Abstract
Imidacloprid-induced hormesis in the form of stimulated reproduction has previously been reported in green peach aphid, Myzus persicae. Changes in gene expression accompanying this hormetic response have not been previously investigated. In this study, expression of stress response (Hsp60), dispersal (OSD, TOL and ANT), and developmental (FPPS I) genes were examined for two generations during imidacloprid-induced reproductive stimulation in M. persicae. Global DNA methylation was also measured to test the hypothesis that changes in gene expression are heritable. At hormetic concentrations, down-regulation of Hsp60 was followed by up-regulation of this gene in the subsequent generation. Likewise, expression of dispersal-related genes and FPPS I varied with concentration, life stage, and generation. These results indicate that reproductive hormesis in M. persicae is accompanied by a complex transgenerational pattern of up- and down-regulation of genes that likely reflects trade-offs in gene expression and related physiological processes during the phenotypic dose-response. Moreover, DNA methylation in second generation M. persicae occurred at higher doses than in first-generation aphids, suggesting that heritable adaptability to low doses of the stressor might have occurred.
Keywords: Hormesis, Myzus persicae, fecundity, gene expression, global DNA methylation
INTRODUCTION
Hormesis is low-dose stimulation coupled with high dose inhibition of a stressor-induced response (Calabrese and Baldwin 2002). Chemical-induced hormesis is widespread across many biological groups and has been studied in medical, evolutionary, and ecological contexts (Calabrese and Baldwin 2003). The biochemical and molecular underpinnings of hormetic responses have been examined using several biological models (Calabrese and Baldwin 2003). Regulation of gene expression is implicit in the hormetic responses (Son et al. 2010), activating adaptive cellular stress response pathways assisted by transcriptional factors such as antioxidant response element (ARE), forkhead box O (FoxO), heat-shock factor (HSF), and nuclear factor-κB (NF-κB).
Gene expression during hormesis has not been well studied in insect-insecticide models, although changes in certain biochemical endpoints have been reported during pesticide-induced stimulations (Cutler 2013; Guedes and Cutler 2013). Stimulated production of total calcium and proteins was seen in Choristoneura fumiferana (Clemens) exposed to sub-lethal doses of organophosphorus and carbamate insecticides (Smirnoff 1983), suggesting that basic metabolites such as sugars, lipids, and total proteins can be measured in addition to organismal and population endpoints to give clues into how insect metabolism changes during hormesis. Larval weight gain and changes in isoesterase profiles were seen in Tribolium castaneum L. exposed to low concentrations of azadirachtin (Mukherjee et al. 1993), and juvenile hormone (JH) III titers were correlated with stimulated fecundity in green peach aphid, Myzus persicae (Sulzer), when exposed to low doses of imidacloprid (Yu et al. 2010). Transcriptional responses of Helicoverpa armigera Hübner to gossypol-induced hormesis were suggested to be a specific transcriptional adaptation rather than general stress response (de la Paz Celorio-Mancera et al. 2011).
The HSF pathway can be triggered by a variety of stressors, including elevated temperatures, heavy metals, viral or microbial infection, and toxins (Son et al. 2010). In agriculture, insecticides are used to kill insect pests but can activate the HSF pathway in insects that survive insecticide exposure (Yoshimi et al. 2002). Other abiotic stressors that disrupt homeostasis can also initiate the HSF pathway. For example, Chironomus tentans Fabricius (Karouna-Renier and Zehr 1999), T. castaneum L. (Mahroof et al. 2005), Cydia pomonella (L.) (Yin et al. 2006), and Liriomyza huidobrensis Blanchard (Huang et al. 2007) accumulated heat shock proteins (Hsp) following heat stress, resulting in thermotolerance. Nutritional stress with heat stress induced higher transcription of Hsp70 than heat stress alone (Salvucci et al. 2000). In M. persicae, Hsp are essential for survival and several biological processes, such as detoxification of xenobiotics (Figueroa et al. 2007; Ramsey et al. 2007).
M. persicae exhibits dimorphism as alate (winged/dispersal) and apterous (wingless) forms (Braendle et al. 2006). The switch to the alate form in M. persicae seems to be triggered by several mechanisms in response to sub-optimal environmental conditions such as high density and poor nutrition (Braendle et al. 2006). Alate production (dispersal) is accompanied by up-regulation of olfactory segment D (OSD), take-out like/JH binding protein (TOL), and mitochondrial adenine nucleotide translocase (ANT) (Ghanim et al. 2006). The role of JH in aphid wing dimorphism is still uncertain (Braendle et al. 2006; Schwartzberg et al. 2008), but is probably governed by environmental and genetic stimuli (Brisson 2010). Imidacloprid-induced reproductive stimulation has been associated with wing dimorphisms in M. persicae (Wang et al. 2008). JH biosynthesis via the mevalonate pathway includes a precursor farnesyl diphosphate synthase I (FPPS I) gene (Zhang and Li 2008) and its expression may regulate reproduction in insects (Cusson et al. 2006).
When first instar M. persicae were continuously exposed to sublethal concentrations of imidacloprid, stimulated fecundity in first and second generation adults was observed (Ayyanath et al. 2013). To understand the molecular underpinnings of this phenotypic response, in the present study we examined gene expression in M. persicae exposed to hormetic concentrations of imidacloprid for two generations. Stress response (Hsp60) (Stanley and Fenton 2000), dispersal (OSD, TOL, and ANT) and developmental (FPPS I) genes were examined. We hypothesized that OSD, TOL, and ANT gene expression would correspond to fecundity responses previously reported (Ayyanath et al. 2013), and that Hsp60 gene expression would be up-regulated in initial generation (G0) second instar nymphs. We also predicted that FPPS I gene expression would be down-regulated (Keeling et al. 2004) during adult exposure corresponding to fecundity responses.
In addition, we examined whether insecticide-induced hormesis in insects results in heritable changes in gene expression. DNA methylation is an important epigenetic mechanism in insects that may provide critical contributions to insect developmental and phenotypic variation (Glastad et al. 2011). Therefore, in addition to measuring gene expression, global DNA methylation was measured in our test aphids. We hypothesized that hypermethylation would occur when Hsp60, FPPS I, OSD, TOL and ANT genes were up-regulating.
METHODS AND MATERIALS
Plants and insects
Potato, Solanum tuberosum L. (cv. Kennebec), was grown in a greenhouse in 12.5 cm diameter pots containing Pro-Mix® (Halifax Seed, Halifax, Nova Scotia) potting soil. Plants were watered as needed. Foliage from these plants was used for insect rearing and experiments. M. persicae was obtained from a population infesting broccoli plants (Brassica oleracea L.) in a pesticide free-greenhouse at the Faculty of Agriculture, Dalhousie University, Truro, NS. Aphid cohorts were maintained on excised leaves in clear plastic boxes (37 L × 24 W × 14 H cm) lined with moistened paper towels. Boxes were held in a growth chamber (22 ± 2° C, 16:8 L:D, 65 ± 5 % RH) and every second day a layer of freshly excised leaves was placed on one end of the box. The infested foliage on the opposite end was discarded when about 80% aphids moved to fresh foliage. Paper towels were replaced every 10 days.
Chemicals
Imidacloprid (Admire® 240 SC, 240 g a.i. L−1; Bayer CropScience Canada, AB, Canada) was suspended in deionized water to obtain a 1000 μg a.i. L−1 stock solution. The working solutions contained 0.15% Triton™ X 100 (Fisher Scientific, Fair Lawn, New Jersey) as an emulsifier. We previously found that concentrations of 25 μg a.i. L−1 adversely affected M. persicae reproduction. Because we were interested in gene regulation at concentrations below the no observable adverse effects concentration (NOAEC), insecticide concentrations of 0.025, 0.1, 0.25, 2.5 and 10 μg a.i. L−1 were used in the current experiments. Controls in these experiments consisted of 0.15% Triton in water.
Leaf-dip exposure
Potato leaf discs (1.8 cm diameter) were excised using a stainless steel cork borer. Using forceps, leaf discs were dipped in control or insecticide solutions for 5 sec, air-dried for 1 h, and then two discs each were placed in 5.5 cm Petri plates lined with a Whatman No. 1 filter paper. Ten first instar M. persicae (∼24 h old) were transferred to each treated leaf disc. Dishes were covered with a Petri plate cover, placed in sealable plastic containers and held in growth chamber as described above.
Aphids were exposed to and reared continuously on treated leaf discs for two generations (G0, G1). Leaf discs were replaced every second day. The experiment was a completely randomized design, with imidacloprid concentration being the main factor of interest. Each container represented a biological replicate of a concentration and had five Petri dishes, each with ten aphids. For gene expression analyses, five aphids from each Petri plate were randomly collected at day 4, 9, 13 and 17, representing G0 second instar, G0 adult, G1 second instar and G1 adult stages, respectively. Excess nymphs were discarded as soon as 10 nymphs per Petri plate were obtained on day 9 and 17. A total of three biological replicates were set up in the experiment. For the global DNA methylation experiment, the same experimental procedure and design was used.
Sample preparation
Gene expression analyses
Collected aphids were flash frozen in liquid nitrogen. Total RNA was isolated using a RNeasy® mini kit (Qiagen, Toronto, Ontario). Quality (A260/280 > 2.0) and quantity of total RNA was assessed with a Nanodrop ND-1000 (NanoDrop Technologies, Wilmington, Delaware) and gel electrophoresis (rRNA band intensity: 28s = 2X 18s). Later, cDNA was synthesized from 1 μg of total RNA using a QuantiTect® Reverse Transcription kit (Qiagen, Toronto, Ontario) and stored at −20° C until further analyses. The primers (Sigma-Aldrich, Oakville Ontario) used for quantitative Real-Time (qRT) PCR are listed in Table 1. Internal controls with cycle of threshold (ct) values closer to the ct of selected genes were chosen to calculate expression fold-change (Pfaffl 2001). For quantification of OSD and TOL genes, the β-actin gene was used as an internal control. Ace was used as an internal control for ANT, Hsp60, and FPPS I genes. qRT PCR was performed on a StepOne™ RT PCR System (Applied Biosystems, Burlington, Ontario) in a 10 μL reaction following the manufacturer’s instructions using SYBR green reagent (Applied Biosystems, Burlington, Ontario). The reaction mixture contained 2X SYBR green reagent master mix, 2 μL cDNA, 2.5 μL ultrapure water, and 0.25 μL each of forward and reverse primers (final concentration of 2.5 μM). Data were analyzed from three independent runs using mixed model analysis of variance (PROC MIXED) (SAS 2008). If means were significantly different, they were separated using a LSD test (α = 0.05). Relative quantification (RQ) of gene expression above or below that of controls is reported.
TABLE 1.
Primer sequences (5′→3′) for selected and internal control genes used to measure gene expression in Myzus persicae exposed to sublethal concentrations of imidacloprid.
| Symbol | Forward sequence | Reverse sequence | Accession# / reference | size (bp) |
|---|---|---|---|---|
| ANT | GCCGGTAATTTAGCATCAGG | CCTTGGACAAACAGTCTCCA | DQ407505 | 151 |
| OSD | TCCCGAAGGAGCTGAACTTA | GCTTAGGGTCCCATTTGTCA | AJ634652 | 164 |
| TOL | AGCGCTTTCTGACGGAAATA | AGCATTCGAAGAAGCGATTG | EB714328 | 177 |
| FPPS I | CGAACAGGCCATTTACCAGT | GACCCATCGCAGTTTTCATT | EU334430 | 107 |
| Hsp60 | AGCATTGACCATGCCATGTA | AAACATCGGTCATTGCATCA | AJ250348 | 122 |
| β-actin | GGTGTCTCACACACAGTGCC | CGGCGGTGGTGGTGAAGCTG | (Puinean et al. 2010) | 90–120 |
| Ace | TAACGTAGTAGTGCCAAAGC | CACTGTAGAGCCATTAGCTG | (Puinean et al. 2010) | 90–120 |
For all the time points, gene expression data was subjected to dose-response modeling (Ayyanath et al. 2013; Cedergreen et al. 2005). Fecundity responses of G0 and G1 adults previously reported (Ayyanath et al. 2013) were correlated/regressed against gene expression in G0 and G1 adults found in the present study using R statistical software with addon package drc (http://www.bioassay.dk).
Global DNA methylation analysis
Samples from aphids were collected as described above, and genomic DNA was isolated using a DNeasy® Blood and Tissue Kit (Qiagen, Toronto, Ontario). From aphids of each concentration and time point, 500 ng of gDNA was digested, using Nuclease P (Sigma-Aldrich, Oakville, Ontario), and phosphorylated, using alkaline phosphatase (Sigma-Aldrich, Oakville, Ontario), to examine global DNA methylation changes using a DNA Methylation EIA™ kit (Cayman Chemical, Ann Arbor, Michigan). A standard curve with r2 > 0.9 was obtained using the kit standard. Data analysis was performed as per the manufacturer instructions after calculating the amount of methylated DNA based on the obtained equation from the standard curve. Relative methylation of DNA to controls was analyzed using PROC MIXED (SAS 2008), with means separations done using a LSD test (α = 0.05).
RESULTS
Gene expression analyses
Continuous exposure to sublethal concentrations of imidacloprid resulted in up- and down-regulation of the genes analyzed, with intra- and inter-generational differences observed (Table 2). Gene expression in treatments is reported as relative quantity (RQ) to that in control aphids and a 2-fold up- or down- regulation was considered as biologically significant (Ghanim et al. 2006).
TABLE 2.
P-values for a two generation (G0, G1) experiment examining effects on gene expression (up- or down-regulation) and global DNA methylation during imidacloprid-induced hormesis in second instar (N) and adult (A) Myzus persicae. Significant treatment effects were subjected to a least square means separation (LSD, α = 0.05) and reported graphically.
| Source of Variation | Generation-time point | |||
|---|---|---|---|---|
| G0-N | G0-A | G1-N | G1-A | |
| Hsp60 gene | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| FPPS I gene | 0.0004 | 0.0001 | 0.0023 | 0.0001 |
| OSD gene | 0.0035 | 0.0001 | 0.0001 | 0.0001 |
| TOL gene | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| ANT gene | 0.0469 | 0.0002 | 0.0001 | 0.0001 |
| Global DNA methylation | 0.0002 | 0.0001 | 0.0040 | 0.0131 |
Models attempting to describe gene expression against concentration did not fit (f > 0.1) for any of the studied genes at imidacloprid concentrations that induced hormesis. A weak relationship was found for OSD (R2 < 0.325; P = 0.0130) and TOL (R2 < 0.312; P = 0.0126) gene regulation and G1 adult fecundity. All other correlations and regressions were not significant.
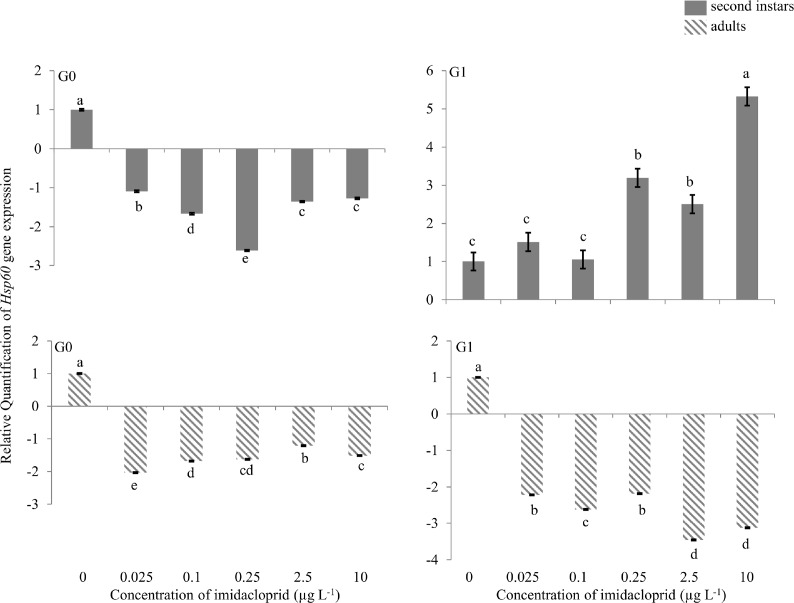
Hsp60 gene
In the first generation (G0), there was 2.5-fold down-regulation of Hsp60 in second instars exposed to 0.25 μg a.i. L−1. In G0 adults, 2.0-fold down-regulation was found in the 0.025 μg a.i. L−1 treatment, and less down-regulation at higher concentrations (Figure 1). In G1, there was 3.2-, 2.5- and 5.3-fold up-regulation of the Hsp60 gene in second instars at 0.25, 2.5 and 10 μg a.i. L−1, respectively. Two- to 3.3-fold down-regulation of the gene was observed at all concentrations in G1 adults.
FIGURE 1.
Heat shock protein (Hsp) 60 gene expression during imidacloprid-induced hormesis in Myzus persicae. Test aphids representing second instars and adults from initial (G0) and succeeding (G1) generations were used for down-stream analyses. Error bars represent standard error of means. For a given time point, bars with different letters above or below them are significantly different (P ≤ 0.05, LSD test).
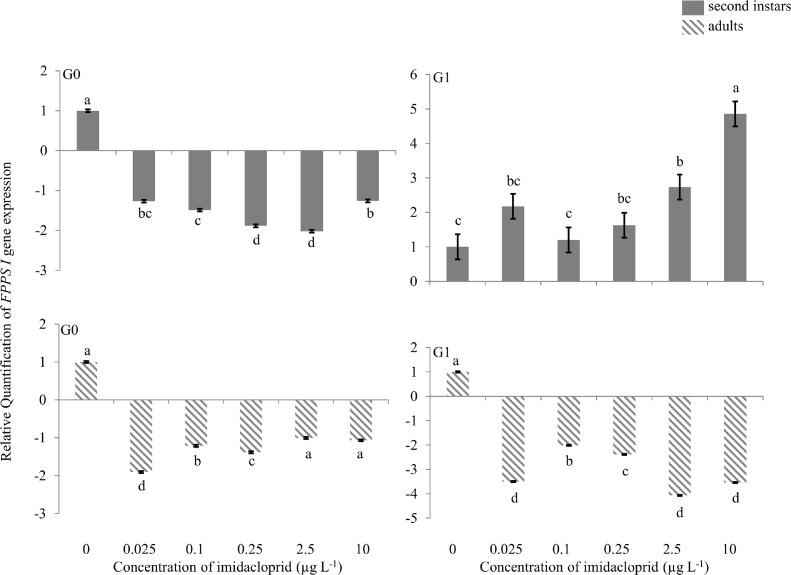
FPPS I gene
Approximately 2.0-fold down regulation of the FPPS I gene was observed in G0 second instars exposed to 0.25 and 2.5 μg a.i. L−1 imidacloprid (Figure 2), and in G0 adults from the 0.025 μg a.i. L−1 treatment. At other imidacloprid concentrations changes in FPPS I gene expression was relatively minor in G0. G1 second instars from the 0.025, 2.5 and 10 μg a.i. L−1 treatments had 2.2-, 2.7 and 5.0-fold up-regulation, respectively, of the FPPS I gene. In G1 adults, ∼2.0- to 5.0-fold down-regulation was observed across the range of imidacloprid treatments.
FIGURE 2.
Farnesyl diphosphate synthase (FPPS I) gene expression during imidacloprid-induced hormesis in Myzus persicae. Test aphids representing second instar nymphs and adults from initial (G0) and succeeding (G1) generations were used for down-stream analyses. Error bars represent standard error of means. For a given time point, bars with different letters above or below them are significantly different (P ≤ 0.05, LSD test).
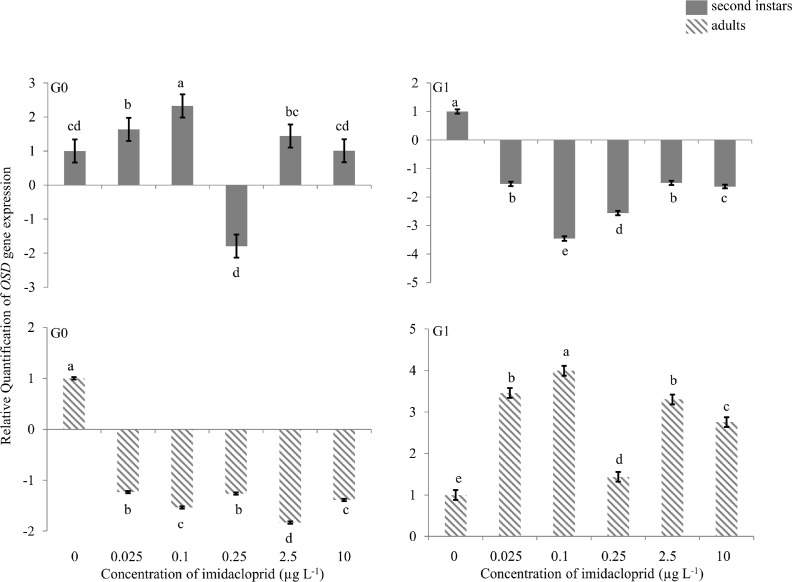
OSD gene
When G0 first instars were exposed to 0.1 μg imidacloprid L−1, 2.3 fold up-regulation of the OSD gene was observed in G0 second instars, and at 0.25 μg a.i. L−1 ∼2.0-fold down-regulation was observed (Figure 3). Exposure of G0 nymphs to other concentrations did not change the expression of the OSD gene. In G0 adults, only at 2.5 μg a.i. L−1 was a change in gene expression observed, with ∼2.0-fold down-regulation. G1 second instars exposed to 0.1 and 0.25 μg a.i. L−1, had 3.3- and 2.5-fold down-regulation, respectively. Other concentrations did not alter OSD gene expression at that time point. All concentrations except 0.25 μg a.i. L−1 up-regulated OSD gene expression in G1 adults; 3.5-, 4.0-, 3.3- and 2.8-fold up-regulation of the gene was observed at 0.025, 0.1, 2.5 and 10 μg a.i. L−1, respectively.
FIGURE 3.
Olfactory Segment-D (OSD) gene expression during imidacloprid-induced hormesis in Myzus persicae. Test aphids representing second instar nymphs and adults from initial (G0) and succeeding (G1) generations were used for down-stream analyses. Error bars represent standard error of means. For a given time point, bars with different letters above or below them are significantly different (P ≤ 0.05, LSD test).
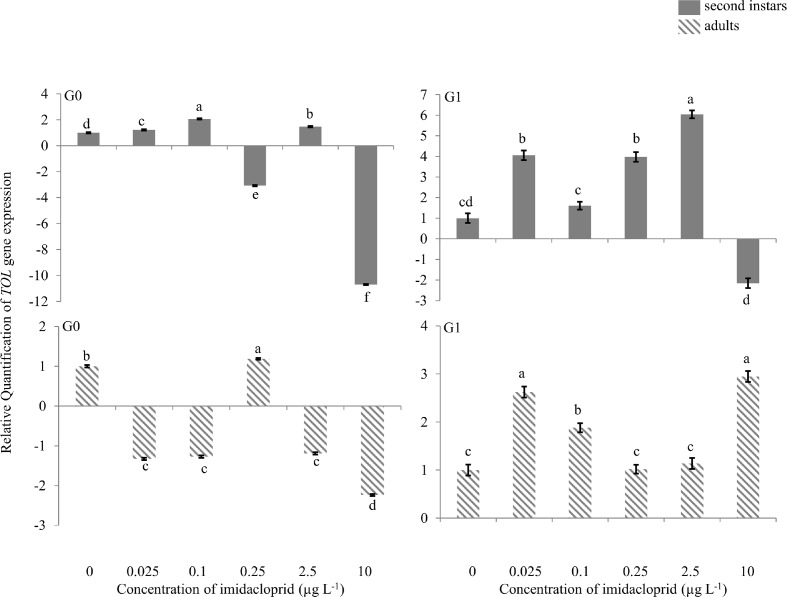
TOL gene
There was 2.0-fold up-regulation of the TOL gene in G0 second instars exposed to 0.1 μg a.i. L−1 imidacloprid (Figure 4), and 3.3- and 10.0-fold down-regulation at 0.25 and 10 μg a.i. L−1, respectively. In G0 adults, a change in TOL gene expression (2.0-fold down-regulation) was only observed at 10 μg a.i. L−1. In G1 second instars, there was 4.0-, 4.0-and 6.0 fold up-regulation at 0.025, 0.25 and 2.5 μg a.i. L−1, respectively, and 2.0-fold down-regulation at 10 μg a.i. L−1. In G1 adults, 2.6- and 3.0-fold up-regulation of the TOL gene was observed at 0.025 and 10 μg a.i. L−1 of imidacloprid.
FIGURE 4.
Take-out like (TOL) gene expression during imidacloprid-induced hormesis in Myzus persicae. Test aphids representing second instar nymphs and adults from initial (G0) and succeeding (G1) generations were used for down-stream analyses. Error bars represent standard error of means. For a given time point, bars with different letters above or below them are significantly different (P ≤ 0.05, LSD test).
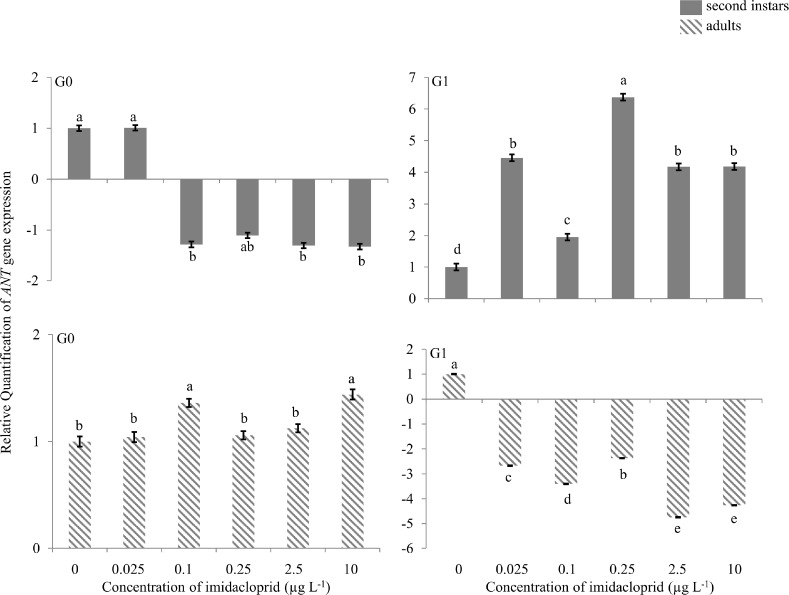
ANT gene
There was no change in expression of the ANT gene in G0 nymphs or adults (Figure 5). In G1 second instars, there was 4.5-, 2.0-, 6.4-, 4.2- and 4.2-fold up-regulation at 0.025, 0.1, 0.25, 2.5 and 10 μg a.i. L−1. In G1 adults, 2.5- to 5.0-fold down-regulation was observed at all imidacloprid exposure concentrations.
FIGURE 5.
Adenosine nucleotide translocase (ANT) gene expression during imidacloprid-induced hormesis in Myzus persicae. Test aphids representing second instar nymphs and adults from initial (G0) and succeeding (G1) generations were used for down-stream analyses. Error bars represent standard error of means. For a given time point, bars with different letters above or below them are significantly different (P ≤ 0.05, LSD test).
Global DNA methylation analysis
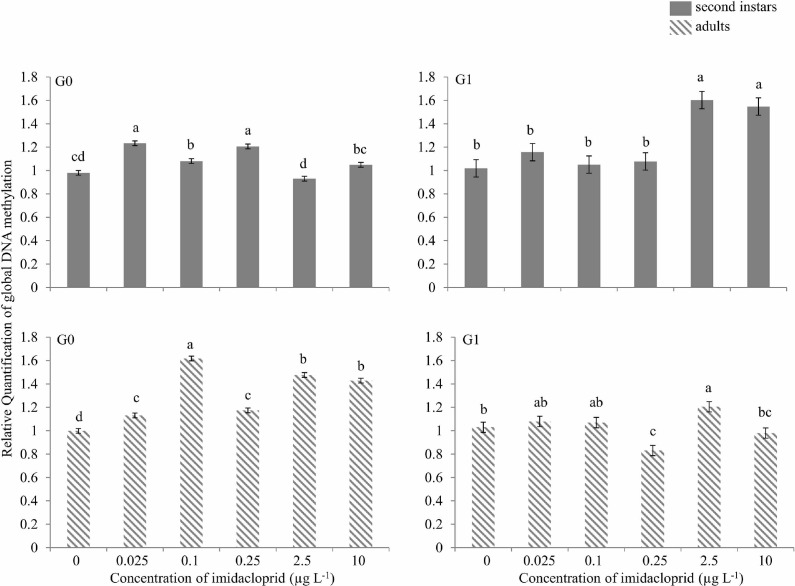
When first instar M. persicae were exposed to sublethal concentrations of imidacloprid and G0 second instars were analyzed for global DNA methylation, approximately 25, 10 and 20% hypermethylation above the control was observed at 0.025, 0.1 and 0.25 μg a.i. L−1, respectively, and about 10% hypomethylation was observed at 2.5 μg a.i. L−1 (Figure 6). No change in methylation was detected at 10 μg a.i. L−1. In G0 adults, 10–60% hypermethylation was seen at various imidacloprid concentrations. In G1 second instars, significant hypermethylation in the order of 50–60% above the control was found only at 2.5 and 10 μg a.i. L−1. In G1 adults, hypomethylation of 25% and 10% occurred at 0.25 and 10 μg a.i. L−1, respectively, while 10% hypermethylation was observed at 2.5 μg a.i. L−1. Other concentrations did not show any change in global DNA methylation relative to control (Figure 6).
FIGURE 6.
Global DNA methylation during imidacloprid-induced hormesis in Myzus persicae. Test aphids representing second instar nymphs and adults from initial (G0) and succeeding (G1) generations were used for down-stream analyses. Error bars represent standard error of means. For a given time point, bars with different letters above them are significantly different (P ≤ 0.05, LSD test).
DISCUSSION
We previously reported intra- and inter-generational differences in reproduction hormesis when green peach aphids were exposed to low doses of imidacloprid (Ayyanath et al. 2013). Using the same insect, insecticide, and doses, here we show that up- or down-regulation of stress, mitochondrial function, dispersal, and developmental genes occurs when first instar M. persicae are continuously exposed for two generations to sublethal concentrations of imidacloprid. In multiple instances, there was evidence of up-regulation of genes in one life stage or generation that was countered by down-regulation of the same genes in subsequent life stages or generations.
Hsp70 expression has been associated with reduced fecundity/reproduction in L. huidobrensis (Huang et al. 2007), Drosophila melanogaster (Hercus et al. 2003), Trialeurodes vaporariorum (Westwood), and Bemisia tabaci (Gennadius) (Cui et al. 2008). This is possibly attributable to accumulation of Hsp and associated traits (thermo-tolerance) at a cost of impaired fecundity. In this study, continuous exposure of M. persicae to sublethal concentrations of imidacloprid previously found to stimulate reproduction had no effect or resulted in down-regulation of the Hsp60 gene in second instars and adults of the initial generation, followed by up-regulation in second instars of the succeeding generation. Hsp expression has been shown to vary depending on the stressor type (Sanders et al. 1995; Veldhuizentsoerkan et al. 1991) and scenario of exposure to a stressor (Helmcke and Aschner 2010).
Down-regulation of Hsp expression in insects not well understood (Mahroof et al. 2005). Alternation of up- and down-regulation occurred across generations and between nymphs and adults within a generation in the present study. For example, up-regulation of Hsp expression in G1 second instars was followed by down-regulation in adults. Similar alternations were found in Hsp70 expression in egg, larva, pupa, and adult of T. castaneum exposed to mild heat stress (Mahroof et al. 2005). Stimulated fecundity in M. persicae exposed to hormetic concentrations of imidacloprid (Ayyanath et al. 2013) could be due to down-regulation of Hsp gene in G0 and G1. Down-regulation of Hsp90 and Hsp70 was reported in rat brains exposed to various concentrations of DDT (Shutoh et al. 2009) and bronchial cancer in smokers with chronic obstructive pulmonary disease (Cappello et al. 2006). In these studies, down-regulation of Hsp expression was suggested to be a recovery response to regain homeostasis following chronic exposure to mild levels of stress. Reduced Hsp70 expression can also be associated with short-term stress, as seen when C. tentanus recovers from exposure to heat shock (Karouna-Renier and Zehr 1999).
Up-regulation of Hsp60 follows an accumulation of unfolded (damaged) proteins resulting from stress or injury in an organism (Parsell and Lindquist 1993), and accumulation of Hsp often results in decreased fecundity (Huang et al. 2007). Hsp60 up-regulation was observed in M. persicae G1 nymphs exposed to higher sublethal concentrations of imidacloprid (0.25–10 μg a.i. L−1), and reduced reproductive outputs were previously seen in G1 aphids exposed to these imidacloprid concentrations. Due to overcompensation following insecticide exposure in G0, the insect probably became conditioned to higher insecticide concentrations in G1 by elevating expression of Hsp60. Alternatively, accumulation of damaged proteins triggered the up-regulation of the gene.
Fecundity, reproduction, and metamorphosis in insects is controlled by JH (Dawson et al. 1987; Hartfelder 2000; Verma 1981). In aphids, the FPPS I gene is an important enzyme regulator and its down-regulation may increase JH titers in females (Keeling et al. 2004). It catalyzes formation of farnesyl diphosphate (FPP), a precursor needed in biosynthesis of JH, alarm pheromones and sex pheromones (Dawson et al. 1987; Vandermoten et al. 2009). JH is known to stimulate reproduction in M. persicae and elevated JH titers in maternal aphids inhibit wing development and promote development of apterous forms (Tamaki 1973; Verma 1981). In pea aphid, Acrythosiphon pisum, the mother that perceives stress cues such as crowding transmits the information to unborn progeny (Brisson 2010). Similarly, it is possible that G0 M. persicae adults stressed by sublethal imidacloprid exposure passed the signal to G1 nymphs, as indicated by the strong up-regulation of the FPPS I gene. This was countered (compensated) in G1 adults, as observed by down-regulation of the FPPS I gene, again coinciding with the hormetic (stress) response previously seen at this stage (Ayyanath et al. 2013).
It was previously shown that stimulated fecundity in M. persicae exposed to hormetic concentrations of imidacloprid resulted in increased JH III titers (Yu et al. 2010). This suggests that increased M. persicae fecundity and JH titers may be correlated with down-regulation of the FPPS I gene, since down-regulation of this gene was previously shown to accompany increase JH titers in an insect (Keeling et al. 2004). These deductions should be made with caution and considered only where the time points used in correlating JH titers and fecundity correspond. In the present study, down-regulation of FPPS I observed in G0 adults exposed to 0.025 μg a.i. L−1 and in G1 adults exposed to 0.025 and 0.1 μg a.i. L−1 was previously associated with higher aphid fecundity (Ayyanath et al. 2013). Significant down-regulation of FPPS I was also seen at imidacloprid concentrations that did not result in stimulated reproduction, suggesting that other genes, also needed for JH production, were not affected by the insecticide treatment.
Dispersal-related genes such as OSD, TOL and ANT are more highly expressed (2–5 fold) in alates than apterous aphids (Ghanim et al. 2006). In the present experiment, OSD, TOL and ANT gene expression varied in apterous individuals exposed continuously to sublethal hormetic concentrations of imidacloprid. Complete wing development was not observed, but at higher concentrations small wing pads appeared in some insects (data not shown).
OSD gene up-regulation has previously been shown to be inversely related to aphid fecundity (Bos et al. 2010). We found that up-regulation of the OSD gene in G0 second instar M. persicae exposed to certain imidacloprid concentrations was followed by down-regulation of the gene in G0 adults, and even greater down-regulation in G1 second instars. Immature G0 aphids stressed at certain imidacloprid concentrations that resulted in up-regulation of the OSD gene may have conditioned subsequent adults for increased reproduction. In G0 adults, OSD gene down-regulation occurred at all concentrations, possibly indicating compensation to the stress, coinciding with increased reproduction at those same concentrations (Ayyanath et al. 2013). Likewise, OSD gene down-regulation in G1 second instars occurred at concentrations of imidacloprid identical to those previously shown to elicit a hormetic reproductive response in M. persicae. Trends similar to OSD gene regulation were observed with TOL. This gene is associated with chemoreception (Fan et al. 2011; Jacobs et al. 2005; Weil et al. 2009) and circadian control of feeding behavior. TOL can be induced by starvation (Fujikawa et al. 2006; Weil et al. 2009), juvenile hormone binding proteins in response to fluctuating JH titers (Bohbot and Vogt 2005) during courtship and mating, or when regulating antennal responses to food, hosts, or pheromones (Dauwalder et al. 2002). The complexity of this gene and its involvement in multiple functions does not readily permit direct correlation with the hormetic responses previously observed (Ayyanath et al. 2013).
The ANT gene regulates mitochondrial proteins that function as carriers of important metabolites involved in a number of mitochondrial processes, mainly catalyzing exchange of ADP for ATP across inner mitochondrial membranes (Zhang et al. 1999). The unresponsiveness of the ANT gene in G0 suggests that exposure to hormetic concentrations of imidacloprid results in no additional energy expenditures for the aphids. However, as with the TOL, Hsp60 and FPPS genes, significant up-regulation of ANT occurred in G1 nymphs, indicating extra energy was expended. This suggests that even if no extra energy was expended in G0 adults, the insect can still have stimulated reproduction, with energy expenditure effects felt in offspring. Down-regulation of the ANT gene in G1 adults probably reflects the cessation of energy requirements and or other regulatory process (up-regulation in second instar) during the development phase in G0 nymphs.
Recent experiments indicate that environmental fluctuations can induce specific and predictable epigenetic-related molecular changes (Vaiserman 2012). It is unclear if insecticide-induced hormesis in insects is an epigenetic process, resulting in heritable changes in gene expression (Glastad et al. 2011; Gressel 2011). DNA methylation is an important epigenetic mechanism for regulation of gene expression. In insects, DNA methylation is thought to play a role in developmental responsiveness to environmental factors and may provide critical contributions to insect developmental and phenotypic variation (Bass and Field 2011; Glastad et al. 2011; Gressel 2011). Increases in hypermethylation, typically reduce DNA transcription and usually result in inactivation of genes, although this is not always the case (Suzuki and Bird 2008). Concurrent amplification and methylation of an esterase gene in greenbug, Schizaphis graminum (Rondani), suggested heritability rendering increased resistance to organophosphorus insecticides (Ono et al. 1999). Similarly, in M. persicae, the E4 gene is important in expression/amplification of insecticide-detoxifying esterases, and reduced DNA methylation in this insect coincides with a loss of E4 gene expression (Field et al. 2004; Hick et al. 1996). In the present study, methylation occurred initially in G0 nymphs and adults, and in at only high imidacloprid concentrations G1 nymphs. This was followed by an absence of methylation at any concentration in G1 adults, possibly indicating a heritable adaptation to the sub-NOAEC concentrations of imidacloprid we tested. This suggests that trans-generationally the insect would be able to cope with higher concentrations, which has repercussions for resistance development (Gressel 2011).
Overall, there are complex patterns of gene expression across generations in insects exposed to hormetic concentrations of insecticide. We interpret the up- or down-regulation of Hsp60, FPPS I, OSD, TOL and ANT genes in G0 to be a priming response (Costantini et al. 2010) in preparation for further adverse conditions, vis a vis insecticide exposure. Imidacloprid resistance development in insects via mutations has been reported in the past (Bass and Field 2011; Wen et al. 2009) and insecticide resistance is associated with amplification and methylation of esterase genes (Field 2000; Field et al. 2004; Ono et al. 1999). We speculate that insecticide-induced hormesis might serve as a precursor to insecticide tolerance and ultimately resistance (Gressel 2011). Further investigation is needed in this area to fully comprehend how insect response to low levels of stress in the form of hormesis relates to induction of detoxification enzymes and methylation changes across generations.
Acknowledgments
Financial support for this project was through the Ontario Graduate Scholarship Program and several University of Guelph internal scholarship donors (scholarships to M-M.A), the Natural Sciences and Engineering Research Council of Canada (NSERC Discovery Grants to G.C.C. and B.P.), and the Canada Foundation for Innovation (Leaders Opportunity Funds to G.C.C. and B.P.)
REFERENCES
- Ayyanath M-M, Cutler G, Scott-Dupree C, Sibley P. Transgenerational shifts in reproduction hormesis in green peach aphid exposed to low concentrations of imidacloprid. PLoS One. 2013;8:e74532. doi: 10.1371/journal.pone.0074532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass C, Field LM. Gene amplification and insecticide resistance. Pest Manag Sci. 2011;67:886–890. doi: 10.1002/ps.2189. [DOI] [PubMed] [Google Scholar]
- Bohbot J, Vogt RG. Antennal expressed genes of the yellow fever mosquito (Aedes aegypti L.); characterization of odorant-binding protein 10 and takeout. Insect Biochem Mol Biol. 2005;35:961–979. doi: 10.1016/j.ibmb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Bos JIB, Prince D, Pitino M, Maffei ME, Win J, Hogenhout SA. A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid) PLoS Genet. 2010;6:e1001216. doi: 10.1371/journal.pgen.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braendle C, Davis GK, Brisson JA, Stern DL. Wing dimorphism in aphids. Heredity. 2006;97:192–199. doi: 10.1038/sj.hdy.6800863. [DOI] [PubMed] [Google Scholar]
- Brisson JA. Aphid wing dimorphisms: linking environmental and genetic control of trait variation. Phil Trans R Soc B. 2010;365:605–616. doi: 10.1098/rstb.2009.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Defining hormesis. Hum Exp Toxicol. 2002;21:91–97. doi: 10.1191/0960327102ht217oa. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Toxicology rethinks its central belief. Nature. 2003;421:691–692. doi: 10.1038/421691a. [DOI] [PubMed] [Google Scholar]
- Cappello F, Di Stefano A, David S, Rappa F, Anzalone R, La Rocca G, D’Anna SE, Magno F, Donner CF, Balbi B, Zummo G. HSP60 and HSP10 down-regulation predicts bronchial epithelial carcinogenesis in smokers with chronic obstructive pulmonary disease. Cancer. 2006;107:2417–2424. doi: 10.1002/cncr.22265. [DOI] [PubMed] [Google Scholar]
- Cedergreen N, Ritz C, Streibig JC. Improved empirical models describing hormesis. Environ Toxicol Chem. 2005;24:3166–3172. doi: 10.1897/05-014r.1. [DOI] [PubMed] [Google Scholar]
- Costantini D, Metcalfe NB, Monaghan P. Ecological processes in a hormetic framework. Ecol Lett. 2010;13:1435–1447. doi: 10.1111/j.1461-0248.2010.01531.x. [DOI] [PubMed] [Google Scholar]
- Cui X, Wan F, Xie M, Liu T. Effects of heat shock on survival and reproduction of two whitefly species, Trialeurodes vaporariorum and Bemisia tabaci biotype B. J Insect Sci. 2008;8:1–10. [Google Scholar]
- Cusson M, Beliveau C, Sen SE, Vandermoten S, Rutledge RG, Stewart D, Francis F, Haubruge E, Rehse P, Huggins DJ, Dowling APG, Grant GH. Characterization and tissue-specific expression of two lepidopteran farnesyl diphosphate synthase homologs: Implications for the biosynthesis of ethyl-substituted juvenile hormones. Proteins. 2006;65:742–758. doi: 10.1002/prot.21057. [DOI] [PubMed] [Google Scholar]
- Cutler GC. Insects, insecticides and hormesis: Evidence and considerations for study. Dose-Response. 2013;11:154–177. doi: 10.2203/dose-response.12-008.Cutler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwalder B, Tsujimoto S, Moss J, Mattox W. The Drosophila takeout gene is regulated by the somatic sex-determination pathway and affects male courtship behavior. Genes Dev. 2002;16:2879–2892. doi: 10.1101/gad.1010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson GW, Griffiths DC, Janes NF, Mudd A, Pickett JA, Wadhams LJ, Woodcock CM. Identification of an aphid sex-pheromone. Nature. 1987;325:614–616. [Google Scholar]
- de la Paz Celorio-Mancera M, Ahn S-J, Vogel H, Heckel DG. Transcriptional responses underlying the hormetic and detrimental effects of the plant secondary metabolite gossypol on the generalist herbivore Helicoverpa armigera. BMC Genomics. 2011;12:575. doi: 10.1186/1471-2164-12-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Francis F, Liu Y, Chen JL, Cheng DF. An overview of odorant-binding protein functions in insect peripheral olfactory reception. Genet Mol Res. 2011;10:3056–3069. doi: 10.4238/2011.December.8.2. [DOI] [PubMed] [Google Scholar]
- Field LM. Methylation and expression of amplified esterase genes in the aphid Myzus persicae (Sulzer) Biochem J. 2000;349:863–868. doi: 10.1042/bj3490863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field LM, Lyko F, Mandrioli M, Prantera G. DNA methylation in insects. Insect Mol Biol. 2004;13:109–115. doi: 10.1111/j.0962-1075.2004.00470.x. [DOI] [PubMed] [Google Scholar]
- Figueroa CC, Prunier-Leterme N, Rispe C, Sepulveda F, Fuentes-Contreras E, Sabater-Munoz B, Simon JC, Tagu D. Annotated expressed sequence tags and xenobiotic detoxification in the aphid Myzus persicae (Sulzer) Insect Sci. 2007;14:29–45. [Google Scholar]
- Fujikawa K, Seno K, Ozaki M. A novel Takeout-like protein expressed in the taste and olfactory organs of the blowfly, Phormia regina. FEBS J. 2006;273:4311–4321. doi: 10.1111/j.1742-4658.2006.05422.x. [DOI] [PubMed] [Google Scholar]
- Ghanim M, Dombrovsky A, Raccag B, Shrerman A. A microarray approach identifies ANT, OS-D and takeout-like genes as differentially regulated in alate and apterous morphs of the green peach aphid Myzus persicae (Sulzer) Insect Biochem Mol Biol. 2006;36:857–868. doi: 10.1016/j.ibmb.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Glastad KM, Hunt BG, Yi SV, Goodisman MAD. DNA methylation in insects: on the brink of the epigenomic era. Insect Mol Biol. 2011;20:553–565. doi: 10.1111/j.1365-2583.2011.01092.x. [DOI] [PubMed] [Google Scholar]
- Gressel J. Low pesticide rates may hasten the evolution of resistance by increasing mutation frequencies. Pest Manag Sci. 2011;67:253–257. doi: 10.1002/ps.2071. [DOI] [PubMed] [Google Scholar]
- Guedes RNC, Cutler GC. Insecticide-induced hormesis and arthropod pest management. Pest Manag Sci. 2013 doi: 10.1002/ps.3669. in press. doi: 101002/ps3669. [DOI] [PubMed] [Google Scholar]
- Hartfelder K. Insect juvenile hormone: from “status quo” to high society. Braz J Med Biol Res. 2000;33:157–177. doi: 10.1590/s0100-879x2000000200003. [DOI] [PubMed] [Google Scholar]
- Helmcke KJ, Aschner M. Hormetic effect of methylmercury on Caenorhabditis elegans. Toxicol Appl Pharmacol. 2010;248:156–164. doi: 10.1016/j.taap.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercus MJ, Loeschcke V, Rattan SIS. Lifespan extension of Drosophila melanogaster through hormesis by repeated mild heat stress. Biogerontology. 2003;4:149–156. doi: 10.1023/a:1024197806855. [DOI] [PubMed] [Google Scholar]
- Hick CA, Field LM, Devonshire AL. Changes in the methylation of amplified esterase DNA during loss and reselection of insecticide resistance in peach-potato aphids, Myzus persicae. Insect Biochem Mol Biol. 1996;26:41–47. doi: 10.1016/0965-1748(95)00059-3. [DOI] [PubMed] [Google Scholar]
- Huang L-H, Chen B, Kang L. Impact of mild temperature hardening on thermo tolerance, fecundity, and Hsp gene expression in Liriomyza huidobrensis. J Insect Physiol. 2007;53:1199–1205. doi: 10.1016/j.jinsphys.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Jacobs SP, Liggins AP, Zhou JJ, Pickett JA, Jin X, Field LM. OS-D-like genes and their expression in aphids (Hemiptera : Aphididae) Insect Mol Biol. 2005;14:423–432. doi: 10.1111/j.1365-2583.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- Karouna-Renier NK, Zehr JP. Ecological implications of molecular biomarkers: assaying sub-lethal stress in the midge Chironomus tentans using heat shock protein 70 (HSP-70) expression. Hydrobiologia. 1999;401:255–264. [Google Scholar]
- Keeling CI, Blomquist GJ, Tittiger C. Coordinated gene expression for pheromone biosyn-thesis in the pine engraver beetle, Ips pini (Coleoptera : Scolytidae) Naturwissenschaften. 2004;91:324–328. doi: 10.1007/s00114-004-0523-y. [DOI] [PubMed] [Google Scholar]
- Mahroof R, Zhu KY, Subramanyam B. Changes in expression of heat shock proteins in Tribolium castaneum (Coleoptera: Tenebrionidae) in relation to developmental stage, exposure time and temperature. Ann Entomol Soc Am. 2005;98:100–107. [Google Scholar]
- Mukherjee SN, Rawal SK, Ghumare SS, Sharma RN. Hormetic concentrations of azadirachtin and isoesterase profiles in Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) Experientia. 1993;49:557–560. [Google Scholar]
- Ono M, Swanson JJ, Field LM, Devonshire AL, Siegfried BD. Amplification and methylation of an esterase gene associated with insecticide-resistance in greenbugs, Schizaphis graminum (Rondani) (Homoptera: Aphididae) Insect Biochem Mol Biol. 1999;29:1065–1073. doi: 10.1016/s0965-1748(99)00082-x. [DOI] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puinean AM, Foster SP, Oliphant L, Denholm I, Field LM, Millar NS, Williamson MS, Bass C. Amplification of a cytochrome P450 gene is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. PLoS Genet. 2010;6:e1000999. doi: 10.1371/journal.pgen.1000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JS, Wilson ACC, de Vos M, Sun Q, Tamborindeguy C, Winfield A, Malloch G, Smith DM, Fenton B, Gray SM, Jander G. Genomic resources for Myzus persicae: EST sequencing, SNP identification, and microarray design. BMC Genomics. 2007;8:423. doi: 10.1186/1471-2164-8-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci M, Stecher D, Henneberry T. Heat shock proteins in whiteflies, an insect that accumulates sorbitol in response to heat stress. J Therm Biol. 2000;25:363–371. doi: 10.1016/s0306-4565(99)00108-4. [DOI] [PubMed] [Google Scholar]
- Sanders BM, Nguyen J, Martin LS, Howe SR, Coventry S. Induction and subcellular localization of two major stress proteins in response to copper in the fathead minnow Pimephales promelas. Comp Biochem Physiol C. 1995;112:335–343. doi: 10.1016/0742-8413(95)02029-2. [DOI] [PubMed] [Google Scholar]
- SAS . SAS Version 9.2. SAS Institute Inc; Cary, NC: 2008. [Google Scholar]
- Schwartzberg EG, Kunert G, Westerlund SA, Hoffmann KH, Weisser WW. Juvenile hormone titres and winged offspring production do not correlate in the pea aphid, Acyrthosiphon pisum. J Insect Physiol. 2008;54:1332–1336. doi: 10.1016/j.jinsphys.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Shutoh Y, Takeda M, Ohtsuka R, Haishima A, Yamaguchi S, Fujie H, Komatsu Y, Maita K, Harada T. Low dose effects of dichlorodiphenyltrichloroethane (DDT) on gene transcription and DNA methylation in the hypothalamus of young male rats: implication of hormesis-like effects. J Toxicol Sci. 2009;34:469–482. doi: 10.2131/jts.34.469. [DOI] [PubMed] [Google Scholar]
- Smirnoff WA. Residual effects of Bacillus thuringiensis and chemical insecticide treatments on spruce budworm (Chroistonuera fumiferana Clemens) Crop Prot. 1983;2:225–230. [Google Scholar]
- Son TG, Cutler RG, Mattson MP, Camandola S. Transcriptional mediators of cellular hormesis. In: Mattson MP, Calebrese EJ, editors. Hormesis: A revolution in biology, toxicology and medicine. Springer; New York: 2010. pp. 69–93. [Google Scholar]
- Stanley K, Fenton B. A member of the Hsp60 gene family from the peach potato aphid, Myzus persicae (Sulzer.) Insect Mol Biol. 2000;9:211–215. doi: 10.1046/j.1365-2583.2000.00174.x. [DOI] [PubMed] [Google Scholar]
- Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9:465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- Tamaki G. Insect developmental inhibitors - effect of reduction and delay caused by juvenile-hormone mimics on production of winged migrants of Myzus persicae (Hemiptera: Aphididae) on peach trees. Can Entomol. 1973;105:761–765. [Google Scholar]
- Vaiserman AM. Hormesis and epigenetics: Is there a link? Ageing Res Rev. 2012;10:413–421. doi: 10.1016/j.arr.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Vandermoten S, Santini S, Haubruge E, Heuze F, Francis F, Brasseur R, Cusson M, Charloteaux B. Structural features conferring dual geranyl/farnesyl diphosphate synthase activity to an aphid prenyltransferase. Insect Biochem Mol Biol. 2009;39:707–716. doi: 10.1016/j.ibmb.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Veldhuizentsoerkan MB, Holwerda DA, Vandermast CA, Zandee DI. Synthesis of stress proteins under normal and heat-shock conditions in gill tissue of sea mussels (Mytilus edulis) after chronic exposure to cadmium. Comp Biochem Physiol C. 1991;100:699–706. doi: 10.1016/0742-8413(91)90063-y. [DOI] [PubMed] [Google Scholar]
- Verma K. 1981. Roles of juvenile hormone in the green peach aphid, Myzus persicae(Sulzer) (Homoptera: Aphididae) p. 124. MSc Thesis, University of British Columbia, Vancouver, BC. [Google Scholar]
- Wang XY, Yang ZQ, Shen ZR, Lu J, Xu WB. Sublethal effects of the selected insecticides on fecundity and wing dimorphism of green peach aphid (Hom: Aphididae) J Appl Entomol. 2008;132:135–142. [Google Scholar]
- Weil T, Korb J, Rehli M. Comparison of queen-specific gene expression in related lower termite species. Mol Biol Evol. 2009;26:1841–1850. doi: 10.1093/molbev/msp095. [DOI] [PubMed] [Google Scholar]
- Wen Y, Liu Z, Bao H, Han Z. Imidacloprid resistance and its mechanisms in field populations of brown planthopper, Nilaparvata lugens Stal in China. Pest Biochem Physiol. 2009;94:36–42. [Google Scholar]
- Yin X, Wang S, Tang J, Hansen D, Lurie S. Thermal conditioning of fifth-instar Cydia pomonella (Lepidoptera: Totricidae) affects HSP70 accumulation and insect mortality. Physiol Entomol. 2006;31:241–247. [Google Scholar]
- Yoshimi T, Minowa K, Karouna-Renier NK, Watanabe C, Sugaya Y, Miura T. Activation of a stress-induced gene by insecticides in the midge, Chironomus yoshimatsui. J Biochem Mol Toxicol. 2002;16:10–17. doi: 10.1002/jbt.10018. [DOI] [PubMed] [Google Scholar]
- Yu Y, Shen G, Zhu H, Lu Y. Imidacloprid-induced hormesis on the fecundity and juvenile hormone levels of the green peach aphid Myzus persicae (Sulzer) Pest Biochem Physiol. 2010;98:238–242. [Google Scholar]
- Zhang YL, Li Z. Two different farnesyl diphosphate synthase genes exist in the genome of the green peach aphid, Myzus persicae. Genome. 2008;51:501–510. doi: 10.1139/G08-037. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Roote J, Brogna S, Davis AW, Barbash DA, Nash D, Ashburner M. Stress sensitive B encodes an adenine nucleotide translocase in Drosophila melanogaster. Genetics. 1999;153:891–903. doi: 10.1093/genetics/153.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]