated anti-rabbit IgG (cat. Nr. 7074, Cell Signalling Technology, Danvers, MA, USA; working dilution: 1:4,000) or horseradish peroxidase-conjugated anti-mouse IgG (cat. Nr. 7076, Cell Signalling Technology, Danvers, MA, USA; working dilution: 1:5,000) as the secondary antibodies at room temperature. The blots were developed with an ECL chemiluminescent as the chromophore (Millipore, Bedford, MA, USA). The public domain NIH ImageJ Software (http://imagej.nih.gov/ij/) was used to evaluate band density, which was expressed as arbitrary units of grey level. The absorbance of GAPDH bands was used as a normalizing factor. For each gel blot, the normalized values were then expressed as percentage of relative normalized controls and used for statistical evaluation.
Histology and immunohistochemistry
For histological analysis, coded 20 μm sections were cut with a cryostat (Leica CM1850, Leica Microsystems Srl, Milan, Italy). For structural studies, sections of the spinal cord were stained with toluidine blue and examined with a Zeiss Axiophot microscope (Carl Zeiss Microscopy, Thornwood, NY, USA). For immunohistochemistry, sections were first incubated in PBS containing 10% horse or goat serum for 1 hour, and then left overnight at 4°C with: rabbit polyclonal anti-TrkA (sc-118, Santa Cruz Biotechnology; working concentration: 1:100); mouse monoclonal anti-p75NTR (sc-56331, Santa Cruz Biotechnology; working concentration: 1:100); rabbit polyclonal anti-leptin (sc-842, Santa Cruz Biotechnology; working concentration: 1:100). Sections, were then exposed to biotinylated rabbit anti-goat IgG Antibody (Vector Laboratories, Burlingame, CA, USA; working concentration: 1:500) for 2 hours at room temperature, and the immunoperoxidase staining was performed using an ABC reagent (Avidin-Biotin Complex solution, Vectastain Elite Kit, Vector Laboratories, Burlingame, CA, USA). The sections incubated with normal IgG were used as non-specific staining controls. Immunostained signals were then visualized with 3,3′-diaminobenzidine (DAB) Peroxidase (HRP) Substrate Kit (Vector Laboratories, Burlingame, CA, USA) and imaged using a Zeiss Axiophot microscope (Carl Zeiss Microscopy, Thornwood, NY, USA). Spinal cord sections (n = 10) of each control and experimental groups were used for a quantitative analysis in randomly selected, non-overlapping fields. For quantitative determination, we used the image analysis program Nikon-Lucia (Nikon Instruments SpA, Firenze, Italy) that automatically selected immunostained cell bodies, but no small cell fragments.
Statistical analysis
Statistical evaluations were performed by the GraphPad 5 software (GraphPad Software Inc., San Diego, CA, USA) and the data were expressed as mean ± SEM. Data from behavioural test (n = 7 for each experimental group), western blot analysis (n = 5 for each experimental group), ELISA (n = 5 for each experimental group) and image analysis (n = 10 for each experimental group) were evaluated by one-way analysis of variance (ANOVA). The Tuckey's honest significant difference (HSD) test was used for comparison between groups. A P value less than 0.05 was considered statistically significant.
Results
IN-NGF increased NGF and TrkA in the spinal cord of healthy rats
As depicted in Figure 1A, 1 day after IN-NGF administration, the level of NGF protein in the spinal cord increased significantly, compared to the level of controls. To investigate whether the elevated presence of NGF affected the expression of NGF receptors, we measured the tissue content of the high-affinity NGF receptor TrkA by western blot analysis and immuhistochemistry (shown in Figure 1B and Figure 1C). Our analysis indicated that the IN-NGF administration enhanced the presence of the TrkA receptor, compared to controls (Figure 1B).
Figure 1.
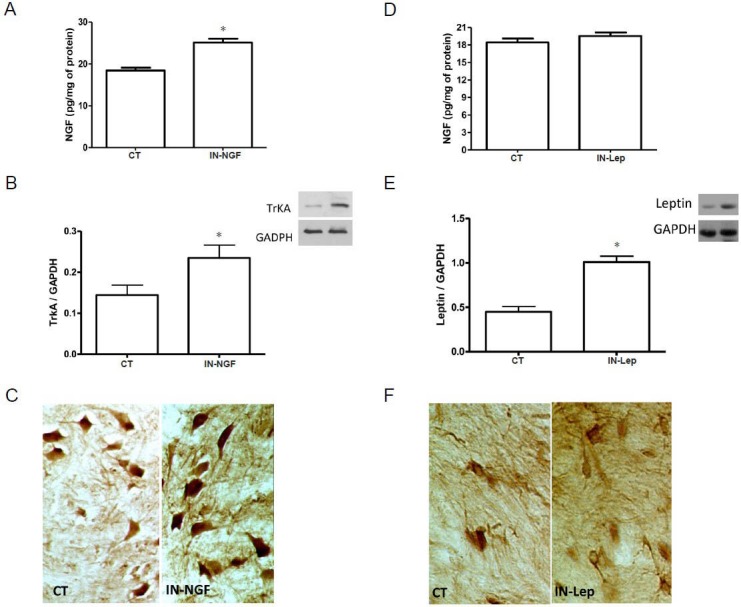
Effects of IN-NGF or IN-Lep on spinal NGF in healthy rats.
(A) Levels of NGF in the spinal cord of rats 24 hours after receiving a single IN administration of vehicle (CT) or NGF (IN-NGF). The increase of NGF in the NGF-treated rats was revealed by ELISA. *P < 0.05, vs. CT. (B) Expression of TrkA in the spinal cord of rats 24 hours after receiving a single IN administration of vehicle (CT) or NGF (IN-NGF). A representative western blot is presented, together with data from densitometry analysis of five separate gel/blot runs (n = 5). As depicted in the panel, TrkA tissue content is increased in the IN-NGF-treated rats. Data are presented as arbitrary unit of grey levels after normalization with GAPDH band integrated optical density. *P < 0.05, vs. CT. (C) Immunohistochemical localization of TrkA in the spinal cord of rats 24 hours after receiving a single IN administration of vehicle (CT) or NGF (IN-NGF). The picture shows the increase of TrkA immunoreactivity in spinal cord neurons in NGF-treated rats. (D) Levels of NGF in the spinal cord of rats 24 hours af-ter receiving a single IN administration of vehicle (CT) or leptin (IN-Lep). As revealed by ELISA, spinal NGF levels were unaffected by IN-Lep. (E, F) Leptin levels in the spinal cord after a single IN administration of vehicle (CT) or leptin (Lep). A representative western blot is presented in panel E, together with data from densitometry analysis of five separate gel/blot runs (n = 5). As depicted in the panel, Lep tissue content is increased in the IN-Lep-treated rats. Data are presented as arbitrary unit of grey levels after normalization with GAPDH band integrated optical density. *P < 0.05, vs. CT. The immunohistochemical localization of leptin in the spinal cord (panel F) revealed that the protein immunoreactivity increases after IN-Lep administration. IN: Intranasal; IN-NGF: intranasal administration of nerve growth factor; IN-Lep: intranasal administration of leptin.
IN-Lep did not affect NGF content in the spinal cord of healthy rats
To explore if the effects of IN-NGF were specifically related to the administration of the neurotrophin, we administered leptin, a neuro-hormone with spinal cord neuroprotective properties (Maruyama et al., 2008; Gezici et al., 2009), but with biochemical characteristics completely unrelated to NGF, in a separate group of rats. It was found that at 24 hours after intranasal delivery of leptin, the spinal content of NGF protein was unchanged (Figure 1D), while leptin increased in the spinal cord as revealed by western blot analysis and immunohistochemistry (Figure 1E, F).
IN-NGF improved locomotor behaviour and increased NGF and NGF receptor content in the spinal cord of SCI rats
To obtain information about the possible functional role of IN-NGF, rats with SCI were daily treated for 3 consecutive weeks with IN-NGF, and thereafter tested for locomotor activity. As depicted in Figure 2A, rats treated with NGF demonstrated improved locomotor behaviour, compared to NGF-untreated rats. The repeated IN-NGF treatments also enhanced the levels of NGF and TrkA receptors (Figure 2B, C). Because other studies have demonstrated that the low-affinity NGF receptor p75 neurotrophin receptor (p75NTR) was implicated in the survival of injured motoneurons (Ferri et al., 2002), we investigated the expression of p75NTR in injured and NGF-treated rats. The results reported in Figure 2D, E indicated that NGF administration enhanced the expression of p75NTR in injured rats, as shown in immunohistochemical analysis (Figure 2D, E).
Figure 2.
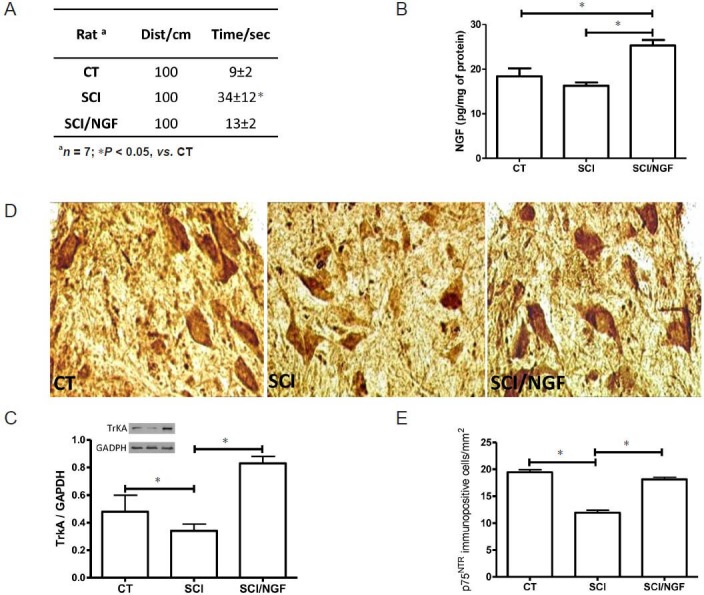
Effects of prolonged treatment with IN-NGF in SCI rats.
(A) Locomotor behaviour of control rats (CT), spinal cord injured rats treated with vehicle solution (SCI) and SCI rats treated with NGF for 3 consecutive weeks (SCI/NGF). The test evaluated the time needed for each rat to reach a distance of 1 meter. The rats with SCI and treated with NGF displayed better locomotor activity compared to rats treated with vehicle. *P < 0.05. (B) Levels of NGF in non-injured spinal cord (CT), in inured spinal cord treated for 3 weeks with vehicle (SCI) and in injured spinal cord treated for 3 weeks with NGF (SCI/NGF). The presence of NGF is significantly enhanced in SCI/NGF group than in both CT and SCI groups. *P < 0.05. (C) A representative western blot is presented, together with data from densitometry analysis of five separate gel/blot runs (n = 5). TrkA is down-regulated by tissue injury in the spinal cord (SCI vs. CT, *P < 0.05), while IN-NGF restored basal TrkA levels (SCI/NGF vs. SCI, *P < 0.05). Data are presented as arbitrary unit of grey levels after normalization with GAPDH band integrated absorbance. *P < 0.05. Immunohistochemical localization (D) of p75NTR in the spinal cord of control rats (CT), spinal cord injured rats treated with vehicle for 3 weeks (SCI) and spinal cord injured rats treated with IN-NGF for 3 weeks (SCI/NGF). The image analysis (E) revealed that p75NTR immunopositive cell number decreased below CT levels after SCI, while IN-NGF normalized the number of p75NTR-positive cells in the spinal cord of SCI rats (SCI/NGF). *P < 0.05. IN-NGF: Intranasal administration of nerve growth factor; SCI: spinal cord injury.
Discussion
It has been shown that molecules with neurotrophic activity can bypass the blood-brain barrier, reaching multiple brain sites (Chen et al., 1998; Koevary et al., 2003) and also spinal cord neurons (Thorne et al., 1995; Illum, 2000; Sofroniew et al., 2001). The existence of a nasal-spinal cord pathway has been shown by radiolabeled studies indicating that intranasally delivered vascular endothelial growth factor or insulin reached the spinal cord (Thorne et al., 1995; Yang et al., 2009). The anatomical connection between the nasal cavity and central nervous system structures consists of two possible routes: one is associated with the peripheral olfactory system connecting the nasal passage with the olfactory bulbs and rostral brain regions; the other goes through the peripheral trigeminal system connecting the nasal passage with the brainstem and spinal cord region (Soligo et al., 2013). Results of our studies show that IN-NGF enhanced the presence of NGF and expression of NGF receptors in intact and injured spinal cord. The evidence that intranasally delivered leptin increased leptin immunoreactivity in the spinal cord, but did not affect spinal NGF levels, suggests that IN-NGF selectively enhances NGF activity in the spinal cord cells. Though it is not clear, from the present experiment, if the rise in spinal NGF after IN-NGF could represent a clue for IN-NGF travel from the nasal cavity through the spinal cord toward the brain or for an indirect stimulation of spinal NGF production elicited by IN-NGF, our data demonstrate that, by intranasal delivery of NGF, it is possible to selectively enhance NGF presence and action at spinal level.
Our data indicate that IN-NGF was able to generate an improvement of motor behaviour in rats with SCI (Bianchi et al., 2012; De Bellis et al., 2012) with a parallel increase of both TrkA and p75NTR, that were found down-regulated 3 weeks after SCI (Montazeri et al., 2013). NGF is produced by and acts upon a variety of neurons of the central nervous system (Thorne et al., 1995; Chen et al., 1998; Connor and Dragunow, 1998; Koevary et al., 2003; Yang et al., 2009), and it is able to guide regenerating axons across a lesion site, as well as to promote regenerative response in injured spinal cord neurons (Tuszynski et al., 1994). Since in our experiments intranasal NGF induced an increase in spinal NGF and NGF receptor content, it is conceivable that the enhanced NGF signalling mediated by TrkA and p75NTR in lesioned spinal cells attenuates the progression of neuronal degeneration and improves motor deficits. Further study is needed to investigate if the mechanism of NGF-mediated neuroprotection in SCI is related to the anti-apoptotic action of TrkA and p75NTR downstream signalling activation (Brandoli et al., 2001).
In conclusion, our observations provide further support to the existence of a nasal-spinal cord route and prospect a possible, rescuing role for intranasally delivered NGF for damaged spinal cord neurons. Our work confirms and extends the results of previously published papers from other groups (Alcala-Barraza et al., 2010), demonstrating that intranasal delivery of neurotrophin could be of great value in the therapy of central nervous system traumatic injury, including those of the spinal cord. Whether intranasal administration of NGF, alone or in combination with other specific active neurotrophic molecules, can reduce and/or delay degenerative events, such as SCI and whether the effect is long lasting need to be further investigated.
Footnotes
Funding: This study was supported by Proj. PRIN prot. 2007AF3XH4_005, “Fondazione Cassa di Risparmio di Roma”, and “Ministero della Salute” Grant No. RF-FGB-2005-150198.
Conflicts of interest: None declared.
Copyedited by Wu ZH, Hu JX, Li CH, Song LP, Zhao M
References
- 1.Alcala-Barraza SR, Lee MS, Hanson LR, McDonald AA, Frey WH, 2nd, McLoon LK. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J Drug Target. 2010;18:179–190. doi: 10.3109/10611860903318134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aloe L, Calzà L. Amsterdam, New York: Elsevier; 2004. NGF and related molecules in health and disease. [Google Scholar]
- 3.Bianchi P, Rocco ML, De bellis A, Aloe L. Effect of intranasal NGF adminstation in injured spinal cord and leptin levels in adult rats. Adipobiology. 2012;4:67–75. [Google Scholar]
- 4.Bocchini V, Angeletti PU. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969;64:787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandoli C, Shi B, Pflug B, Andrews P, Wrathall JR, Mocchetti I. Dexamethasone reduces the expression of p75 neurotrophin receptor and apoptosis in contused spinal cord. Brain Res Mol Brain Res. 2001;87:61–70. doi: 10.1016/s0169-328x(00)00284-9. [DOI] [PubMed] [Google Scholar]
- 6.Brown A, Ricci MJ, Weaver LC. NGF mRNA is expressed in the dorsal root ganglia after spinal cord injury in the rat. Exp Neurol. 2007;205:283–286. doi: 10.1016/j.expneurol.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Chen XQ, Fawcett JR, Rahman YE, Ala TA, Frey IW. Delivery of nerve growth factor to the brain via the olfactory pathway. J Alzheimers Dis. 1998;1:35–44. doi: 10.3233/jad-1998-1102. [DOI] [PubMed] [Google Scholar]
- 8.Connor B, Dragunow M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res Brain Res Rev. 1998;27:1–39. doi: 10.1016/s0165-0173(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 9.Davis-Lopez de Carrizosa MA, Morado-Diaz CJ, Morcuende S, de la Cruz RR, Pastor AM. Nerve growth factor regulates the firing patterns and synaptic composition of motoneurons. J Neurosci. 2010;30:8308–8319. doi: 10.1523/JNEUROSCI.0719-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bellis A, Rocco ML, Bianchi P, Aloe L. Effect of intranasal NGF administration in rats with injured spinal cord. Prog Neurosci. 2012;1:83–90. [Google Scholar]
- 11.Di Fausto V, Fiore M, Tirassa P, Lambiase A, Aloe L. Eye drop NGF administration promotes the recovery of chemically injured cholinergic neurons of adult mouse forebrain. Eur J Neurosci. 2007;26:2473–2480. doi: 10.1111/j.1460-9568.2007.05883.x. [DOI] [PubMed] [Google Scholar]
- 12.Di Marco A, Demartis A, Gloaguen I, Lazzaro D, Delmastro P, Ciliberto G, Laufer R. Leptin receptor-mediated regulation of cholinergic neurotransmitter phenotype in cells of central nervous system origin. FEBS J. 2000;267:2939–2944. doi: 10.1046/j.1432-1033.2000.01308.x. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Martos CM, Gonzalez P, Rodriguez FJ. Acute leptin treatment enhances functional recovery after spinal cord injury. PLoS One. 2012;7:e35594. doi: 10.1371/journal.pone.0035594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferri CC, Ghasemlou N, Bisby MA, Kawaja MD. Nerve growth factor alters p75 neurotrophin receptor-induced effects in mouse facial motoneurons following axotomy. Brain Res. 2002;950:180–185. doi: 10.1016/s0006-8993(02)03035-4. [DOI] [PubMed] [Google Scholar]
- 15.Gezici AR, Ergun R, Karakas A, Gunduz B. Serum leptin levels following acute experimental spinal cord injury. J Spinal Cord Med. 2009;32:416–421. doi: 10.1080/10790268.2009.11753205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N, Casadesus G. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer's disease. J Alzheimers Dis. 2010;19:1155–1167. doi: 10.3233/JAD-2010-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey J. Leptin: a diverse regulator of neuronal function. J Neurochem. 2007;100:307–313. doi: 10.1111/j.1471-4159.2006.04205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11:1–18. doi: 10.1016/s0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 20.Koevary SB, Lam V, Patsiopoulos G, Lake S. Accumulation of porcine insulin in the rat brain and cerebrospinal fluid following ocular application. J Ocul Pharmacol Ther. 2003;19:377–384. doi: 10.1089/108076803322279435. [DOI] [PubMed] [Google Scholar]
- 21.Krenz NR, Weaver LC. Nerve growth factor in glia and inflammatory cells of the injured rat spinal cord. J Neurochem. 2000;74:730–739. doi: 10.1046/j.1471-4159.2000.740730.x. [DOI] [PubMed] [Google Scholar]
- 22.Lambiase A, Tirassa P, Micera A, Aloe L, Bonini S. Pharmacokinetics of conjunctivally applied nerve growth factor in the retina and optic nerve of adult rats. Invest Ophthalmol Vis Sci. 2005;46:3800–3806. doi: 10.1167/iovs.05-0301. [DOI] [PubMed] [Google Scholar]
- 23.Lambiase A, Pagani L, Di Fausto V, Sposato V, Coassin M, Bonini S, Aloe L. Nerve growth factor eye drop administrated on the ocular surface of rodents affects the nucleus basalis and septum: biochemical and structural evidence. Brain Res. 2007;1127:45–51. doi: 10.1016/j.brainres.2006.09.102. [DOI] [PubMed] [Google Scholar]
- 24.Lambiase A, Aloe L, Centofanti M, Parisi V, Mantelli F, Colafrancesco V, Manni GL, Bucci MG, Bonini S, Levi-Montalcini R. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma. Proc Natl Acad Sci U S A. 2009;106:13469–13474. doi: 10.1073/pnas.0906678106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levi-Montalcini R. The nerve growth factor: thirty-five years later. Biosci Rep. 1987;7:681–699. doi: 10.1007/BF01116861. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Chen SS, Dan QQ, Rong R, Zhou X, Zhang LF, Wang TH. Crucial roles of NGF in dorsal horn plasticity in partially deafferentated cats. Growth Factors. 2011;29:49–56. doi: 10.3109/08977194.2010.549129. [DOI] [PubMed] [Google Scholar]
- 27.Malik-Hall M, Dina OA, Levine JD. Primary afferent nociceptor mechanisms mediating NGF-induced mechanical hyperalgesia. Eur J Neurosci. 2005;21:3387–3394. doi: 10.1111/j.1460-9568.2005.04173.x. [DOI] [PubMed] [Google Scholar]
- 28.Maruyama Y, Mizuguchi M, Yaginuma T, Kusaka M, Yoshida H, Yokoyama K, Kasahara Y, Hosoya T. Serum leptin, abdominal obesity and the metabolic syndrome in individuals with chronic spinal cord injury. Spinal Cord. 2008;46:494–499. doi: 10.1038/sj.sc.3102171. [DOI] [PubMed] [Google Scholar]
- 29.Montazeri F, Esmaeili A, Miroliaei M, Moshtaghian SJ. Messenger RNA expression patterns of p75 neurotrophin receptor and tropomyosin-receptor-kinase A following spinal cord injury. J Spinal Cord Med. 2013;36:231–236. doi: 10.1179/2045772312Y.0000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan W, Banks WA, Kastin AJ. Permeability of the blood-brain barrier to neurotrophins. Brain Res. 1998;788:87–94. doi: 10.1016/s0006-8993(97)01525-4. [DOI] [PubMed] [Google Scholar]
- 32.Sharma HS. Selected combination of neurotrophins potentiate neuroprotection and functional recovery following spinal cord injury in the rat. Acta Neurochir Suppl. 2010;106:295–300. doi: 10.1007/978-3-211-98811-4_55. [DOI] [PubMed] [Google Scholar]
- 33.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 34.Soligo M, Nori SL, Protto V, Florenzano F, Manni L. Acupuncture and neurotrophin modulation. Int Rev Neurobiol. 2013;111:91–124. doi: 10.1016/B978-0-12-411545-3.00005-5. [DOI] [PubMed] [Google Scholar]
- 35.Thorne RG, Emory CR, Ala TA, Frey WH., 2nd Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 1995;692:278–282. doi: 10.1016/0006-8993(95)00637-6. [DOI] [PubMed] [Google Scholar]
- 36.Tuszynski MH, Peterson DA, Ray J, Baird A, Nakahara Y, Gage FH. Fibroblasts genetically modified to produce nerve growth factor induce robust neuritic ingrowth after grafting to the spinal cord. Exp Neurol. 1994;126:1–14. doi: 10.1006/exnr.1994.1037. [DOI] [PubMed] [Google Scholar]
- 37.Yang JP, Liu HJ, Wang ZL, Cheng SM, Cheng X, Xu GL, Liu XF. The dose-effectiveness of intranasal VEGF in treatment of experimental stroke. Neurosci Lett. 2009;461:212–216. doi: 10.1016/j.neulet.2009.06.060. [DOI] [PubMed] [Google Scholar]


