Abstract
We reviewed the beneficial or harmful effects of low-dose ionizing radiation on several diseases based on a search of the literature. The attenuation of autoimmune manifestations in animal disease models irradiated with low-dose γ-rays was previously reported by several research groups, whereas the exacerbation of allergic manifestations was described by others. Based on a detailed examination of the literature, we divided animal disease models into two groups: one group consisting of collagen-induced arthritis (CIA), experimental encephalomyelitis (EAE), and systemic lupus erythematosus, the pathologies of which were attenuated by low-dose irradiation, and another group consisting of atopic dermatitis, asthma, and Hashimoto’s thyroiditis, the pathologies of which were exacerbated by low-dose irradiation. The same biological indicators, such as cytokine levels and T-cell subpopulations, were examined in these studies. Low-dose irradiation reduced inter-feron (IFN)-gamma (γ) and interleukin (IL)-6 levels and increased IL-5 levels and the percentage of CD4+CD25+Foxp3+Treg cells in almost all immunological disease cases examined. Variations in these biological indicators were attributed to the attenuation or exacerbation of the disease’s manifestation. We concluded that autoimmune diseases caused by autoantibodies were attenuated by low-dose irradiation, whereas diseases caused by antibodies against external antigens, such as atopic dermatitis, were exacerbated.
Keywords: low-dose irradiation, immune response, autoimmune disease, allergic diseases, cytokines, T-cell subpopulation
INTRODUCTION
Even very low radiation doses were initially considered to be harmful until the 1980s, and a linear no-threshold (LNT) dose-response relationship was reported. However, epidemiological studies revealed that the prevalence of cancer in areas with high natural radiation background was not high and various animal experiments demonstrated that low-dose irradiation was not always harmful. Since Luckey first proposed the physiological stimulatory effects of radiation in 1982, much evidence has accumulated in support of this concept. These beneficial and stimulatory effects, including stimulation of the growth rate (Luckey 1982), enhancement of survival after lethal high-dose irradiation (Yonezawa et al. 1990), prolongation of life span (Ducoff 1975), activation of immune functions (Liu et al. 1987; Anderson et al. 1988; Liu 1989; Ishii et al. 1995, 1996), delay in tumor growth or metastasis (Hashimoto et al. 1999; Kojima et al. 2004), delay of death due to radiation-induced acute myeloid leukemia by an earlier exposure to a small adapting dose of radiation (Mitchel et al. 1999), reducing the risk of both spontaneous spinal osteosarcomas and lymphomas by a single low-dose and low-dose-rate exposure (Mitchel et al. 2003) in animal experiments, prevention of chemically induced hepatotoxicity (Kojima et al. 2000b) and prevention of type-I diabetes in NOD mice (Takahashi et al. 2000), have been referred to as ‘radiation hormesis’ (Luckey 1991).
Several studies have since investigated the relationship between low-dose irradiation and the immune response (Gerber et al. 1983; Liu et al. 1987; Ibuki and Goto 1994). The results obtained from animal experiments demonstrated that low-dose whole-body irradiation enhanced the immune response by augmenting the proliferative reactive response of T cells to mitogenic stimulation (Kojima et al. 2000a; Pandey et al. 2005), altering cytokine release (Liu et al. 2003; Cheda et al. 2008) and immune cell populations (Ina and Sakai 2005), and/or augmenting T cell activation capacity by dendritic cells (Shigematsu et al. 2007). Regarding autoimmunity, the potentiation of T cell-mediated immunity in autoimmune-prone mice following exposure to low-dose irradiation was examined at the end of the 20th century (James and Makinodan 1988; James et al. 1990), and several research groups subsequently focused on the effects of low-dose irradiation on autoimmune diseases such as systemic lupus erythematosus (Ootsuyama et al. 2003; Tanaka et al. 2005; Tago et al. 2008), multiple sclerosis (Tsukimoto et al. 2008), rheumatoid arthritis (Nakatsukasa et al. 2008, 2010; Weng et al. 2010) and autoimmune thyroiditis (Nagayama et al. 2008) after the start of the 21st century.
Some groups have also focused on the effects of low-dose irradiation on allergic diseases, such as asthma (Fang et al. 2005) and atopic dermatitis (Fang et al. 2006). These studies originated from the proposal that low-dose irradiation could alter cytokine levels and the percentage of T cell subpopulations, which may in turn contribute to the attenuation of pathologies. If autoimmune reactions can indeed be controlled by low-dose irradiation, new treatment options may be developed in the future.
Based on the results of the studies described above, we attempted to focus on the effect of low-dose irradiation on autoimmune diseases and allergic diseases, classify these effects, and summarize their mechanisms.
Meanwhile, in our referenced papers, whole-body 0.5 Gy/exposure in animal was considered to be low dose. Many clinical applications concerning low dose radiation therapy (LD-RT) have been reported (Cuttler 2004; Rödel et al. 2012), and most of experimental anti-inflammatory effects have displayed a maximum between 0.3 Gy and 1.0 Gy. The dose range seems to be reasonable according to the clinical experience with LD-RT over more than on century (Kern and Keilholz 2009). Therefore the authors of our referenced papers might take clinically low dose irradiation into consideration because they finally aimed to develop new therapies for above diseases. Moreover, there are some reports that investigated the mechanism of LD-RT using the animal models (Hildebrandt et al. 2000, 2003; Rödel et al. 2007). 0.5 Gy/exposure of γ- or x-ray irradiation was also regarded as low-dose in these animal studies. From above reasons, we consider 0.5 Gy/ exposure as low-dose irradiation in this review.
The findings presented in this review will be useful for the development of new therapies for immunological disorders.
THE EFFECT OF LOW-DOSE γ-IRRADIATION ON IMMUNE DISEASES
The effect of low-dose ionizing radiation on diseases resulting from immunological disorders was divided into two groups based on a literature search: the attenuation and exacerbation of pathologies. Table 1 shows the two types of immunologically related diseases influenced by low-dose irradiation using animal models. The beneficial effect of low-dose irradiation has been reported in rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and multiple sclerosis (MS), whereas symptoms were exacerbated by low-dose irradiation in atopic dermatitis (AD), asthma, and Hashimoto’s thyroiditis (HT).
Table 1.
Two types of diseases influenced by low-dose irradiation.
| Status | Diseases | Authors |
|---|---|---|
| Attenuation of pathology | Rheumatoid arthritis (Collagen-induced arthritis) | Nakatsukasa et al. 2008, 2010; Weng et al. 2010 |
| Systemic lupus erythematosus | Ootsuyama et al. 2003; Tanaka et al. 2005; Tago et al. 2008 | |
| Multiple sclerosis | Tsukimoto et al. 2008 | |
| Exacerbation of pathology | Atopic Dermatitis | Fang et al. 2006 |
| Asthma | Fang et al. 2005; Park et al. 2013b | |
| Hashimoto’s thyroiditis | Nagayama et al. 2008 |
Collagen-induced Arthritis (CIA)
Rheumatoid arthritis (RA) is an autoimmune disease that causes pain, swelling, and stiffness in the joints, and may lead to joint destruction and chronic disability. It is characterized by the accumulation and proliferation of inflammatory cells in the synovial lining, synovial over-growth, and the formation of pannus tissues (Feldmann et al. 1996; Gravallese 2002; Firestein 2003). As the histopathology and above-mentioned clinical features of CIA are similar to those of human RA, CIA is the most widely used animal model of autoimmunity (Myers et al. 1997). Several biological indicators of RA have been reported in the living body, and are the clinical score of pathology, autoantibody concentrations, cytokine levels, and T cell subpopulations. Autoantibodies, such as rheumatoid factor and the anti-type II collagen (CII) antibody, play a key role in the pathogenesis of inflammatory arthritis (Cho et al. 2007; Rowley et al. 2008). Among the cytokines, tumor necrosis factor-α (TNF-α), inter-leukin-6 (IL-6) (Chu et al. 1991; Ridderstad et al. 1991), and interleukin-17 (IL-17) (Nakae et al. 2003; Lubberts et al. 2005; Afzali et al. 2007) have been closely associated with inflammation in RA. Proper immune functions are strongly dependent on numerical changes in various lymphocyte subsets (Askonas 1988). Bogdándi et al. (2010) showed that low-dose irradiation caused quantitative alterations in these cell numbers.
The schedules for immunization and irradiation are shown in Figs. 1A, 1B, 1C and 1D. The results of clinical score reported by Nakatsukasa et al. (2008, 2010) and Weng et al. (2010) are summarized in Table 2A, and indicated that low-dose irradiation delayed the onset, reduced the incidence, and attenuated the pathology of CIA in DBA/1J mice.
FIG. 1A.
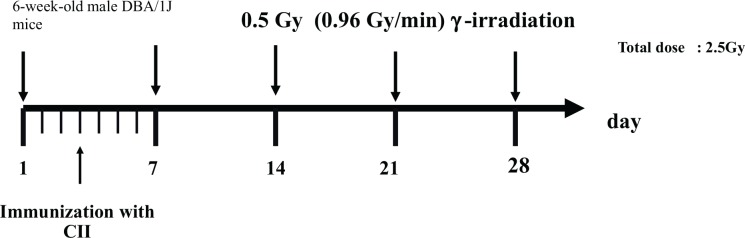
Schedule for immunization with bovine type II Collagen (CII) and irradiation. (Nakatsukasa et al. 2008).
FIG. 1B.
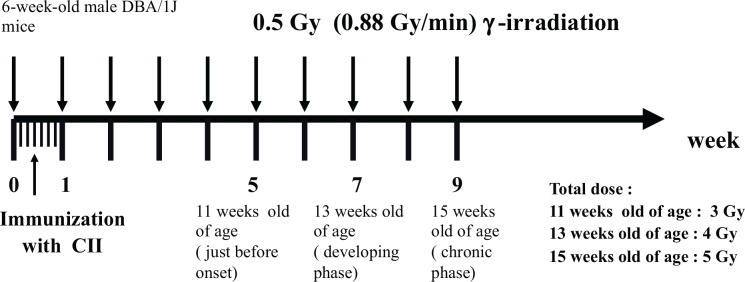
Schedule for immunization with bovine type II Collagen (CII) and irradiation. (Nakatsukasa et al. 2010).
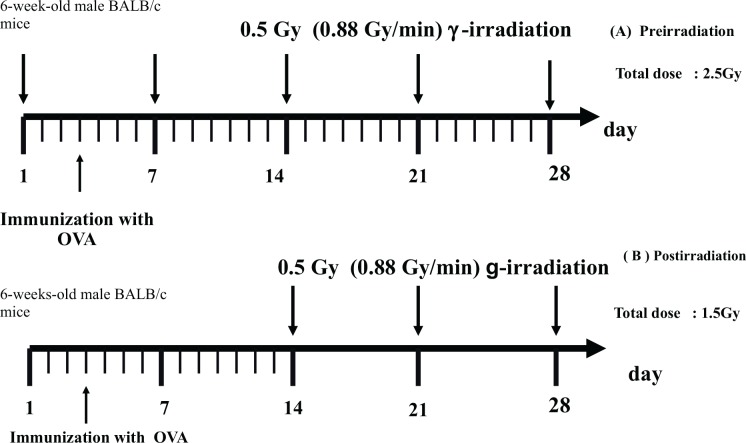
FIG. 1C.
Schedule for immunization with ovalbumin (OVA) and irradiation. (A) Preirradtiation (B) Postirradiation (Nakatsukasa et al. 2010).
FIG. 1D.
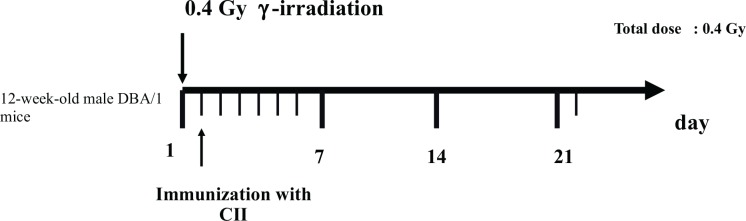
Schedule for immunization with bovine type II Collagen (CII) and irradiation. (Weng et al. 2010).
Table 2A.
Changes in the severity of diseases after low-dose irradiation.
| Authors of referenced studies | Irradiation | Clinical score (by an established scoring system) |
|---|---|---|
| Nakatsukasa et al. 2008 | Whole-body 0.5Gy/week (0.96Gy/min) for 5 weeks (from 6w to 10 w old) | Not increased until 50 days after immunization with collagen (almost score 0). Without irradiation, increased 30–40 days after immunization (above score 2). |
| Nakatsukasa et al. 2010 | Whole-body 0.5Gy/week (0.88Gy/min) for 5 weeks (from 5w to 9 w old) | The severity score was significantly suppressed 6 weeks and 8 weeks after immunization with collagen (score 0.5 for irradiated and score 2 for non-irradiated mice at 6 weeks, score 2.8 for irradiated and score 4.5 for non-irradiated mice at 8 weeks). |
| Weng et al. 2010 | Whole-body 0.4Gy 24h before a collagen injection | The severity score was less than 2 21 days after the collagen injection. Most mice in the CIA control group showed signs with a severity more than 2. |
Nakatsukasa et al. (2008, 2010) reported that anti-CII antibody production was detected in the serum of CIA mice, but not in that of normal mice, and was suppressed by low-dose irradiation. They also examined tumor necrosis factor levels and cytokines produced by splenocytes of which spllen was harvested from mice at 3 days after the last irradiation. Splenocytes from both control and irradiated mice were not stimulated by any antigen ex vivo and these findings have been summarized in Table 2B. TNF-α and interferon (IFN)-gamma (γ) levels were high in CIA mice, but were significantly lower in irradiated CIA mice than in non-irradiated CIA mice. They also showed that IL-6 increased in CIA and decreased to normal levels following treatment with irradiation. Because IL-6 is known to induce the activation of B cells (Kishimoto 1989), they also suggested that 0.5Gy γ-irradiation suppressed autoantibody production in mice with CIA by suppressing IL-6 production. These results indicate that the anti-CII antibody, which is an autoantibody, and IL-6 play an important role in developing the pathology of CIA. They concluded that γ-irradiation suppressed anti-CII antibody levels and the production of IL-6, thereby contributing to the attenuation of arthritis. From the above results, they speculated that if γ-irradiation suppressed the production of any antibodies, including those directed against an external antigen, low-dose γ-irradiation may be harmful during infection. They then attempted to examine the effect of repeated 0.5Gy γ-irradiation on antibody production against an external antigen. Ovalbumin (OVA) was injected into BALB/c mice as an external antigen and immunoglobulin levels were measured during repeated irradiation. The results revealed different changes in IL-6 concentrations from those in CIA mice. IL-6 production was significantly increased in the control OVA-immunized group and was further increased in the irradiated OVA-immunized group. These findings were the reverse of those observed in CIA mice (Tables 2B and 2C), and indicated that this difference was due to the antigen. CIA has been attributed to an internal antigen, whereas OVA was an external antigen. IL-17 levels were significantly increased in the CIA and OVA-immunized groups, but were significantly decreased by irradiation in both groups (Tables 2B and 2C). These findings indicated different effects on CIA and OVA-immunized mice. In CIA mice, the suppression of IL-17 production together with the up-regulation of regulatory T (Treg) cell population may contribute to attenuating the pathology of CIA by repeated 0.5-Gy γ irradiation. However, an increase in the production of Th2-type cytokines, such as IL-10 or IL-6 (Table 2C) together with up-regulation of the plasma cell percentage (Table 2D), may have contributed to the enhancement in antibody production in OVA-immunized mice (Kishimoto 1989; Rousset et al. 1992). Therefore, although IL-17 was suppressed by irradiation, the effect of antibody production may overcome that of IL-17.
Table 2B.
Changes in TNF and cytokine levels in CIA mice with or without irradiation.
| Authors of referenced studies | TNF and cytokines | Non-irradiated CIA group versus normal group | Irradiated CIA group versus non-irradiated CIA group |
|---|---|---|---|
| Nakatsukasa et al. 2008 | TNF-α | ↑ | ↓ |
| IFN-γ | ↑ | ↓ | |
| IL-6 | ↑ | ↓ | |
| Nakatsukasa et al. 2010 | IL-6 | ↑ | ↓ (4 and 6 weeks after immunization) |
| → (8 weeks after immunization) | |||
| IL-17 | ↑ | ↓ (4 and 6 weeks after immunization) | |
| → (8 weeks after immunization) |
↑ : significantly increased
↓ : significantly decreased.
Table 2C.
Changes in cytokine production by repeated 0.5Gy irradiation in OVA (ovalbumin)-immunized mice.
| Authors of referenced studies | Cytokines | Non-irradiated OVA immunized group versus normal group | Irradiated OVA immunized group versus non-irradiated OVA immunized group |
|---|---|---|---|
| Nakatsukasa et al. 2010 | IL-4 | ↑ | ↗ |
| IL-5 | → | ↗ | |
| IL-6 | ↑ | ↑ | |
| IL-10 | → | ↑ | |
| IL-17 | ↑ | ↓ |
↑ : significantly increased
↓: significantly decreased
→ : not significantly different (same level)
↗ : not significant but increasing tendency.
Table 2D.
Changes in the percentage (%) of the lymphocyte population by irradiation in OVA-immunized mice.
| Authors of referenced studies | Lymphocytes | OVA immunized group versus normal group | Irradiated OVA immunized group versus non-irradiated OVA immunized group | Status after irradiation | Sampling date |
|---|---|---|---|---|---|
| Nakatsukasa et al. 2010 | CD3+ | → | → | Not changed | Splenocytes were obtained 4 days after final irradiation. (10 weeks old) |
| CD4+ | → | ↑ | Increased post-irradiation | ||
| CD8+ | → | ↓ | Decreased pre-irradiation | ||
| CD19+ | → | → | Not changed | ||
| CD3–CD19–CD38+ (plasma cell) | ↓ | ↑ | Reduced level was increased to normal levels | ||
| CD4+CD25+Foxp3+ (Treg cell) | → | ↑ | Increased pre-irradiation |
↑ : significantly increased
↓ : significantly decreased
→ : not significantly different (same level).
As for lymphocyte phenotypes in the spleen when CD4+CD25+Foxp3+T cells were considered as Treg cells, the percentage of Treg cells was significantly increased by irradiation in these three studies (Tables 2D and 2E). On the contrary, following irradiation, the absolute number of total lymphocytes decreased and involved CD4+CD25+Foxp3+ and CD4+FoxP3− T cells (Weng et al, 2010) (Table 2E). They suggested that this was because collagen immunization induces lymphocytosis. The increased proportion of Tregs in the irradiated CIA group as compared to non-irradiated CIA group (Table 2E) indicated that Tregs were more resistant in this dose of irradiation and also have a selective advantage in the homeostatic reconstitution over conventional T cells. The up-regulation of Treg cells appears to be characteristic of these experimental conditions. Some studies have indicated quantitative abnormalities in Treg cells related to the pathogenesis of CIA (Morgan et al. 2003, 2005). Treg cells are the main effectors of immunological tolerance and the up-regulation of Treg cells may contribute to attenuating the pathology of CIA.
Table 2E.
Changes in the percentage (%) of the lymphocyte population by irradiation.
| Authors of referenced studies | Lymphocytes | Non-irradiated CIA group versus normal group | Irradiated CIA group versus non-irradiated CIA group | Status after irradiation | Sampling date |
|---|---|---|---|---|---|
| Nakatsukasa et al. 2008 | CD4+ | ↓ | → | Not changed | Splenocytes were obtained 4 days after the final irradiation. (10 weeks old) |
| CD8+ | ↓ | → | Not changed | ||
| CD19+(B cell) | → | → | Not changed | ||
| CD3–CD19–CD38+ (plasma cell) | ↑ | ↓ | Reduced to normal levels | ||
| Pan NK+ (NK cell) | ↓ | → | Reduced level was not changed by irradiation | ||
| CD4+CD25+Foxp3+ (Treg cell) | → | ↑ | Increased | ||
| Nakatsukasa et al. 2010 | CD4+CD25+Foxp3+ (Treg cell) | → | ↑ | Increased | Splenocytes were obtained 4, 6, and 8 weeks after col- lagen immu- nization (irradi- ation was con- ducted every week from 3 days before immunization) |
| Weng et al. 2010 | Absolute number of total lymphocytes | Not examined | ↓ | Decreased | Peripheral blood 12 days or 24 days after irradiation (collagen injection was conducted 24 hr after irradiation) |
| Number of CD4+CD25+Foxp3+ (Treg cell) | Not examined | ↓ | Decreased | ||
| Number of CD4+Foxp3+ (containing effector T cells) | Not examined | ↓ | Decreased | ||
| % of Treg cells | Not examined | ↑ | Increased |
↑ : significantly increased
↓ : significantly decreased
→ : not significantly different (same level).
Systemic lupus erythematosus (SLE)
SLE, which is characterized by spontaneous severe total-body lymphadenopathy, hypergammaglobulinemia, autoantibody production, and immune complex formation, leads to many autoimmune diseases, such as glomerulonephritis, hepatitis, and inflammation in the blood vessels, joints, lung, skin, intestine and brain. MRL/MpJ-lpr/lpr (hereafter MRL-lpr/lpr) mice and MRL/gld mice have generally been used as an animal model for SLE (Kelley and Roths 1985; Cohen and Eisenberg 1991). MRL-lpr/lpr mice have a mutation in a cell surface protein, Fas, that mediates apoptosis (Chu et al. 1993),. Whereas MRL/gld mice develope a phenotype of autoimmune diseases similar to that of the lpr mice, having the mutation within FasL gene (Roths et al. 1984; Watson et al. 1992). Tanaka et al. (2005) and Tago et al. (2008) examined the effect of repeated 0.5Gy γ-ray irradiation on autoimmune disease in MRL-lpr/lpr mice. The schedule for irradiation is shown in Fig. 2A. These researchers belonged to the same research group; therefore, Tago et al. (2008) took over unresolved issues from Tanaka et al. (2005). At first, Tanaka et al. (2005) focused on the kidney and salivary gland, i.e., glomerulonephritis and sialoadenitis, and showed homological improvements in disease-specific damage by repeated 0.5Gy γ-ray irradiation. They also demonstrated that mice with splenomegaly and lymphadenopathy, which were representative symptoms in MRL-lpr/lpr mice, recovered and were similar to normal mice after the last irradiation (0.5Gy each time ; 5 times per week for 4 weeks) (Tanaka et al. 2005). The abnormal proliferation of CD3+CD4−CD8−CD45R/B220+ T cells has been observed in MRL-lpr/lpr mice. These cells lack the expression of both CD4 and CD8 and expressed the CD45R/B220 isoform, which has commonly been observed on B cells (Budd et al. 1987). This abnormal cell proliferation has been regarded as the cause of autoimmune manifestation (Cohen and Eisenberg, 1991). Tanaka et al. (2005) showed a marked increase in the percentage of CD3+CD4−CD8−CD45R/B220+ T cells, while that of CD3+CD4+ T cells significantly decreased in MRL-lpr/lpr mice. However, 3 weeks after the last irradiation, the percentage of CD3+CD4−CD8−CD45R/B220+ T cells harvested from the spleen significantly decreased and that of CD3+CD4+ T cells recovered to normal levels (Table 3A).
FIG. 2A.
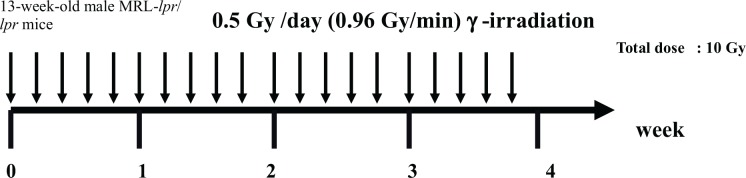
Schedule for irradiation. (Tanaka et al. 2005, Tago et al. 2008).
Table 3A.
Changes in the percentage (%) of the lymphocyte population by irradiation.
| Authors of referenced studies | Lymphocytes | Non-irradiated MRL-lpr/lpr group versus normal group | Irradiated MRL-lpr/lpr group versus non-irradiated MRL-lpr/lpr group | Status after irradiation | Sampling date |
|---|---|---|---|---|---|
| Tanaka et al. 2005 | CD3+CD4+ | ↓ | ↑ | Reduced level was increased | Splenocytes were obtained 3 weeks after last irradiation. |
| CD3+CD8+ | ↓ | ↓ | Reduced level was further slightly decreased | ||
| CD3+CD4– CD8–B220+ |
↑ | ↓ | Markedly increased level was decreased | ||
| Pan NK+ (NK cell) | ↘ | ↑ | Slightly reduced level was increased | ||
| Tago et al. 2008 | CD3+CD4–
CD8–B220+ |
↑ | ↓ | Increased level was decreased at 20 weeks old | Population of splenocyte subsets was obtained in 12- to 18-week-old mice |
| CD4+ | Not examined | ↑ | Significantly increased | ||
| CD19+ | Not examined | ↓ | Significantly decreased | ||
| CD8+ | Not examined | → | Not changed | ||
| Ootsuyama et al. 2003 | CD4–CD8 | ↑ (Non-irradiated MRL/gld group versus normal group) | ↓ (Irradiated MRL/gld group versus non-irradiated MRL/gld group) | Markedly increased level was decreased by irradiation (0.2Gy or 0.5Gy, 5 times/week for 4 weeks) | Splenocytes were obtained 3 days after last irradiation. |
↑ : significantly increased
↓ : significantly decreased
→ : not significantly different (same level)
↗ : not significant but increasing tendency
↗ : not significant but decreasing tendency.
The same results were also reported by Tago et al. (2008). They demonstrated that the progression of splenomegaly in MRL-lpr/lpr mice was suppressed (Tago et al. 2008). Since splenomegaly results from an increase in the number of CD3+CD4−CD8−CD45R/B220+ T cells (Watanabe-Fukunaga et al. 1992), the suppression of splenomegaly was due to a reduction in the number of these abnormal cells. They examined what contributed to this phenomenon, and showed that irradiation reduced the rate of proliferation of CD3+CD4−CD8−CD45R/B220+ T cells or induced their death. The results obtained from the quantification of lactate dehydrogenase (LDH) release indicated that irradiation did not induce selective CD3+CD4−CD8−CD45R/B220+ T cell death. On the other hand, by measuring [3H]thymidine incorporation, the rate of proliferation of CD3+CD4−CD8−CD45R/B220+ T cells was decreased by irradiation, which suggested that irradiation did not kill cells, but decreased their proliferation rate.
Regarding kidney damage, Tanaka et al. (2005) focused on NOx production in the macrophages of MRL-lpr/lpr mice. NOx was defined as NO2−, NO3−, etc. NOx production may injure the kidney and excessive NOx was suggested to be regulated by IL-12 (Huang et al. 1996). They attempted to explain the amelioration of kidney damage, such as glomerulonephritis, from the lower levels of IL-12 observed following irradiation (Tanaka et al. 2005). IL-12 production by peritoneal macrophages was examined in this study, and the results showed that IL-12 production was significantly lower in mice treated with repeated irradiation than in control MRL-lpr/lpr mice (Table 3B). Moreover, significantly high NOx− production in the macrophages of MRL-lpr/lpr mice was lowered by irradiation in parallel with IL-12. They indicated that a reduction in IL-12 production led to lower NOx− production and improved kidney damage.
Table 3B.
Changes in cytokine levels in MRL-lpr/lpr mice with or without irradiation.
| Authors of referenced studies lpr/lpr group | Cytokines | Non-irradiated MRL-lpr/lpr group versus normal group | Irradiated MRL-lpr/lpr group versus non-irradiated MRL- |
|---|---|---|---|
| Tanaka et al. 2005 | IFN-γ | ↓ | ↓ |
| IL-4 | ↓ | → | |
| IL-5 | ↓ | ↑ | |
| IL-12 | ↓ | ↓ |
↑ : significantly increased
↓ : significantly decreased
→ : not significantly different (same level).
Tago et al. (2008) focused on IL-6 because it has been shown to induce the activation of B cells. They found that the production of IL-6 in splenocytes from irradiated mice was lower than that in disease control mice. They also measured the production of the anti-single-stranded DNA antibody in the serum of mice, and found anti-single-stranded DNA antibody levels were lower in irradiated mice. Anti-single-stranded DNA antibodies were shown to be pathogenic in the kidney by cross-reaction with a glomerular antigen (Swanson et al. 1996; Qing et al. 2006). They indicated that the activation of B cells was suppressed by irradiation through a decrease in IL-6 levels, followed by the amelioration of kidney damage after repeated 0.5 Gy γ-irradiation.
On the other hand, Ootsuyama et al. (2003) analyzed the relationship between changes in the T-cell subpopulation and the amelioration of autoimmune diseases using MRL/MpTn-gld/gld (MRL/gld mice) as an animal model. The schedule for irradiation is shown in Fig. 2B. They examined histological changes in arthritis in knee joints and arthritis in the kidney. Inflammatory conditions in knee joints and arthritis in the kidneys were ameliorated after γ-ray irradiation at 0.2 Gy/exposure 5 times per week for 4 weeks. The effect of extended exposure on the composition of the splenic T-cell subpopulation was then examined. The results showed that the abnormal percentage in CD4−CD8− T cells observed in untreated MRL/gld mice showed a clear decrease after 0.2 Gy or 0.5Gy/exposure 5 times per week for 4 weeks. Furthermore, they indicated that irradiation-induced apoptosis was specifically observed in CD4−CD8− T cells and that this phenomenon did not appear to need a Fas-FasL system. They demonstrated that apoptosis through the Fas-FasL pathway may hardly occur in response to non-specific stimulus such as ionizing irradiation.
FIG. 2B.
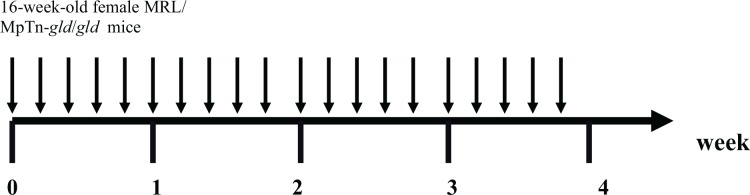
Schedule for irradiation. (Ootsuyama et al. 2003) Radiation dose pattern : (1) 0.05 Gy/day (0.0013Gy/min) × 5 days/week for 4 weeks (Total dose: 1 Gy); (2) 0.2 Gy/day (1.04Gy/min) × 5 days/week for 4 weeks (Total dose: 4 Gy); (3) 0.5 Gy/day (1.04Gy/min) × 5 days/week for 4 weeks (Total dose: 10 Gy).
Ootsuyama et al. (2003) suggested that the CD4−CD8− T cells that accumulated were more sensitive to irradiation than other T-cell subpopulations, and that a decrease in these abnormal cells with extended exposure to low-dose irradiation led to the amelioration of autoimmune diseases.
Multiple Sclerosis (MS)
Multiple sclerosis (MS) is an autoimmune disease that affects more than 1 million people worldwide and severely compromises motor and sensory function. The representative clinical feature of this disease is a disorder in the central nervous system marked by weakness, numbness, a loss of muscle coordination, and problems with vision, speech, and bladder control. The etiology of this chronic neuroinflammatory disease remains unknown and there is currently no fully effective treatment (Lutton et al. 2004; Kipp et al. 2012). Based on histopathological observations, the penetration of leukocytes into the central nervous system and clinical symptoms of relapses, remission, and progressive paralysis in MS have been attributed to the loss of myelin and neurons.
The animal model for MS is experimental autoimmune encephalomyelitis (EAE) and all current therapies have demonstrated efficacy in EAE models. EAE is produced by an intradermal injection of a protein component of central nervous system myelin protein, such as myelin basic protein (MBP) (Seil 1972; Zamvil et al. 1986).
MS therapy was approached by Tsukimoto et al. (2008) through fractionated low dose γ-ray irradiation. The schedule for immunization and irradiation is shown in Fig. 3. They investigated therapeutic effects related to cytokines, MBP-specific antibodies, and regulatory T-cell by analysis after irradiation. Previous studies have shown an increase in IL-6 levels in the serum of MS patients (Stelmasiak et al. 2001; Koutsouraki et al. 2011; Chen et al. 2012). IL-6 has been implicated in neuroinflammatory reactions and is involved in multiple physiological central nervous system (CNS) processes such as neuron homeostasis, astrogliogenesis, and neuronal differentiation (Spooren et al. 2011). TNF-α also has a crucial role in neuroinflammation, and elevated TNF-α levels have been reported in EAE mice (Haji et al. 2012). Regarding MS patients, TNF-α levels were significantly higher in the cerebrospinal fluid (CSF) and in plasma than those of the controls (Obradović et al. 2012). Moreover, IL-17 and IL-17-secreting CD4+ T (Th17) cells were shown to play a pivotal role in the pathogenic mechanism of MS (Aranami and Yamamura 2008). The proportion of Th17 cells in patients with MS was markedly high and serum IL-17 levels were increased in patients with MS (Wang et al. 2011).
FIG. 3.
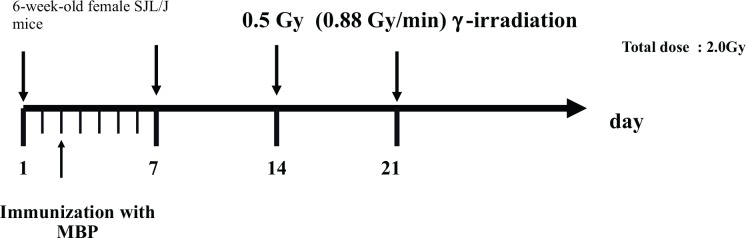
Schedule for immunization with myelin basic protein (MBP) and irradiation. (Tsukimoto et al, 2008).
Tsukimoto et al. (2008) showed that the incidence of pathological changes and the clinical scores of such changes were lower in irradiated EAE mice than in non-irradiated EAE mice. This result suggests that irradiation delays the development of pathology and attenuates pathological symptoms in EAE mice. The effect of irradiation on the production of pro-inflammatory cytokines was then examined, and revealed that the elevated production of TNF-α in EAE mice was slightly suppressed by irradiation. However, this slight decrease in TNF-α production may be involved in the therapeutic effect of repeated 0.5-Gy γ-irradiation on EAE. In contrast, the higher IL-6 levels in EAE mice were significantly reduced by irradiation (Table 4). IL-6 induces the activation of B cells; therefore, the production of autoantibodies involved in the pathology of EAE was induced by IL-6. In Tsukimoto et al.’s report (2008), IL-6 levels were reduced by irradiation; therefore, the production of autoantibodies was suppressed. Anti-MBP antibody levels in the serum of mice were lower in irradiated EAE mice than in non-irradiated EAE mice. Moreover, the markedly higher level of IL-17 in EAE mice than in normal mice was also significantly suppressed by irradiation (Table 4). These results have been summarized in Table 4. IL-6 has recently been reported to play an important role in regulating the balance between IL-17-producing Th17 cells and Treg cells; therefore, IL-6 may work as a regulator of the Treg/Th17 balance (Kimura and Kishimoto, 2010). When CD4+CD25+Foxp3+ T cells were considered as Treg cells in Tsukimoto et al.’s study (2008), the percentage of Treg cells in the CD4+ T cells of irradiated EAE mice was significantly higher than that in the CD4+ T cells of non-irradiated EAE mice. The increased level of IL-6 in EAE mice was suppressed, which indicated that Th-17 cells and IL-17 were regulated and Treg cells were upregulated by irradiation. These phenomena may contribute to attenuating the pathology of EAE.
Table 4.
The effect of irradiation on cytokine levels in EAE mice.
| Authors of referenced studies | Cytokines | Non-irradiated EAE mice versus normal mice | Irradiated EAE mice versus non-irradiated EAE mice |
|---|---|---|---|
| Tsukimoto et al. 2008 | TNF-α | ↗ | ↘ |
| IL-6 | ↑ | ↓ | |
| IL-17 | ↑ | ↓ |
↑ : significantly increased
↓ : significantly decreased
↘ : not significant but increasing tendenc
↗ : not significant but decreasing tendency.
Atopic dermatitis (AD)
AD is a chronically relapsing inflammatory skin disease with altered immune responses. The immune system is involved through lymphocyte-mediated inflammation in the skin, which creates eczema, and the increased incidence of type I and possibly type IV allergies induced by environmental allergens (Hanifin 1982; Thestrup-Pedersen 1989). Elevated IgE and eosinophilia are the representative features of AD patients and may reflect the increased response of type-2 T-helper (Th2) cytokines with a decrease in IFN-γ production. IL-4 and IL-5 regulate IgE synthesis and eosinophil activity in AD, and IL-5 levels, in particular, appear to change in parallel with the clinical severity; a significant decrease in IL-5 levels was observed in AD when the clinical severity decreased (Kondo et al. 2001). Fang et al. (2006) investigated the effect of repeated small-dose γ-ray irradiation on AD using an animal model. They attempted to examine whether small-dose γ-ray irradiation was useful for the treatment of AD because previous reports demonstrated several small doses of γ-ray irradiation inhibited xenograft tumor growth in mice (Kojima et al. 2002, 2004). These anti-tumor effects were mediated by radiation-induced elevations in the IFN-γ / IL-4 (type1- helper (Th-1) cytokine / Th-2 cytokine) ratio. Fang et al. (2006) considered that such cytokine polarization may also contribute to the treatment of AD. NC/Nga mice, which were established as an inbred strain by Kondo in 1957 based on Japanese fancy mice, were shown to be suitable as an animal model of AD (Kondo et al. 1969; Festing 1996), and were subsequently treated with a mite antigen. This animal model was shown to manifest clinical and immunological aspects similar to patients with AD (Sasakawa et al. 2001).
Fang et al. (2006) attempted to induce AD models in NC/Nga mice, which were irradiated with 0.5 Gy of γ-rays 2 days before each stimulation with the mite antigen, twice a week from the age of 7 to 11 weeks old (total 10 times) (Fig. 4). Assays of total IgE and cytokine production by spleen lymphocytes were performed and flow cytometric analysis was conducted on the subpopulations of spleen lymphocytes. The total IgE level in the AD disease-control group increased after the 3rd stimulation with the mite antigen, reached a plateau at around the 5th stimulation, and remained significantly higher until the 10th stimulation. The irradiated group showed significantly higher IgE values than the disease-control group at each point after the 3rd stimulation. This result suggested that B cells were stimulated by repeated small-dose irradiation. Regarding cytokines, IL-5 levels in the disease-control group of NC/Nga mice significantly increased to 20 times higher than those in the normal group, and these levels increased further by irradiation to approximately 1.5 times higher than those in the disease-control group. Increasing IL-5 levels may result in the severe pathologies of AD, as described above. IFN-γ levels were lower in irradiation-treated group than in the disease-control group (Table 5A). Therefore, the ratio of IFN-γ/IL-4 in the disease-control group of NC/Nga mice was significantly lower than that in the non-stimulated normal group, and this ratio was significantly reduced by irradiation. The subpopulation of spleen lymphocytes was examined and slightly lower percentages of CD3+, CD3+CD4+ and CD3+CD8+ T cells were observed in the disease control group, and the percentage of CD3+, and CD3+CD4+ was significantly higher in the irradiation-treated group than in the disease control group (Table 5B). An increase in the percentage of CD19+ cells was only seen in the disease-control group, and this increase was inhibited by irradiation (Table 5B).
FIG. 4.
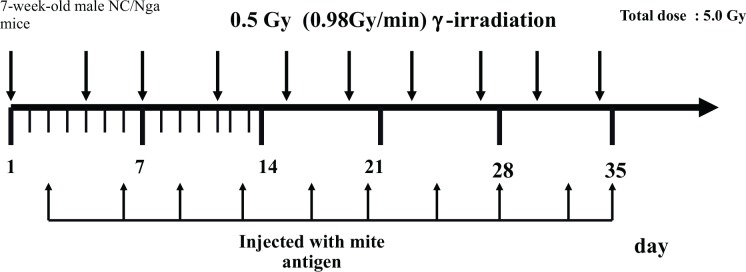
Schedule for injection with mite antigen and irradiation. (Fang et al. 2006).
Table 5A.
The effect of irradiation on cytokine levels in atopic dermatitis in NC/Nga mice.
| Authors of referenced studies | Cytokines | Non-irradiated atopic dermatitis (AD)control mice versus normal mice | Irradiated AD mice versus non-irradiated AD control mice |
|---|---|---|---|
| Fang et al. 2006 | IL-4 | ↗ | ↗ |
| IL-5 | ↑ | ↑ | |
| IFN-γ | ↓ | ↘ |
↑ : significantly increased
↓ : significantly decreased
→ : not significantly different (same level)
↗ : not significant but increasing tenden
↘ : not significant but decreasing tendency.
Table 5B.
Changes in the percentage (%) of the lymphocyte population by irradiation in NC/Nga mice.
| Authors of referenced studies | Lymphocytes | Non-irradiated AD mice group versus normal mice | Irradiated AD mice group versus non-irradiated AD mice group | Status after irradiation | Sampling date |
|---|---|---|---|---|---|
| Fang et al. 2006 | CD3+ | ↓ | ↑ | Significantly higher than the disease control | Splenocytes were obtained 24 hr after the last stimulation (11 weeks of age) |
| CD3+CD4+ | → | ↑ | Significantly higher than the disease control | ||
| CD3+CD8+ | → | → | Not changed | ||
| CD19 + | ↑ | ↓ | Increased level was reduced | ||
| Pan-NK +cell | → | → | Not changed |
↑: significantly increased
↓ : significantly decreased
→ : not significantly different (same level).
They concluded that repeated low-dose ionizing radiation may exacerbate the immune response in AD, which is an immune hypersensitivity disease.
Asthma
Asthma is characterized by inflammation, reversible airway obstruction, increased airway responsiveness to various stimuli, and the production of a large quantity of IgE antibodies by B cells and a decrease in the IFN-γ/IL-4 ratio (Okudaira et al. 1983; Nawata et al. 1984; Goldstein et al. 1994). Fang et al. (2005) attempted to examine the effect of γ-ray irradiation on asthma using an animal model because the immuno-modulatory effects of whole-body γ-ray irradiation may act in opposition to the pathogenic mechanism of asthma. They used a murine model of asthma, namely, BALB/c mice that were sensitized and challenged with ovalbumin (OVA) (Krinzman et al. 1996; Ikeda et al. 2002). The schedule for sensitization and challenge with OVA and irradiation is shown in Fig. 5A. Total IgE content, cytokine levels, and the lymphocyte subpopulation were examined in the murine asthma model. The total IgE content in OVA-induced asthmatic mice significantly increased and reached a maximum level approximately 30 days after the start of the experiment. When the effect of 10 repeats of 0.5Gy γ-ray irradiation on total IgE levels was examined in OVA-induced asthma model mice on day 24, the value of the irradiated group was approximately two times higher than that of the disease control group. Many previous studies have demonstrated that the allergic inflammation associated with asthma is due to T-lymphocyte activation with the predominant production of IL-4 and IL-5 (Krug et al. 1996; Park et al. 1996; Humbert et al. 1997). The production of IL-4 and IL-5 in the asthma disease-control group was significantly higher than that in the non-sensitized normal-group in a study by Fang et al. (2005). These cytokine levels were also significantly higher in the irradiated group than in the disease-control group. When 0.5 Gy γ-ray irradiation was repeated 10 times, both of these cytokines increased further (Table 6A). IFN-γ levels in the disease-control group were also significantly higher than those in the non-sensitized group; however, these levels were reduced to normal values following irradiation (Table 6A). Therefore the IFN-γ /IL-4 ratio of the irradiated group was markedly reduced, which suggested the exacerbation of asthma. The effect of repeated 0.5 Gy γ -ray irradiation on the percentage change in the lymphocyte subpopulation was then examined in asthmatic mice. The percentages of CD3+, CD4+, and CD8+ T cells after treatment with OVA were significantly lower than those in the normal group, whereas those of CD19+ and Pan−NK were slightly increased compared with the normal group, however, the increase in percentages of these cells were not significant (Table 6B). As a whole, the percentage of T cells could be regarded as decreasing. There is a report that asthmatic patients showed a statistically significant increase in CD4+ cells in blood (Mota-Pinto et al. 2011), however, there are few reports that examined the absolute number of T cells in other organs. Therefore the decreased percentage of T cells in spleen cannot be known where they are going. The statistical difference between before and after treatment was so small that the changes of T cell numbers in other organs might not be detected. These decreases in the percentages of CD3+ and CD4+ T cells were blocked by irradiation. The percentages of CD8+ T cells and Pan−NK were almost the same following irradiation, while that of CD19+ T cells was decreased significantly in comparison with that in the disease-control group. The activation, differentiation and multiplication of T helper cells (CD3+CD4+) play a central roles in the immune enhancement (Lara-Marquez et al. 1998, 2001; Robinson et al. 1992). The increased percentage of CD3+CD4+ after irradiation might have relation to the above immune enhancement.
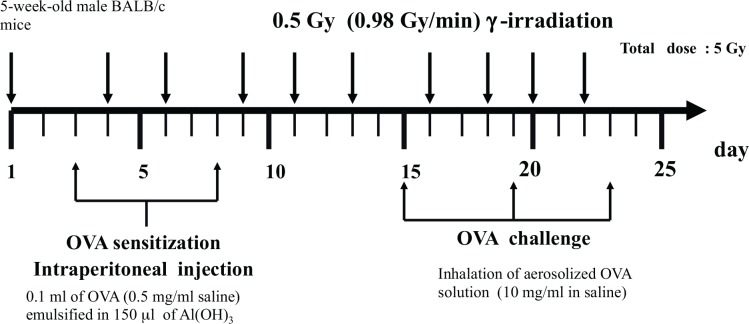
FIG. 5A.
Schedule for sensitization and challenge with OVA and irradiation. (Fang et al. 2005).
Table 6A.
The effect of irradiation on cytokine levels in asthma model mice.
| Authors of referenced studies | Cytokines | Non-irradiated asthma mice versus normal mice | Irradiated asthma mice versus non-irradiated asthma mice |
|---|---|---|---|
| Fang et al. 2005 | IL-4 | ↑ | ↑ |
| IL-5 | ↑ | ↑ | |
| IFN-γ | ↑ | ↓ (Reduced to normal levels) |
↑ : significantly increased
↓ : significantly decreased
→ : not significantly different (same level).
Table 6B.
Changes in the percentage (%) of the lymphocyte population by irradiation in asthma model mice.
| Authors of referenced studies | Lymphocytes | Non-irradiated asthma mice versus normal mice | Irradiated asthma mice versus non-irradiated asthma mice | Status after irradiation | Sampling date |
|---|---|---|---|---|---|
| Fang et al. 2005 | CD3+ | ↓ | ↑ | Increased (to normal levels) | Splenocytes were obtained 24 hr after the final inhalation on day 23. |
| CD4+ | ↓ | ↑ | Increased (to normal levels) | ||
| CD8+ | ↓ | → | Not changed | ||
| CD19+ | ↗ | ↓ | Decreased | ||
| Pan-NK cell | ↗ | → | Not changed |
↑ : significantly increased
↓ : significantly decreased
→ : not significantly different (same level)
↗ : not significant but increasing tendency
↘ : not significant but decreasing tendency.
From the above results, it was concluded that repeated γ-ray irradiation may exacerbate asthma.
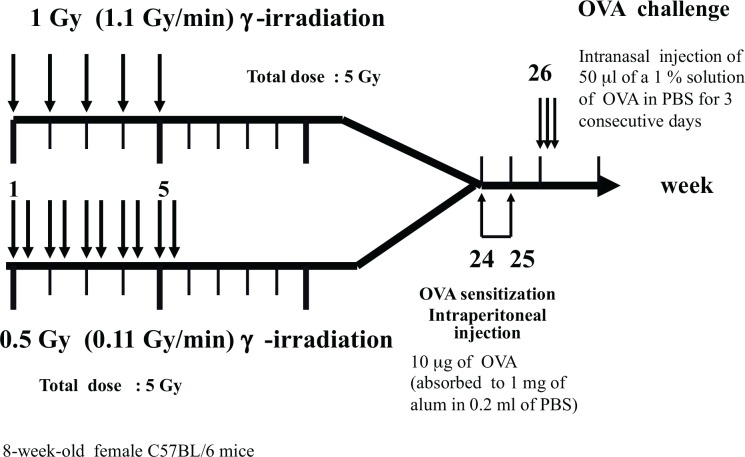
A recent study (Park et al. 2013b) showed similar results to those of Fang et al. (2005). Park et al. (2013b) demonstrated that fractionated irradiation induced chronic allergic airway inflammation; therefore, irradiation resulted in the exacerbation of asthma. They irradiated C57BL/6 mice repeatedly at 1 or 0.5Gy for 6 months and, following irradiation, sensitized and challenged these mice with OVA over a short period of time (Fig. 5B). In their irradiation protocol, sensitization and the challenge were performed following irradiation, and not at the same time. In Fang et al. (2005)’s protocol, the first sensitization was performed 3 days after the first 0.5Gy irradiation, following which sensitization and the challenge were simultaneously or alternately conducted with irradiation. The protocol used by Park et al. (2013b) (Fig. 5B) was not completely the same as that of Fang et al. (2005) (Fig. 5A)., however, both had the same starting point. The two research groups started from irradiation, after which they sensitized mice.
FIG. 5B.
Schedule for sensitization and challenge with OVA and irradiation. (Park et al. 2013b).
Park et al. (2013b) concluded that fractionated irradiation induced chronic allergic airway inflammation through increasing macrophages infiltration into the lung and production of active TGF-β.
Hashimoto’s thyroiditis (HT)
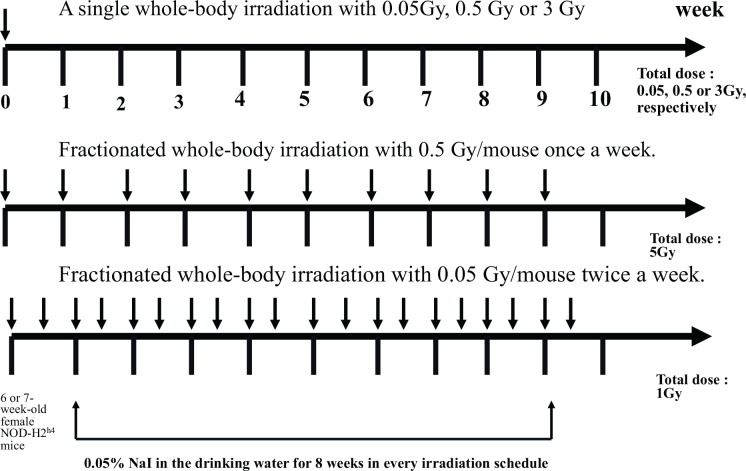
Hashimoto’s disease was first introduced as struma lymphomatosa, a disease of the thyroid gland, by Hashimoto in 1912 (Hashimoto 1912). Hashimoto’s thyroiditis (HT) is characterized by destruction of the thyroid gland by the cellular immune response and consequent hypothyroidism. The thyroid glands were shown to be enlarged in a symmetrical fashion and the degree of enlargement was not ascertainable from a pathological examination alone. Microscopically, typical cases showed follicles greatly diminished in size and lined with highly eosinophilic epithelia (Marshall and Meissner 1955). Lymphocytic infiltration and degenerative changes in follicular epithelia have also been reported (Biörklund and Söderström 1976; Sato et al. 1977). HT is considered to be an autoimmune disease and previous studies examined thyroid-related autoanti-bodies (Jasani et al. 1999; Nakamura et al. 2008). As significant correlations between irradiation and thyroid autoimmunity were observed in studies conducted at Chernobyl (Vykhovanets et al. 1997; Pacini et al. 1998), Nagayama et al. (2008) attempted to evaluate how irradiation affected thyroid autoimmunity in a mouse model. They used a spontaneous model of non-obese diabetic-H2h4 (NOD-H2h4) mice for HT, which develop antithyrogloblin autoantibodies and intrathyroidal lymphocyte infiltration when supplied with iodine in their drinking water (Many et al. 1996; Rasooly et al. 1996). The schedule for irradiation is shown in Fig. 6. Nagayama et al. (2008) evaluated the effect of irradiation on the histology of the thyroid gland. Although naive mice showed little intrathyroidal lymphocyte infiltration, NaI-treated mice developed mild to severe thyroiditis. These results indicated that the development of thyroiditis was accompanied by the appearance of anti-Tg autoantibodies in the sera. Their irradiation protocol was divided into 2 arms, one was single dose irradiation (0.05, 0.5 or 3 Gy/mouse) and the other was fractionated (0.05 Gy twice a week or 0.5 Gy once a week for nine weeks) doses of γ–irradiation. As a result of irradiation, a single dose of whole-body irradiation with 0.5 Gy one week prior to the beginning of NaI significantly enhanced the severity of thyroiditis and titers of anti-Tg autoantibodies. However, non of other irradiation protocols that are shown in Fig. 6 did not affect the degree of thyroiditis or titers of anti-Tg autoantibodies. They also examined whether an adaptive response was observed in this model, namely, whether the induction of radioresistance to subsequent higher doses of radiation occurred by pretreatment with low radiation doses. The results obtained revealed that thyroiditis was enhanced in all groups of mice that received 0.5 Gy whole-body irradiation with or without pretreatment with a very low dose (0.05 Gy) of irradiation one or two weeks before, and demonstrated the lack of an adaptive response. Regarding the effect of irradiation on immune cells (splenocytes), they examined the total number of spleen cells, CD4+ T cells, CD8+ T cells, CD19+ B cells, CD4+CD25+ T cells, and CD4+Foxp3+ T cells. A single dose of whole-body irradiation with 0.05Gy or 0.5 Gy had little effect on the number of spleen cells, except for a slight decrease of approximately 30% in the number of splenocytes with 0.5 Gy irradiation. The above results demonstrated that low-dose irradiation exacerbated thyroid autoimmunity in NOD-H2h4 mice, as demonstrated by elevated thyroiditis scores and anti-Tg autoantibody titers.
FIG. 6.
Schedule for irradiation. (Nagayama et al. 2008).
They also studied the irradiation effect on the pathology of Grave’s disease, which represented hyperthyroidism caused by the production of excessive hormones; however, no effect was observed in the Grave’s disease animal model.
DISCUSSION
As described above, the effect of low-dose ionizing radiation on immunological diseases was divided into two groups; the attenuation and exacerbation of pathology. The pathologies of RA, SLE, and MS were attenuated by low-dose irradiation, whereas, the pathologies of AD, asthma, and HT were exacerbated by low-dose irradiation.
The effect of low-dose irradiation was evaluated on diseases caused by immunological disorders in our literature search by four biological indicators: the severity of pathologies, cytokine levels, autoantibody production, and T-cell subpopulations, which changed according to the progression of pathologies and, therefore, played important roles in evaluating these diseases.
Diseases in which pathological improvements are expected with low-dose irradiation
Severity of pathologies
The pathological severity of CIA, the pathology of which resembled that of human RA, was examined using an established scoring system (Inglis et al. 2007). As shown in Table 2A, Nakatsukasa et al. (2008) reported that the severity score of arthritis in irradiated CIA mice did not increase until 50 days after immunization with collagen, whereas the score increased 30 to 40 days after immunization without irradiation. They also indicated that the incidence of CIA was reduced by irradiation. A detailed examination of pathological changes was conducted 4, 6, and 8 weeks after immunization in their 2010 report. These time points of examination corresponded to the pathological stages of just before the onset, the developing phase, and the chronic phase of CIA, respectively. They indicated that the severity score was significantly suppressed 6 weeks and 8 weeks after immunization with collagen; therefore, low-dose irradiation was considered to be effective before the chronic stage of this disease. An effective result was obtained in Weng et al.’s study (2010), even though irradiation was administered just once before the collagen injection. They demonstrated that most mice in the CIA control group showed signs of arthritis, with a severity score equal or more than 2, 21 days after immunization with collagen, while all mice in the irradiated group scored less than 2. Based on the results of these three studies, the beneficial effect of low-dose irradiation appears to have been observed in a relatively earlier phase of the disease. When irradiation was administered once, its effect appeared to have been maintained for 3 weeks after immunization with collagen. On the other hand, the beneficial effect of repeated low-dose irradiation appears to have lasted for 8 weeks after immunization; however, no significant effect was reported after 8 weeks.
As for SLE, Tanaka et al. (2005) showed photomicrographs of tissue sections including the kidney or salivary gland of MRL-+/+ and MRL-lpr/lpr mice. From their results, non-irradiated MRL-lpr/lpr mice exhibited severe histological damage to the kidney or salivary gland; however, this damage was markedly suppressed by repeated low-dose irradiation. These findings were not evaluated by an established scoring system, but by the clearly attenuated changes observed in tissue sections.
Tsukimoto et al. (2008) evaluated EAE pathology by clinical score based on the degree of paralysis. They indicated that the severity of EAE was attenuated by low-dose irradiation.
Cytokine levels
The profile of cytokine secretion reflects the functional integrity of the immune system because cytokines are the most important mediators by which cells of the immune system communicate.
Many studies have investigated the the relationship between IL-6 and RA because IL-6 plays a key role in the local and systemic manifestation of RA. IL-6 concentrations were higher in the synovial fluid (Westacott et al. 1990; Harigai et al. 1991; Ridderstad et al. 1991) and serum (Spirchez et al. 2012) of patients with RA, and quantitative analysis of IL-6 cytokine gene expression in RA by in situ hybridization of synovial fluid cells (Firestein et al. 1990; Tan et al. 1990) supported these findings. IL-6 is not only a proinflammatory cytokine, but may also interact in complex ways with cells involved in bone remodeling. Abdel Meguid et al. (2013) indicated that IL-6 played an important role in increasing osteoclastic activity and subsequent bone resorption in patients with RA, and suggested that blocking IL-6 with IL-6 inhibitors may be effective in inhibiting the inflammatory process and preventing the bone complications associated with RA diseases. Table 2B shows that IL-6 levels were significantly higher in the non-irradiated CIA mice group than those in the normal mice group. However, these levels were significantly reduced after repeated low-dose irradiation. Changes in the concentration of IL-6 have been used to as evidence for the attenuation observed in histological examinations. On the other hand, because no change in IL-6 was observed 8 weeks after immunization, the beneficial effect of repeated low-dose irradiation appears to occur in the earlier phase of the disease. This correlated well with the evaluation by clinical score. TNF-α levels were also significantly higher in the non-irradiated CIA mice group than in the normal mice group, and significantly decreased following irradiation (Table 2B). TNF-α was shown to be localized in the inflamed synovial tissue of patients with RA (Chu et al. 1991), is involved in the cytokine network in RA, and contributes to IL-6 production by synovial fibroblasts in vivo (Harigai et al. 1991). In our literature search, we found that TNF-α levels significantly increased in the non-irradiated CIA mice group, but significantly decreased after irradiation. This phenomenon was similar to the changes observed in IL-6 levels. Therefore, changes in TNF-α levels may contribute to those in IL-6 level (Table 2B).
According to the report by Tsukimoto et al. (2008), the increase in IL-6 levels in EAE mice was significantly reduced by irradiation (Table 4). This was the same pattern of change as that observed in CIA. Moreover, IL-17 levels also increased in non-irradiated EAE mice and significantly decreased with irradiation. A previous study demonstrated that IL-17 increased IL-6 release; therefore, IL-6 may behave in a similar manner to that of IL-17 (Wang et al. 2008a). IL-17 showed similar behavior that of IL-6 in CIA (Table 2B). The effect of low-dose irradiation on interleukin levels in CIA and EAE were similar (Table 2B and Table 4). However, the levels of interleukins observed in OVA-immunized mice were different from those in CIA or EAE mice. IL-6 production was significantly higher in non-irradiated OVA-immunized mice than in non-immunized mice, and a further increase was reported in the irradiated group (Table 2C). Since IL-6 is known to induce the activation of B cells, leading to the production of antibodies by plasma cells (Kishimoto 1989), an enhancement in antibody production occurred in irradiated OVA-immunized mice. Nakatsukasa reported that a further significant increase in IgG production was observed in pre-irradiated OVA-immunized mice and slight increases were observed in IgE production. On the other hand, production of the anti-CII autoantibody was reported to be lower in irradiated mice with CIA than in non-irradiated mice (Nakatsukasa et al. 2008, 2010). The suppression of IL-6 production by low-dose irradiation may contribute to the suppression of anti-CII autoantibody production because IL-6 levels were significantly lower in irradiated CIA mice than in non-irradiated CIA mice (Table 2B). IL-6 levels were shown to be closely related with antibody production; however, differences existed depending on whether the antigen was external or internal. OVA is an external antigen, whereas CII is considered to be internal antigen. They indicated that 0.5Gy irradiation enhances antibody production in response to an external antigen, and could exacerbate the pathology of the disease. However, attenuation of the disease pathology may be expected when the disease is caused by an autoantibody against an internal antigen.
Anti-MBP antibody levels were measured in the serum of EAE mice because this was regarded as a pathogenic autoantibody produced by activated B cells. Tsukimoto et al. (2008) indicated that anti-MBP antibody levels were lower in irradiated EAE mice than in non-irradiated EAE mice. Since the anti-MBP antibody is an antibody against an internal antigen, attenuation of the disease pathology could also be expected in EAE, similar to that in CIA.
T-cell subpopulations
Proper immune functions are dependent on the presence of a critical number of cells. The effects of low-dose irradiation on the immune system were examined by determining time-dependent numerical changes in various lymphocyte subsets (Bogdándi et al. 2010). Bogdándi et al. particularly reported the numerical changes of CD4+CD25+Foxp3+ Treg cells, following low-dose irradiation in C57BL/6 mice. Nakatsukasa et al. (2008, 2010) and Weng et al. (2010) also reported the percentages of these cells were significantly higher in irradiated CIA mice and irradiated OVA-immunized mice (Tables 2D and 2E). The up-regulation of Treg cells in irradiated mice was also reported in MRL-lpr/lpr mice, used as an animal model for SLE (Tago et al. 2008), and EAE mice, used as an animal model for MS (Tsukimoto et al. 2008). On the other hand, Bogdándi et al. (2010) reported no significant change in the percentage of CD4+CD25+Foxp3+ Treg cells in normal C57BL/6 mice after one single 0.5Gy γ-irradiation. A difference may exist between normal mice and disease mouse models. The up-regulation of Treg cells after low-dose irradiation was observed in all mouse models of autoimmune diseases from our referenced studies. The up-regulation of Treg cells after low-dose irradiation may be a common event in autoimmune disease mouse models. Treg cells are crucial mediators of autoimmune tolerance, and a deficiency or dysfunction in these cells may contribute to the development of autoimmune diseases (Sakaguchi 2005; Liu and Leung 2006). However, expansion of the Treg cell population could also prevent autoimmune attacks in these diseases (Viglietta et al. 2004; Matarese et al. 2005). Tregs regulate ongoing immune responses under normal conditions and prevent autoimmunity, imbalanced function or number of these Tregs, and can lead to decreased autoimmunity (Cools et al. 2007). The suppressive function of CD4+CD25+Tregs was lower in patients with autoimmune diseases such as MS or RA than healthy donors (Viglietta et al. 2004; Anderson and Isaacs 2008). In addition, some autoimmune diseases were shown to have reduced levels of CD4+CD25+Tregs in the peripheral blood (Longhi et al. 2006). Although the population of CD4+CD25+Foxp3+ Treg cells in the disease mice of CIA (Table 2E) or EAE (Tsukimoto et al. 2008) was not significantly lower than that in normal mice with irradiation, the up-regulation of Treg cells reported in the low-dose irradiated disease mice of our referenced studies may have contributed to the control of autoimmunity and attenuation of pathologies. The application of a physiological regulatory system could be a rational strategy to treat autoimmune diseases. Several reports have demonstrated Treg cell expansion using some drugs (Wang et al. 2008b; Wekerie 2008; Daniel et al. 2010; Asanuma et al. 2011). If Treg populations are controlled by repeated low-dose irradiation, the same treatment effect may be obtained without using drugs. Repeated low-dose irradiation may be effective and a good treatment strategy for some patients, especially those with allergies to such drugs or decreased liver and kidney functions.
Meanwhile, total doses irradiated to CIA mice were 2.5 Gy-5 Gy in Nakatsukasa et al.’s reports (2008, 2010) (Figs. 1A, 1B and 1C) and 0.4 Gy in Weng et al.’s report (2010) (Fig. 1D). These levels were within the limit of the clinical application’s levels which have been established empirically. Namely, these were single doses of 0.3–1.0 Gy in 4–5 fractions for acute and 1-3 fractions for chronic diseases per week to total doses of 3–5 Gy and 12 Gy, respectively (Seegenschmiedt et al, 1997). In animal studies, there are reports that indicated the anti-inflammatory effect and the reduction of clinical symptoms if the local irradiations were given with daily fractionated doses of 5 x0.5 Gy, total dose was 2.5 Gy (Hildebrandt et al, 2000, 2003). In Nakatsukasa et al.’s reports (2008, 2010), the whole body irradiation was conducted, therefore somewhat higher doses than that of local irradiation might be applied. In the report of SLE, total irradiation doses were within the limit of 10 Gy (Ootsuyama et al, 2003, Tago et al, 2008, Tanaka et al, 2005) (Figs. 1A and 1B). It seems to be somewhat higher but within the limit of clinical application’s levels (Seegenschmiedt et al, 1997). Total irradiation doses in CIA, SLE and EAE were considered to be appropriate to examine the effect of low dose irradiation on inflammatory diseases.
Diseases in which pathological exacerbation is expected with low-dose irradiation
The pathological severity of AD and asthma were not evaluated by histological examinations in our referenced three studies (Fang et al. 2005, 2006; Nagayama et al. 2008). They evaluated pathological exacerbation following low-dose irradiation based on cytokine levels and T-cell sub-populations. On the other hand, although HT was evaluated by histological examinations using severity scores, cytokine levels were not measured. Regarding HT, the severity score, anti-Tg antibody levels, and T-cell subpopulation were examined to evaluate pathological changes.
Cytokine levels
AD is characterized by elevated serum IgE levels and expression of the cytokines IL-4, IL-5, and IFN-γ (Leung 1993; Spergel et al. 1999). The synthesis of IgE was shown to be regulated by IL-4, IFN-γ, and IL-2. IL-4 enhanced the production of IgE, whereas IFN-γ inhibited IL-4-mediated IgE production (Takahashi et al. 1992). The mechanisms by which IgE synthesis was increased in AD may be related to either increased IL-4 production and/or decreased IFN-γ (Jujo et al. 1992; Nakazawa et al. 1997). On the other hand, AD skin lesions have been shown to express IL-5 mRNA and protein (Tanaka et al. 1994), and IL-5 also regulates IgE synthesis and promotes eosinophil development (Leung 1998). From these studies, Fang et al. (2006) attempted to determine time-dependent changes in total IgE and IL-4, IL-5, and IFN-γ levels produced by spleen lymphocytes with or without low-dose irradiation. As a result, the slightly increased level of IL-4 in disease control NC/Nga mice was also further increased after low-dose irradiation (Table 5A). The difference in IL-4 levels was slight; however, significantly increased IL-5 levels were observed in disease control mice, and these levels were significantly higher in irradiation-treated disease mice than in disease control mice. On the other hand, IFN-γ levels in the disease control group of NC/Nga mice were significantly lower than those in the normal group, and irradiation further lowered these levels (Table 5A). IgE levels were shown to increase concomitantly with changes in cytokine levels; therefore, they concluded that low-dose irradiation exacerbated the pathology of AD. IgE plays an important role in allergies, acting as an initiating factor, and is involved in its persistence and exacerbation. Although they did not perform pathological examinations, such as scoring, they concluded that IgE and cytokine levels were reliable.
Several studies were undertaken to examine which cytokine was involved in the pathogenesis of asthma. IL-4, IL-5, IL-6, TNF-α, and IFN-γ are considered to play pivotal roles in the pathological characteristics of asthma, which has been attributed to the presence of inflammatory cell infiltrates in the bronchial mucosa consisting of activated mast cells, eosinophils, and T-cells (Bradding et al. 1994; Tang et al. 1995; Möller et al. 1996; Nagai et al. 1996). IL-4 and IL-5, in particular, have been shown to play important roles in IgE production and eosinophilia. IL-4 is essential for IgE production, and IL-5 is a major factor involved in the production and activation of eosinophils (Sewell and Mu 1996). For example, IL-4 deficient mice had less eosinophils in the bronchoalveolar lavage fluid and markedly reduced peribronchial inflammation than that in wild type mice after immunization with aerosolized OVA (Brusselle et al. 1994). IL-4 knockout mice were also unable to develop allergic eosinophilic airway infiltration and did not produce specific IgE antibodies (Pauwels et al. 1997). A previous study demonstrated that anti-IL-4 inhibited IgE production in a murine model of atopic asthma and indicated anti-IL-4 may be a prophylactic agent for asthma (Zhou et al. 1997). Eosinophilia, lung damage, and airway hyperreactivity normally resulting from an aeroallergen challenge were abolished in IL-5-deficient mice (Foster et al. 1996). IL-5 is considered to be one of the central mediators in the pathogenesis of allergic asthma. On the other hand, IFN-γ was shown to inhibit eosinophil infiltration by inhibiting the production of IL-5 in a mouse model (Shi et al. 1996). They indicated that IFN-γ may be important in the treatment of asthma. Fang et al. (2005) attempted to measure the levels of IL-4, IL-5, and IFN-γ produced by spleen lymphocytes. The production of IL-4 and IL-5 in the disease-control group was significantly higher than that in the non-sensitized normal group. These cytokine levels in the irradiated group were significantly higher than those in the disease-control-group. IFN-γ levels in the disease-control group were also significantly higher than those in the non-sensitized group; however, γ-ray irradiation clearly lowered these elevated levels to normal values (Table 6A). Considering the change in cytokine levels, repeated irradiation with γ-rays may exacerbate the pathology of asthma because the studies described above demonstrated the roles of these cytokines in asthma. Fang et al. (2005) reported the exacerbation of asthma based on changes in the levels of these cytokines. The same result was obtained in Park et al.’s report (2013b). They measured IFN-γ, IL-4, and IL-17 levels as representative cytokines in the inflammatory site, and showed that IFN-γ levels in the bronchoalveolar lavage fluid (BALF) of irradiated mice was lower than that of non-irradiated mice. Moreover, IL-4 levels in the BALF of irradiated mice significantly increased in the group exposed to 5Gy split into 0.5Gy. IL-17 levels were also significantly higher than those of non-irradiated mice. They attributed this exacerbation in the pathology of asthma to the higher level of IL-4 in irradiated mice because IL-4 preferentially induces switching to IgG1 and IgE. They also indicated that fractionated irradiation induced chronic allergic airway inflammation by increasing the influx of macrophages and activating transforming growth factor (TGF)-β levels.
T-cell subpopulations
The influence of irradiation on subpopulations of spleen lymphocytes was examined by Fang et al. (2005, 2006) in AD and asthma model mice. Similar results were obtained in both pathologies. The percentage of CD3+ cells was significantly lower in AD mice than in normal mice, while that of CD19+ was higher than that in normal mice. After irradiation with γ-rays, the percentages of CD3+ and CD4+ cells were significantly higher than those in the disease group, whereas those of CD8+ T cells and Pan-NK remained unchanged. The percentage of CD19+ was significantly lower than that in the disease-control group (Table 5B). Lower percentages of CD3+, CD3+CD4+, and CD3+CD8+ T cells were observed in asthma model mice, and irradiation increased these percentages. The percentage of CD19+ was increased in disease mice, and this level was significantly reduced following irradiation (Table 6B). Similar findings were observed for changes in the T-cell subpopulation before and after irradiation in AD and asthma. These results indicate that irradiation may activate T cell-polarization to helper cells (CD3+CD4+). Since AD and asthma are characterized by the production of large quantities of IgE antibodies by B cells, the increased percentage of CD4+ T cells, which help B cells produce antibodies, may contribute to the exacerbation of these pathologies.
Regarding HT, although the number of splenocytes slightly decreased after a single dose of whole-body γ-irradiation with 0.5Gy, no significant changes were observed in the percentages of T-cell subpopulations. Nagayama et al.2008 showed that a single dose of whole-body γ-irradiation with 0.5Gy one week prior to beginning NaI significantly enhanced the severity of thyroiditis and titers of anti-Tg autoantibodies. They concluded that low-dose irradiation exacerbated thyroid autoimmunity in NOD-H2h4 mice, as demonstrated by the elevated thyroiditis scores and anti-Tg autoantibody titers. Only one-time low-dose irradiation could exacerbate the pathology of HT, which was different from reports concerning repeated low-dose irradiation by Fang et al. (2005, 2006). Low-dose whole-body γ-irradiation may have a systemic effect on this disease even if the frequency of irradiation is only once.
There were no data concerning Treg cells in the above three reports; however, the Treg population in asthma mice model was recently reported by Park et al. (2013a). They showed the Treg cell population was higher in irradiated OVA mice than in OVA or control mice. They suggested that Treg cells enhancement/migration by irradiation may exert a suppressive function in airway inflammation and tissue remodeling in allergic asthma. Their irradiation protocol of Park et al. (2013a) (Fig. 7) was completely different from that of Fang et al. (2005) (Fig. 5A) or Park et al. (2013b) (Fig. 5B). They firstly sensitized mice twice with OVA on day 1 and day 15, and after sensitization, mice received whole-body 0.667Gy (4.72Gy/min) γ-irradiation once daily for 3 consecutive days 24 h before each challenge. If one wants to examine the effect of irradiation on the existing immune condition, the schedule for sensitization and challenge with OVA and irradiation conducted by Park et al. (2013a) is perfect fit as they first induced the disease and then started the irradiation (Fig. 7). Total radiation dose of the work by Park et al. (2013a) was 2Gy (Fig. 7), whereas those of Fang et al. (2005) and Park et al. (2013b) were 5Gy (Figs. 5A and 5B). Although there are few data about asthma other than the above three reports, irradiation schedules and doses seem to be reasonable because they were intermediate levels between the local irradiation doses to arthritic joints in animal model that reported by Hildebrandt et al. (2000) and clinical therapy doses reported by Liebmann et al. (2004). In our referenced papers, smaller total irradiation dose might be good for attenuation of disease. Moreover, higher Treg cell population after irradiation might help attenuate allergic asthma as well as the phenomenon that were seen in CIA (Table 2E). Meanwhile, the sensitivity to irradiation may be strain-dependent. Shankar et al. (1999) indicated that the modification of immune response by radiation was different between BALB/c and C57BL/6 mice. Park et al. (2013b) and Park et al. (2013a) used C57BL/6 mice, on the other hand, Fang et al. (2005) used BALB/c mice. In their result, although the total irradiation doses were different, the attenuation might be seen when the induction of disease was firstly conducted and then irradiation was administered after that (Figs. 5A and 7). On the other hand, the exacerbation might be seen when the irradiation was firstly administered and then the induction of disease was conducted (Fig. 5B). As this difference may be caused by the irradiation protocol, total irradiation dose or animal strain, further examinations are needed to elucidate whether this phenomenon can be applied to therapy.
FIG. 7.
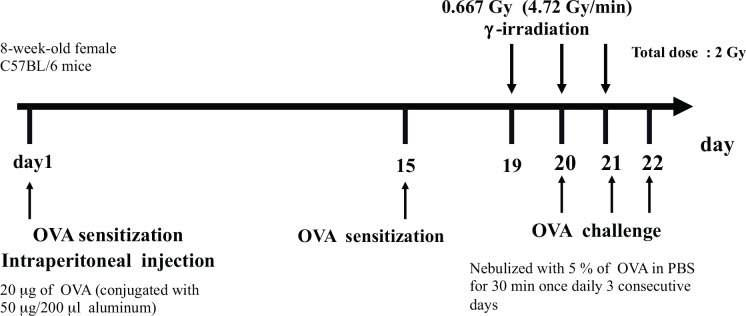
Schedule for sensitization and challenge with OVA and irradiation. (Park et al. 2013a).
Expected mechanisms of pathological improvement or exacerbation of diseases
According to our referenced papers, it is clear that the low dose fractionated irradiation lowered IL-6 levels that were high in CIA or EAE mice (Tables 2B and 4) and up-regulated Treg cells in CIA mice (Table 2E). This phenomenon could be preferable to attenuation of autoimmune diseases such as CIA or EAE. In SLE, it is clear that abnormal CD3+CD4−CD8−B220+ cells were lowered by low-dose fractionated irradiation (Table 3A). The phenomenon could be preferable to attenuation of SLE. In exacerbated cases, low-dose fractionated irradiation lowered IFN-γ levels that were high in asthma mice (Table 6A) and up-regulated CD4+ cells in AD or asthma mice (Tables 5B and 6B). The phenomenon could be the evidence of exacerbation of diseases. However, these were events after irradiation; therefore any other mechanisms have not been elucidated.
However, the results of these animal studies may provide useful information about the mechanism and evidence of LD-RT that has been empirically performed for a long time. The information in this review is worth considering together with that of the review article of Rödel et al. (2007, 2012) when the LD-RT is conducted.
CONCLUSION
From the referenced studies, the effect of low-dose γ-irradiation was shown to be different depending on the cause of the immunological disease. If the cause is due to an antibody against an internal antigen, for example, CIA, SLE, and MS, which are generally referred to as autoimmune diseases, the effect of low-dose irradiation may be preferable. In contrast, if the cause is due to an external antigen, for example, AD and asthma, which are generally referred to as allergic diseases, the effect of low-dose irradiation may be unfavorable.
The effect of low-dose γ-irradiation may be the attenuation or exacerbation of pathologies depending on the source of the antigen causing the disease. Therefore, the cause should be taken into consideration when low-dose γ-irradiation is used as a therapy for immunological diseases.
In conclusion, LD-RT could be clinically applied for RA, SLE and MS from our referenced animal study papers because the irradiation reduces IL-6 levels, up-regulates Treg cells in CIA or down-regulate abnormal cells which are characteristic to SLE. On the other hand, it may be difficult for clinical application of LD-RT in AD or asthma because IF-γ level was lowered by irradiation in asthma and up-regulation of CD4+ cells were observed by irradiation in AD or asthma mice.
REFERENCES
- Abdel Meguid MH, Hamad YH, Swilam RS, Barakat MS. Relation of interleukin-6 in rheumatoid arthritis patients to systemic bone loss and structural bone damage. Rheumatol Int. 2013;33:697–703. doi: 10.1007/s00296-012-2375-7. [DOI] [PubMed] [Google Scholar]
- Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148:32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AE, Isaacs JD. Tregs and rheumatoid arthritis. Acta Reumatol Port. 2008;33:17–33. [PubMed] [Google Scholar]
- Anderson RE, Williams WL, Tokuda S. Effect of low-dose irradiation upon T cell subsets involved in the response of primed A/J mice to Sal cells. Int J Radiat Biol Stud Relat Stud Phys Chem Med. 1988;53:103–118. doi: 10.1080/09553008814550471. [DOI] [PubMed] [Google Scholar]
- Aranami T, Yamamura T. Th17 cells and autoimmune encephalomyelitis (EAE/MS) Allergol Int. 2008;57:115–120. doi: 10.2332/allergolint.R-07-159. [DOI] [PubMed] [Google Scholar]
- Asanuma S, Tanaka J, Sugita J, Kosugi M, Shiratori S, Wakasa K, Shono Y, Shigematsu A, Kondo T, Kobayashi T, Asaka M, Imamura M. Expansion of CD4(+)CD25(+) regulatory T cells from cord blood CD4(+) cells using the common γ-chain cytokines (IL-2 and IL-15) and rapamycin. Ann Hematol. 2011;90:617–624. doi: 10.1007/s00277-010-1121-z. [DOI] [PubMed] [Google Scholar]
- Askonas BA. T-cell differentiation and effector functions. Immunol Suppl. 1988;1:51–52. [PubMed] [Google Scholar]
- Biörklund A, Söderström N. Human auto-immune thyroiditis. Acta Otolayngol. 1976;82:204–207. doi: 10.3109/00016487609120884. [DOI] [PubMed] [Google Scholar]
- Bogdándi EN, Balogh A, Felgyinszki N, Szatimári T, Persa E, Hildebrandt G, Sáfrány G, Lumniczky K. Effect of low-dose radiation on the immune system of mice after total-body irradiation. Radiat Res. 2010;174:480–489. doi: 10.1667/RR2160.1. [DOI] [PubMed] [Google Scholar]
- Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH, Holgate ST. Interleukin-4,-5, and -6 tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994;10:471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- Brusselle GG, Kips JC, Tavemier JH, van der Heyden JG, Cuvelier CA, Pauwels RA, Bluethmann H. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy. 1994;24:73–80. doi: 10.1111/j.1365-2222.1994.tb00920.x. [DOI] [PubMed] [Google Scholar]
- Budd RC, Schreyer M, Miescher GC, MacDonald HR. T cell lineages in the thymus of lpr/lpr mice. Evidence for parallel pathways of normal and abnormal T cell development. J Immunol. 1987;139:2200–2210. [PubMed] [Google Scholar]
- Cheda A, Nowosielska EM, Wrembel-Wargocka J, Janiak MK. Production of cytokines by peritoneal macrophages and splenocytes after exposures of mice to low doses of X-rays. Radiat Environ Biophys. 2008;47:275–283. doi: 10.1007/s00411-007-0147-7. [DOI] [PubMed] [Google Scholar]
- Chen YC, Yang X, Miao L, Liu ZG, Li W, Zhao ZX, Sun XJ, Jiang GX, Chen SD, Cheng Q. Serum level of interleukin-6 in Chinese patients with multiple sclerosis. J Neuroimmunol. 2012;249:109–111. doi: 10.1016/j.jneuroim.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Cho YG, Cho ML, Min SY, Kim HY. Type II collagen autoimmunity in a mouse model of human rheumatoid arthritis. Autoimmun Rev. 2007;7:65–70. doi: 10.1016/j.autrev.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Chu CQ, Field M, Feldmann M, Maini RN. Localization of tumor necrosis factor alpha in synovial tissues and at the cartilage-pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991;34:1125–1132. doi: 10.1002/art.1780340908. [DOI] [PubMed] [Google Scholar]
- Chu JL, Drappa J, Parnassa A, Elkon KB. The defect in Fas mRNA expression in MRL/lpr mice is associated with insertion of the retrotransposon ETn. J Exp Med. 1993;178:723–730. doi: 10.1084/jem.178.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PL, Eisenberg RA. Lpr and gld : single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- Cools N, Ponsaerts P, Van Tendeloo VF, Berneman ZN. Regulatory T cells and human disease. Clin Dev Immunol. 2007;10:1155–1165. doi: 10.1155/2007/89195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttler JM. Low-dose irradiation therapy to cure gas gangrene infections. Int J Low Radiation. 2004;1:318–328. [Google Scholar]
- Daniel C, Wennhold K, Kim HJ, von Boehmer H. Enhancement of antigen-specific Treg vaccination in vivo. Pro Natl Acad Sci. 2010;107:16246–16251. doi: 10.1073/pnas.1007422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducoff HS. Forms of increased longevity of Tribolium after X-irradiation. Exp Gerontol. 1975;10:189–193. doi: 10.1016/0531-5565(75)90031-5. [DOI] [PubMed] [Google Scholar]
- Fang S, Muto Y, Tago F, Shimura N, Kojima S. Effect of repeated small-dose γ-ray irradiation on atopic dermatitis in NC/Nga mice. J Health Sci. 2006;52:406–411. [Google Scholar]
- Fang S, Tago F, Tanaka T, Shimura N, Muto Y, Goto R, Kojima S. Repeated irradiations with γ-rays at a dose of 0.5 Gy may exacerbate asthma. J Radiat Res. 2005;46:151–156. doi: 10.1269/jrr.46.151. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- Festing MFW. In: Genetic variants and strains of the laboratory mouse. Lyon MF, Rastan S, Brown SDM, editors. Oxford University Press; Oxford; 1996. p. 1537. [Google Scholar]
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- Firestein GS, Alvaro-Gracia JM, Maki R. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990;144:3347–3353. [PubMed] [Google Scholar]
- Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber M, Pioch Y, Dubois JB, Serrou B. Effects of low doses of irradiation on the T-cell-mediated cytotoxic response. Ann Immunol. 1983;134C:149–157. doi: 10.1016/s0769-2625(83)80158-5. [DOI] [PubMed] [Google Scholar]
- Goldstein RA, Paul WE, Metcalfe DD, Busse WW, Reece ER. NIH conference. Asthma. Ann Intem Med. 1994;121:698–708. doi: 10.7326/0003-4819-121-9-199411010-00011. [DOI] [PubMed] [Google Scholar]
- Gravallese EM. Bone destruction in arthritis. Ann Rheum Dis. 2002;61(Suppl 2):ii84–ii86. doi: 10.1136/ard.61.suppl_2.ii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji N, Mandolesi G, Gentile A, Sacchetti L, Fresegna D, Rossi S, Musella A, Sepman H, Motta C, Studer V, De Chiara V, Bernardi G, Strata P, Centonze D. TNF-α-mediated anxiety in a mouse model of multiple sclerosis. Exp Neurol. 2012;237:296–303. doi: 10.1016/j.expneurol.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Hanifin JM. Atopic dermatitis. J Am Acad Dermatol. 1982;6:1–13. doi: 10.1016/s0190-9622(82)70001-5. [DOI] [PubMed] [Google Scholar]
- Harigai M, Hara M, Kitani A, Norioka K, Hirose T, Hirose W, Suzuki K, Kawakami M, Masuda K, Shinmei M, et al. Interleukin 1 and tumor necrosis factor-alpha synergistically increase the production of interleukin 6 in human synovial fibroblast. J Clin Lab Immunol. 1991;34:107–113. [PubMed] [Google Scholar]
- Hashimoto H. Zur kenntus der lymphomatösen veränderung der Schildrüse (Struma Lymphomatosa) Arch f kin Chir. 1912;97:219. [Google Scholar]
- Hashimoto S, Shirato H, Hosokawa M, Nishioka T, Kuramitsu Y, Matushita K, Kobayashi M, Miyasaka K. The suppression of metastases and the change in host immune response after low-dose total-body irradiation in tumor-bearing rats. Radiat Res. 1999;151:717–724. [PubMed] [Google Scholar]
- Hildebrandt G, Jahns J, Hindemith M, Spranger S, Sack U, Kinne RW, Madaj-sterba P, Wolf U, Kamprad F. Effects of low dose radiation therapy on adjuvant induced arthritis in rats. Int J Radiat Biol. 2000;76:1143–1153. doi: 10.1080/09553000050111613. [DOI] [PubMed] [Google Scholar]
- Hildebrandt G, Radlingmayr A, Rosenthal S, Rothe R, Jahns J, Hikdemith M, Rödel F, Kamprad F. Low-dose radiotherapy (LD-RT) and the modulation of iNOS expression in adjuvant-induced arthritis in rats. Int J Radiat Biol. 2003;79:993–1001. doi: 10.1080/09553000310001636639. [DOI] [PubMed] [Google Scholar]
- Huang FP, Feng GJ, Lindop G, Scott DI, Liew FY. The role of interleukin 12 and nitric oxide in the development of spontaneous autoimmune disease in MRL/MP-lpr/lpr mice. J Exp Med. 1996;183:1447–1459. doi: 10.1084/jem.183.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert M, Corrigan CJ, Kimmitt P, Till SJ, Kay AB, Durham SR. Relationship between IL-4 and Il-5 mRNA expression and disease severity in atopic asthma. Am J Respir Crit Care Med. 1997;156:704–708. doi: 10.1164/ajrccm.156.3.9610033. [DOI] [PubMed] [Google Scholar]
- Ibuki Y, Goto R. Enhancement of concanavalin A-induced proliferation of splenolymphocytes by low-dose-irradiated macrophages. J Radiat Res. 1994;35:83–91. doi: 10.1269/jrr.35.83. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Kaneko A, Yamamoto M, Ishige A, Sasaki H. Possible involvement of suppression of Th2 differentiation in the anti-allergic effect of Sho-seiryu-to in mice. Jpn J Pharmacol. 2002;90:328–336. doi: 10.1254/jjp.90.328. [DOI] [PubMed] [Google Scholar]
- Ina Y, Sakai K. Activation of immunological network by chronic low-dose-rate irradiation in wild-type mouse strains: analysis of immune cell populations and surface molecules. Int J Radiat Biol. 2005;81:721–729. doi: 10.1080/09553000500519808. [DOI] [PubMed] [Google Scholar]
- Inglis JJ, Notley CA, Essex D, Wilson AW, Feldmann M, Anand P, Williams R. Collagen-induced arthritis as a model of hyperalgesia. Arthritis and Rheumatism. 2007;56:4015–4023. doi: 10.1002/art.23063. [DOI] [PubMed] [Google Scholar]
- Ishii K, Hosoi Y, Ono T, Sakamoto K. Enhanced proliferation and IL-1 production of mouse splenocytes by low-dose whole-body X-irradiation. Physil Chem Phys Med NMR. 1996;28:7–14. [PubMed] [Google Scholar]
- Ishii K, Yamaoka K, Hosoi Y, Ono T, Sakamoto K. Enhanced mitogen-induced proliferation of rat splenocytes by low-dose whole-body X-irradiation. Physiol Chem Phys Med NMR. 1995;27:17–23. [PubMed] [Google Scholar]
- James SJ, Makinodan T. T cell potentiation in normal and autoimmune-prone mice after extended exposures to low doses of ionizing radiation and/or caloric restriction. Int J Radiat Biol Relat Stud Phys Chem Med. 1988;53:137–152. doi: 10.1080/09553008814550491. [DOI] [PubMed] [Google Scholar]
- James SJ, Enger SM, Peterson WJ, Makinodan T. Immune potentiation after fractionated exposure to very low doses of ionizing radiation and/or caloric restriction in autoimmune-prone and normal C57Bl/6 mice. Clin Immunol Immunopathol. 1990;55:427–437. doi: 10.1016/0090-1229(90)90129-e. [DOI] [PubMed] [Google Scholar]
- Jasani B, Temynck T, Lazarus JH, Phillips DI, Avrameas S, Parkes AB. Natural antibody status in patients with Hashimoto’s thyroiditis. 1999;51:9–20. [PubMed] [Google Scholar]
- Jujo K, Renz H, Abe J, Gelfand EW, Leung DY. Decreased interferon gamma and increased interleukin-4 production in atopic dermatitis promotes IgE synthesis. J Allergy Clin Immunol. 1992;90:323–331. doi: 10.1016/s0091-6749(05)80010-7. [DOI] [PubMed] [Google Scholar]
- Kelley VE, Roths JB. Interaction of mutant lpr gene with background strain influences renal disease. Clin Immunol Immunopathol. 1985;37:220–229. doi: 10.1016/0090-1229(85)90153-9. [DOI] [PubMed] [Google Scholar]
- Kern PM, Keilholz L. Radio-immunological mechanisms of anti-inflammatory treatment: is there a way from the past into the future? Autoimmunity. 2009;42:337–339. doi: 10.1080/08916930902831027. [DOI] [PubMed] [Google Scholar]
- Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- Kipp M, van der Valk P, Amor S. Pathology of multiple sclerosis. CNS Neurol Disord Drug Targets. 2012;11:506–517. doi: 10.2174/187152712801661248. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- Kojima S, Ishida H, Takahashi M, Yamaoka K. Elevation of glutathione induced by low-dose gamma rays and its involvement in increased natural killer activity. Radiat Res. 2002;157:275–280. doi: 10.1667/0033-7587(2002)157[0275:eogibl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kojima S, Matsumori S, Ishida H, Yamaoka K. Possible role of elevation of glutathione in the acquisition of enhanced proliferation of mouse splenocytes exposed to small-dose γ-rays. Int J Radiat Biol. 2000a;76:1641–1647. doi: 10.1080/09553000050201136. [DOI] [PubMed] [Google Scholar]
- Kojima S, Nakayama K, Ishida H. Low-dose γ-rays activate immune functions via induction of glutathione and delay tumor growth. J Radiat Res. 2004;45:33–39. doi: 10.1269/jrr.45.33. [DOI] [PubMed] [Google Scholar]
- Kojima S, Shimomura H, Matsumori S. Effect of pre-irradiation with low-dose gamma-rays on chemically induced hepatotoxicity and glutathione depletion. Anticancer Res. 2000b;20:1583–1588. [PubMed] [Google Scholar]
- Kondo K, Nagami T, Tadokoro S. In: Comparative cellular and species radiosensitivity. Bond PV, Sugawara T, editors. Igakushoin; Tokyo: 1969. p. 20. [Google Scholar]
- Kondo S, Yazawa H, Jimbow K. Reduction of serum interleukin-5 levels reflect clinical improvement in patients with atopic dermatitis. J Dermatol. 2001;28:237–243. doi: 10.1111/j.1346-8138.2001.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Koutsouraki E, Hatzifilipou E, Michmizos D, Cotsavasiloglou C, Costa V, Baloyannis S. Increase in interleukin-6 levels is related to depressive phenomena in the acute (relapsing) phase of multiple sclerosis. J Neuropsychiatry Clin Nerosci. 2011;23:442–448. doi: 10.1176/jnp.23.4.jnp442. [DOI] [PubMed] [Google Scholar]
- Krinzman SJ, De Sanctis GT, Cernadas M, Kobzik L, Listman JA, Christiani DC, Perkins DL, Finn PW. T cell activation in a murine model of asthma. Am J Physiol. 1996;271:L476–483. doi: 10.1152/ajplung.1996.271.3.L476. [DOI] [PubMed] [Google Scholar]
- Krug N, Madden J, Redington AE, Lackie P, Djukanovic R, Schauer U, Holgate ST, Frew AJ, Howarth PH. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am J Respir Cell Mol Biol. 1996;14:319–326. doi: 10.1165/ajrcmb.14.4.8600935. [DOI] [PubMed] [Google Scholar]
- Lara-Marquez ML, Deykin A, Krinzman S, Listman J, Israel E, He H, Christiani DC, Perkins DL, Finn PW. Analysis of T-cell activation after bronchial allergen challenge in patients with atopic asthma. J Allergy Clin Immunol. 1998;101:699–708. doi: 10.1016/s0091-6749(98)70180-0. [DOI] [PubMed] [Google Scholar]
- Lara-Marquez ML, Moan MJ, Cartwright S, Listman J, Israel E, Perkins DL, Christiani DC, Finn PW. Atopic asthma: differential activation phenotypes among memory T helper cells. Clin Exp Allergy. 2001;31:1232–1241. doi: 10.1046/j.1365-2222.2001.01146.x. [DOI] [PubMed] [Google Scholar]
- Leung DY. Role of IgE in atopic dermatitis. Curr Opin Immunol. 1993;5:956–962. doi: 10.1016/0952-7915(93)90112-6. [DOI] [PubMed] [Google Scholar]
- Leung DY. Molecular basis of allergic diseases. Mol Genet Metab. 1998;63:157–167. doi: 10.1006/mgme.1998.2682. [DOI] [PubMed] [Google Scholar]
- Liebmann A, Hindemith M, Jahns J, Madaj-Sterba P, Weisheit S, Kamprad F, Hildebrandt G. Low-dose X-irradiation of adjuvant-induced arthritis in rats. Efficacy of different fractionation schedules. Strahlenther Onkol. 2004;180:165–172. doi: 10.1007/s00066-004-1197-2. [DOI] [PubMed] [Google Scholar]
- Liu H, Leung BP. CD4+CD25+ regulatory T cells in health and disease. Clin Exp Pharmacol Physiol. 2006;33:519–524. doi: 10.1111/j.1440-1681.2006.04401.x. [DOI] [PubMed] [Google Scholar]
- Liu SZ. Radiation hormesis. A new concept in radiological science. Chin Med J. 1989;102:750–755. [PubMed] [Google Scholar]
- Liu SZ, Liu WH, Sun JB. Radiation hormesis: its expression in the immune system. Health Phys. 1987;52:579–583. doi: 10.1097/00004032-198705000-00008. [DOI] [PubMed] [Google Scholar]
- Liu XD, Ma SM, Liu SZ. Effects of 0.075Gy x-ray irradiation on the expression of IL-10 and IL-12 in mice. Phys Med Biol. 2003;48:2041–2049. doi: 10.1088/0031-9155/48/13/315. [DOI] [PubMed] [Google Scholar]
- Longhi MS, Hussain MJ, Mitry RR, Arora SK, Mieli-Vergani G, Vergani D, Ma Y. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J Immunol. 2006;176:4484–4491. doi: 10.4049/jimmunol.176.7.4484. [DOI] [PubMed] [Google Scholar]
- Lubberts E, Koenders MI, van den Berg WB. The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res Ther. 2005;7:29–37. doi: 10.1186/ar1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey TD. Physiological benefits from low levels of ionizing radiation. Health Phys. 1982;43:771–789. doi: 10.1097/00004032-198212000-00001. [DOI] [PubMed] [Google Scholar]
- Luckey TD. Radiation Hormesis. Boca Raton, Fla: CRC Press; 1991. [Google Scholar]
- Lutton JD, Winston R, Rodman TC. Multiple sclerosis: etiological mechanisms and future directions. Exp Biol Med. 2004;229:12–20. doi: 10.1177/153537020422900102. [DOI] [PubMed] [Google Scholar]
- Many MC, Maniratunga S, Denef JF. The non-obese diabetic (NOD) mouse: an animal model for autoimmune thyroiditis. Exp Clin Endocrinol Diabetes. 1996;104:17–20. doi: 10.1055/s-0029-1211673. [DOI] [PubMed] [Google Scholar]
- Marshall SF, Meissner WA. Struma lymphomatosa (Hashimoto’s disease) Ann Surg. 1955;141:737–746. doi: 10.1097/00000658-195505000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese G, Carrieri PB, La Cava A, Perna F, Sanna V, De Rosa V, Aufiero D, Fontana S, Zappacosta S. Leptin increase in multiple sclerosis associates with reduced number of CD4(+)CD25+regulatory T cells. Proc Natl Acad Sci. 2005;102:5150–5155. doi: 10.1073/pnas.0408995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel REJ, Jackson JS, McCann RA, Boreham DR. The adaptive response modifies latency for radiation-induced myeloid leukemia in CBA/H mice. Radat Res. 1999;152:273–279. [PubMed] [Google Scholar]
- Mitchel REJ, Jackson JS, Morrison DP, Carlisle SM. Low doses of radiation increase the latency of spontaneous lymphomas and spinal osteosarcomas in cancer-prone, radiation-sensitive Trp53 heterozygous mice. Radiat Res. 2003;159:320–327. doi: 10.1667/0033-7587(2003)159[0320:ldorit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Möller GM, de Jong TA, van der Kwast TH, Overbeek SE, Wierenga-Wolf AF, Thepen T, Hoogsteden HC. Immunolocalization of interleukin-4 in eosinophils in the bronchial mucosa of atopic asthmatics. Am J Respir Cell Mol Biol. 1996;14:439–443. doi: 10.1165/ajrcmb.14.5.8624248. [DOI] [PubMed] [Google Scholar]
- Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, de Vries RR, Toes RE. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212–2221. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- Morgan ME, Sutmuller RP, Witteveen HJ, van Duivenvoorde LM, Zanelli E, Melief CJ, Snijders A, Offringa R, de Vries RR, Toes RE. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–1460. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- Mota-Pinto A, Todo A, Alves V, Santos A, Santos M. Regulatory T cells in elderly patients with asthma. J Investig Allergol Clin Immnol. 2011;21:199–206. [PubMed] [Google Scholar]
- Myers LK, Rosloniec EF, Cremer MA, Kang AH. Collagen-induced arthritis, an animal model of autoimmunity. Life Sci. 1997;61:1861–1878. doi: 10.1016/s0024-3205(97)00480-3. [DOI] [PubMed] [Google Scholar]
- Nagai H, Yamaguchi S, Tanaka H. The role of interleukin-5 (IL-5) in allergic airway hyper-responsiveness in mice. Ann N Y Acad Sci. 1996;796:91–96. doi: 10.1111/j.1749-6632.1996.tb32570.x. [DOI] [PubMed] [Google Scholar]
- Nagayama Y, Kaminoda K, Mizutori Y, Saitoh O, Abiru N. Exacerbation of autoimmune thyroiditis by a single low dose of whole-body irradiation in non-obese diabetic-H2h4 mice. Int J Radiat Biol. 2008;84:761–769. doi: 10.1080/09553000802345910. [DOI] [PubMed] [Google Scholar]
- Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Usa T, Motomura M, Ichikawa T, Nakao T, Kawasaki E, Tanaka M, Ishikawa K, Equchi K. Prevalence of interrelated autoantibodies in thyroid diseases and autoimmune disorders. J Endocrinol Invest. 2008;31:861–865. doi: 10.1007/BF03346432. [DOI] [PubMed] [Google Scholar]
- Nakatsukasa H, Tsukimoto M, Ohshima Y, Tago F, Masada A, Kojima S. Suppressing effect of low-dose gamma-ray irradiation on collagen-induced arthritis. J Radiat Res. 2008;49:381–389. doi: 10.1269/jrr.08002. [DOI] [PubMed] [Google Scholar]
- Nakatsukasa H, Tsukimoto M, Tokunaga A, Kojima S. Repeated gamma irradiation attenuates collagen-induced arthritis via up-regulation of regulatory cells but not by damaging lymphocytes directly. Radiat Res. 2010;174:313–324. doi: 10.1667/RR2121.1. [DOI] [PubMed] [Google Scholar]
- Nakazawa M, Sugi N, Kawaguchi H, Ishii N, Nakajima H, Minami M. Predominance of type 2 cytokine-producing CD4+ and CD8+ cells in patients with atopic dermatitis. J Allergy Clin Immunol. 1997;99:673–682. doi: 10.1016/s0091-6749(97)70030-7. [DOI] [PubMed] [Google Scholar]
- Nawata Y, Koike T, Yanagisawa T, Iwamoto I, Itaya T, Yoshida S, Tomioka H. Anti-IgE autoantibody in patients with bronchial asthma. Clin Exp Immunol. 1984;58:348–356. [PMC free article] [PubMed] [Google Scholar]
- Obradović D, Kataranovski M, Dincić E, Obradović S, Colić M. Tumor necrosis factor-alpha and interleukin-4 in cerebrospinal fluid and plasma in different clinical forms of multiple sclerosis. Vojnosanit Pregl. 2012;69:151–156. [PubMed] [Google Scholar]
- Okudaira H, Hongo O, Ogita T, Haida M, Yamaguchi N, Miyamoyo T. Serum IgE and IgE antibody levels in patients with bronchial asthma, atopic dermatitis, eosinophilic granulomas of the soft tissue (Kimura’s disease) and other diseases. Ann Allergy. 1983;50:51–54. [PubMed] [Google Scholar]
- Ootusyama A, Okazaki R, Norimura T. Effect of extended exposure to low-dose radiation on autoimmune diseases of immunologically suppressed MRL/MpTn-gld/gld mice. J Radiat Res. 2003;44:243–247. doi: 10.1269/jrr.44.243. [DOI] [PubMed] [Google Scholar]
- Pacini F, Vorontsova T, Molinaro E, Kuchinskaya E, Agate L, Shavrova E, Astachova L, Chiovato L, Pinchera A. Prevalence of thyroid autoantibodies in children and adolescents from Belarus exposed to the Chernobyl radioactive fallout. Lancet. 1998;352:763–766. doi: 10.1016/s0140-6736(97)11397-6. [DOI] [PubMed] [Google Scholar]
- Pandey R, Shankar BS, Sharma D, Sainis KB. Low dose radiation induced immunomodulation: effect on macrophages and CD8+ cells. Int J Radiat Biol. 2005;81:801–812. doi: 10.1080/09553000500531886. [DOI] [PubMed] [Google Scholar]
- Park CS, Ra DJ, Lee SM, Jeong SW, Uh S, Kim HT, Kim YH. Interleukin-4 and low-affinity receptor for IgE on B cells in peripheral blood of patients with atopic bronchial asthma. J Allergy Clin Immunol. 1996;97:1121–1128. doi: 10.1016/s0091-6749(96)70267-1. [DOI] [PubMed] [Google Scholar]
- Park BS, Hong GU, Ro JY. Foxp3+-Treg cells enhanced by repeated low-dose gamma-irradiation attenuate ovalbumin-induced allergic asthma in mice. Radiat Res. 2013a;179:570–583. doi: 10.1667/RR3082.1. [DOI] [PubMed] [Google Scholar]
- Park H-R, Jo S-K, Yu D-K, Jung U. Fractionated irradiations lead to chronic allergic airway inflammation through increasing the influx of macrophages. Inflamm Res. 2013b;62:27–36. doi: 10.1007/s00011-012-0547-2. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Brusselle GJ, Kips JC. Cytokine manipulation in animal models of asthma. Am J Respir Crit Care Med. 1997;156:S78–81. doi: 10.1164/ajrccm.156.4.12-tac-1. [DOI] [PubMed] [Google Scholar]
- Qing X, Zavadil J, Crosby MB, Hogarth MP, Hahn BH, Mohan C, Gilkeson GS, Bottinger EP, Putterman C. Nephritogenic anti-DNA antibodies regulate gene expression in MRL/lpr mouse glomerular mesangial cells. Arthritis Rheum. 2006;54:2198–2210. doi: 10.1002/art.21934. [DOI] [PubMed] [Google Scholar]
- Rasooly L, Burek CL, Rose NR. Iodide-induced autoimmune thyroiditis in NOD-H-2h4 mice. Clin Immunol Immunopathol. 1996;81:287–292. doi: 10.1006/clin.1996.0191. [DOI] [PubMed] [Google Scholar]
- Ridderstad A, Abedi-Valugerdi M, Möller E. Cytokines in rheumatoid arthritis. Ann Med. 1991;23:219–223. doi: 10.3109/07853899109148051. [DOI] [PubMed] [Google Scholar]
- Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Eng J Med. 1992;30:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Rödel F, Frey B, Gaipl U, Keilholz L, Fournier C, Manda K, Schöllnberger H, Hildebrandt G, Rödel C. Modulation of inflammatory immune reactions by low-dose ionizing radiation : molecular mechanisms and clinical application. Curr Med Chem. 2012;19:1741–1750. doi: 10.2174/092986712800099866. [DOI] [PubMed] [Google Scholar]
- Rödel F, Keilholz L, Herrmann M, Sauer R, Hildebrandt G. Radiobiological mechanisms in inflammatory diseases of low-dose radiation therapy. Int J Radiat Biol. 2007;83:357–366. doi: 10.1080/09553000701317358. [DOI] [PubMed] [Google Scholar]
- Roths JB, Murphy ED, Eicher EM. A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp Med. 1984;159:1–20. doi: 10.1084/jem.159.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset F, Garcia E, Defrance T, Péronne C, Vezzio N, Hsu DH, Kastelein R, Moore KW, Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley MJ, Nandakumar KS, Holmdahl R. The role of collagen antibodies in mediating arthritis. Mod Rheumatol. 2008;18:429–441. doi: 10.1007/s10165-008-0080-x. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immuno-logical tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- Sasakawa T, Higashi Y, Sakuma S, Hirayama Y, Sasakawa Y, Ohkubo Y, Goto T, Matsumoto M, Matsuda H. Atopic dermatitis-like skin lesions induced by topical application of mite antigens in NC/Nga mice. Int Arch Allergy Immunol. 2001;126:239–247. doi: 10.1159/000049520. [DOI] [PubMed] [Google Scholar]
- Sato T, Takata I, Taketani T, Saida K, Nakajima H. Concurrence of Grave’s diseases and Hashimoto’s thyroiditis. Arch Dis Child. 1977;52:951–955. doi: 10.1136/adc.52.12.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegenschmiedt MH, Keilholz L, Martus P, Goldmann A, Wölfel R, Henning F, Sauer R. Prevention of heterotopic ossification about the hip: final results of two randomized trials in 410 patients using either preoperative or postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1997;39:161–171. doi: 10.1016/s0360-3016(97)00285-x. [DOI] [PubMed] [Google Scholar]
- Seil FJ. Multiple sclerosis. Current etiological concepts. Calif Med. 1972;116:25–33. [PMC free article] [PubMed] [Google Scholar]
- Sewell WA, Mu HH. Dissociation of production of interleukin-4 and interleukin-5. Immunol Cell Biol. 1996;74:274–277. doi: 10.1038/icb.1996.48. [DOI] [PubMed] [Google Scholar]
- Shankar B, Premachandran S, Bharambe SD, Sundaresan P, Sainis KB. Modification of immune response by low dose ionizing radiation: role of apoptosis. Immunol Lett. 1999;68:237–245. doi: 10.1016/s0165-2478(99)00074-7. [DOI] [PubMed] [Google Scholar]
- Shi H, Zhong X, Long X, Chen Y. Effect of interferon-gamma on antigen-induced eosinophil infiltration into the mouse airway. Clin Med J. 1996;109:215–218. [PubMed] [Google Scholar]
- Shigematsu A, Adachi Y, Koike-Kiriyama N, Suzuki Y, Iwasaki M, Koike Y, Nakano K, Mukaide H, Imamura M, Ikehara S. Effects of low-dose irradiation on enhancement of immunity by dendritic cells. J Radiat Res. 2007;48:51–55. doi: 10.1269/jrr.06048. [DOI] [PubMed] [Google Scholar]
- Spergel JM, Mizoguchi E, Oettgen H, Bhan AK, Geha RS. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999;103:1103–1111. doi: 10.1172/JCI5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirchez M, Samasca G, Iancu M, Bolba C, Miu N. Relation of interleukin-6, TNF-alpha and interleukin-1alpha with disease activity and severity in juvenile idiopathic arthritis patients. Clin Lab. 2012;58:253–260. [PubMed] [Google Scholar]
- Spooren A, Kolmus K, Laureys G, Clinckers R, De Keyser J, Haegeman G, Gerlo S. Interleukin-6, a mental cytokine. Brain Res Rev. 2011;67:157–183. doi: 10.1016/j.brainresrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Stelmasiak Z, Koziol-Montewka M, Dobosz B, Rejdak K. IL-6 and sIL-6R concentration in the cerebrospinal fluid and serum of MS patients. Med Sci Monit. 2001;7:914–918. [PubMed] [Google Scholar]
- Swanson PC, Yung RL, Blatt NB, Eagan MA, Norris JM, Richardson BC, Johnson KJ, Glick GD. Ligand recognition by murine anti-DNA autoantibodies. II. Genetic analysis and pathogenicity. J Clin Invet. 1996;97:1748–1760. doi: 10.1172/JCI118602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tago F, Tsukimoto M, Nakatsukasa H, Kojima S. Repeated 0.5-Gy gamma irradiation attenuates autoimmune disease in MRL-lpr/lpr mice with suppression of CD3+CD4−CD8−B220+T-cell proliferation and with up-regulation of CD4+CD25+Foxp3+ regulatory T cells. Radiat Res. 2008;169:59–66. doi: 10.1667/RR1013.1. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Kojima S, Yamaoka K, Niki E. Prevention of type I diabetes by low-dose gamma irradiation in NOD mice. Radiat Res. 2000;154:680–685. doi: 10.1667/0033-7587(2000)154[0680:potidb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Sasaki Y, Hama K, Furue M, Ishibashi Y. Production of IL-4, IL-2, IFN-gamma, and TNF-alpha by peripheral blood mononuclear cells of patients with atopic dermatitis. J Dermatol Sci. 1992;3:172–180. doi: 10.1016/0923-1811(92)90032-7. [DOI] [PubMed] [Google Scholar]
- Tan PL, Farmiloe S, Yeoman S, Watson JD. Expression of the interleukin 6 gene in rheumatoid synovial fibroblasts. J Rheumatol. 1990;17:1608–1612. [PubMed] [Google Scholar]
- Tanaka T, Tago F, Fang S, Shimura N, Kojima S. Repeated 0.5-Gy γ-ray irradiation attenuates autoimmune manifestations in MRL-lpr/lpr mice. Int J Radiat Biol. 2005;81:731–740. doi: 10.1080/09553000500519790. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Delaporte E, Dubucquoi S, Gounni AS, Porchet E, Capron A, Capron M. Interleukin-5 messenger RNA and immunoreactive protein expression by activated eosinophils in lesional atopic dermatitis skin. J Invest Dermatol. 1994;103:589–592. doi: 10.1111/1523-1747.ep12396899. [DOI] [PubMed] [Google Scholar]
- Tang ML, Coleman J, Kemp AS. Interleukin-4 and interferon-gamma production in atopic and non-atopic children with asthma. Clin Exp Allergy. 1995;25:515–521. doi: 10.1111/j.1365-2222.1995.tb01088.x. [DOI] [PubMed] [Google Scholar]
- Thestrup-Pedersen K. Immunology of atopic dermatitis. Acta Derm Venereol Suppl. 1989;151:77–83. [PubMed] [Google Scholar]
- Tsukimoto M, Nakatsukasa H, Sugawara K, Yamashita K, Kojima S. Repeated 0.5-Gy γ irradiation attenuates experimental autoimmune encephalomyelitis with up-regulation of regulatory T cells and suppression of IL17 production. Radiat Res. 2008;170:429–436. doi: 10.1667/rr1352.1. [DOI] [PubMed] [Google Scholar]
- Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vykhovanets EV, Chernyshov VP, Slukvin II, Antipkin YG, Vasyuk AN, Klimenko HF, Strauss KW. 131I dose-dependent thyroid autoimmune disorders in children living around Chernobyl. Clin Immunol Immunopathol. 1997;84:251–259. doi: 10.1006/clin.1997.4379. [DOI] [PubMed] [Google Scholar]
- Wang GY, Sun B, Kong QF, Zhang Y, Li R, Wang JH, Wang DD, Lv GX, Li HL. IL-17 eliminates the therapeutic effects of myelin basic protein-induced nasal tolerance in experimental autoimmune encephalomyelitis by activating IL-6. Scand J Immunol. 2008a;68:589–597. doi: 10.1111/j.1365-3083.2008.02174.x. [DOI] [PubMed] [Google Scholar]
- Wang HH, Dai YQ, Qiu W, Lu ZQ, Peng FH, Wang YG, Bao J, Li Y, Hu XQ. Interleukin-17-secreting T cells in neuromyelitis optica and multiple sclerosis during relapse. J Clin Neurosci. 2011;18:1313–1317. doi: 10.1016/j.jocn.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Wang J, Takeuchi H, Sonobe Y, Jin S, Mizuno T, Miyakawa S, Fujiwara M, Nakamura Y, Kato T, Muramatsu H, Muramatsu T, Suzumura A. Inhibition of midkine alleviates experimental autoimmune encephalomyelitis through the expansion of regulatory T cell population. Proc Natl Acad Sci. 2008b;105:3915–3920. doi: 10.1073/pnas.0709592105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Watson ML, D’eustachio P, Mock BA, Steinberg AD, Morse HC, 3d, Oakey RJ, Howard TA, Rochelle JM, Seldin MF. A linkage map of mouse chromosome I using and interspecific cross segregating for the gld autoimmunity mutation. Mammal Genome. 1992;2:158–171. doi: 10.1007/BF00302874. [DOI] [PubMed] [Google Scholar]
- Wekerie T. T-regulatory cells-what relationship with immunosuppressive agents? Transplant Proc. 2008;40:S13–16. doi: 10.1016/j.transproceed.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Weng L, Williams RO, Vieira PL, Screaton G, Feldmann M, Dazzi F. The therapeutic activity of low-dose irradiation on experimental arthritis depends on the induction of endogenous regulatory T cell activity. Ann Rheum Dis. 2010;69:1519–1526. doi: 10.1136/ard.2009.121111. [DOI] [PubMed] [Google Scholar]
- Westacott CI, Whicher JT, Bames IC, Thompson D, Swan AJ, Dieppe PA. Synovial fluid concentration of five different cytokines in rheumatic diseases. Ann Rheum Dis. 1990;49:676–681. doi: 10.1136/ard.49.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa M, Takeda A, Misonoh J. Acquired radioresistance after low dose X-radiation in mice. J Radiat Res. 1990;31:256–262. doi: 10.1269/jrr.31.256. [DOI] [PubMed] [Google Scholar]
- Zamvil SS, Mitchell DJ, Moore AC, Kitamura K, Steinman L, Rothbard JB. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986;324:258–260. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]
- Zhou CY, Crocker IC, Koenig G, Romero FA, Townley RG. Anti-inteleukin-4 inhibits immunogloblin E production in a murine model of atopic asthma. J Asthma. 1997;34:195–201. doi: 10.3109/02770909709068189. [DOI] [PubMed] [Google Scholar]