Abstract
While contradictory reports are available on the yield of dicentric chromosomes (DC) in blood samples stored at different temperature and stimulated to enter into cell cycle, various times gap followed by exposure, limited information is available on the micronucleus (MN) assay. As scoring the micronuclei frequency from the blood lymphocytes of exposed individuals is an alternative to the gold standard DC assay for triage applications, we examined radiation induced MN yield in delayed mitogenic stimulation after irradiation of in vitro. Peripheral blood lymphocytes (PBL) were exposed to low LET (60Co) radiation dose (0.1 to 5Gy) and incubated at 37°C for 2, 6 and 24 hours. The MN frequency obtained in blood samples stimulated 2 hours post-irradiation showed a dose dependent increase and used to construct the dose-response curve. Further, the results also showed that blood samples stimulated twenty four hours of post-irradiation, a significant reduction (p<0.05) in MN frequencies were obtained when compared to that of blood samples stimulated two hours and six hours after post-irradiation (0.5, 1, 3 and 5Gy). The observed result suggests that the prolonged PBL storage without mitogenic stimulation could lead to interphase cell death and a delayed blood sampling could results in underestimation of dose in biological dosimetry.
Keywords: Micronuclei, Mitogenic stimulation, Peripheral blood lymphocytes, NDI, 60Co
1. INTRODUCTION
Human peripheral blood lymphocytes (PBL) is a readily available sample which makes it simple and easy to assess the extent of cytogenetic damages caused by genotoxic stress or radiation exposures in particular for biodosimetry. Estimation of absorbed dose in exposed individuals is done by comparing the yield of chromosomal aberrations to the already established in vitro dose response curve generated by exposing the blood samples to gamma or X-irradiations using dicentric chromosomal (DC) assay (IAEA 2001). DC assay is the current gold standard assay in use for biodosimetry and is adopted at all reference biodosimetry laboratories. The analysis process requires skilled personnel and is inherently time consuming due to which a laboratory cannot handle more than few subjects during radiation emergencies. Cytokinesis blocked micronucleus (CBMN) assay is an alternative assay used for radiation biodosimetry (IAEA 2001). Recent reports from various laboratories have demonstrated its potential applicability for dose assessment. CBMN assay is now being considered as an attractive tool for triage biodosimetry, because of its advantages such as simplicity of scoring, accuracy, and most importantly ease of automation using microscopy and flow cytometry (Hayashi et al 2000; Bryce et al 2008; Willems et al 2010). Moreover, now a days the protocols are standardized in laboratories present all over the world by conducting an inter lab comparison, to minimize variations, by sharing of workloads and by conducting validations between the labs (Garcia et al 1995; Fenech et al 2003; Yoshida et al 2007; Beinke et al 2011). Majority of the laboratories have an established in vitro dose response curve, generated by irradiation of blood samples at 37°C and initiation of cultures one or two hour of post-irradiation.(Sreedevi and Rao 1994; Koksal et al 1996; Solomon et al 1997; Paillole and Voisin 1998). In case of radiation accidents, the chances of exposures to large population is unavoidable; hence, for medical management and triage biodosimetry applications, the blood samples from exposed individuals has to be transported to distant biodosimetry labs. In such situations, blood samples while being transported could be exposed to a wide range of temperature variations and also there may exist a delay of sampling and culture initiation that can influences the yield of aberrations and dose estimation. It has been reported that the yield of DC depends on temperature, time (Virsik-Peuckert and Harder 1986) and storage of exposed blood at refrigerated conditions or 20°C (48 hours) leads to the accumulation of damages (Moroni et al 2008). Factors such as prolonged culture time with PHA, delayed mitogenic stimulation and late arising first division metaphase after exposure of human PBL in G0 influenced presence of increased chromosomal aberrations and MN frequency (Holmberg et al 1998; Hoffmann et al 2002; Krishnaja and Sharma 2006). Contrarily, it was reported that exposed blood maintained at 22°C or 5°C, did not show any changes in the yield of MN (Lee et al 1999). Thus there is no consistency and conflicting results have been reported on the influences of temperature and PHA stimulation on the yield of MN. Therefore, in this present study an in vitro dose response curve was constructed for MN aberrations, analysed in PBL in two hours of post-exposure to gamma radiation initiated cultures; further the MN frequency was examined in blood samples stored at 37°C for six and twenty four hours of post irradiation to evaluate the influences of storage time on the yield of MN frequency and to relate delayed blood sampling for dosimetry applications.
2. MATERIALS AND METHODS
2.1. In vitro irradiation
The study was approved by the Institutional Ethics Committee (Ref no: IEC-N1/09/OCT/12/26). About 10 ml of heparinized peripheral blood was collected from three healthy volunteers (2 male and 1 female) aged 21, 26 and 38yrs respectively, with informed consent and aliquoted into vials containing one ml each. The aliquots of blood samples were exposed to a range of doses (0.1 to 5Gy) of gamma radiation (60Co) at a dose rate of 0.7Gy/min and a vial of unexposed blood was kept as control. The exposed and unexposed blood was incubated at 37°C for 2 hours, and used to initiate culture for MN assay and to construct the in vitro dose response curve. In parallel, another set of cultures were initiated six and twenty four hours post-irradiation to examine the MN yield due to delayed mitogenic stimulation of exposed samples. All the irradiation was carried out at 37°C ± 2°C in the teletherapy unit available at INMAS (Bhabhatron II, Panacea Med. Tech. Pvt. Ltd., Bangalore, India). The sample irradiation was performed by authorized person and dose rate was calibrated using ionization chamber as a part of routine quality procedure of the Institute.
2.2. CBMN assay
The exposed and unexposed blood samples were processed for culture and harvesting as described earlier (Fenech 2007) with modifications. Blood cultures were initiated using 1ml whole blood in 80% RPMI-1640(Invitrogen, USA) medium, supplemented with 20% FBS (Invitrogen, USA), 20μg/ml PHA (Invitrogen, USA) and standard antibiotics (Invitrogen, USA) (Penicillin and Streptomycin at final concentrations of 100IU/mL and 100mg/mL respectively) at 37°C in a 5% CO2 incubator (CO2 Cell, Germany). At 44 hours post incubation, Cytochalasin-B (Sigma, USA) (6μg/ml) was added aseptically and incubation was continued till 72 hours. The cells were harvested by treating with chilled hypotonic solution (0.075M KCl) and fixing with Carnoy’s fixative (5:1 methanol and acetic acid). Finally, the cells were washed twice with fixative and dropped on to clean glass slides. The slides were air-dried, stained with 8% giemsa in phosphate buffer (pH 6.8) and coded; one thousand binucleated cells were scored (40 X magnifications in Axio Imager.M2, Zeiss) for each dose per individual adopting the scoring criteria described earlier (Fenech 2007).
2.3. Construction of in vitro dose response curve
From the aberration yields, the distribution pattern in cells was ascertained by the standard ‘u’ test as described in the recent IAEA publication (IAEA 2011). Curve fitting was done with the help of the statistical software program known as “Poly Fit” developed by National Radiation Protection Board (NRPB), UK (Edwards and Dennis 1973). The essence of this program is to fit the data points by a weighted least squares method, thereby taking into account the scoring effort for each point.
2.4. Calculation of nuclear division index (NDI)
The NDI provides a measure of the proliferative status of the viable cell fraction. It was calculated by scoring 500 viable cells and then grouped into cells with 1, 2, 3 or 4 nuclei, and calculate the NDI using the formula:
| (1) |
where M1–M4 represent the number of cells with 1–4 nuclei and N is the total number of viable cells scored (excluding necrotic and apoptotic cells) (Fenech 2007).
2.5. Statistical analysis
One way ANOVA (Origin 7 software) was used to compare the MN frequencies obtained from the mean value of two donors at the different time intervals. The observed results are significant at p<0.05. The distribution of aberrations in cells was studied by the method of Papworth and adapted by Savage (1970). This was done by the standard ‘u’ test using the formula:
| (2) |
where, N is the total number of cells scored, d is the coefficient of dispersion (N-1) σ2/y, Y is the mean number of observed aberrations, σ2/y is the relative variance and Var d is the variance of d given by 2(N - 1) (1-1/NY). This method makes use of the fact that the variance (σ) equals the mean (Y) i.e., the variance divided by mean is equal to 1, so that u=0.
3. RESULTS
3.1. MN frequency obtained from the PBL exposed to gamma irradiation and cultured two hours post-irradiation
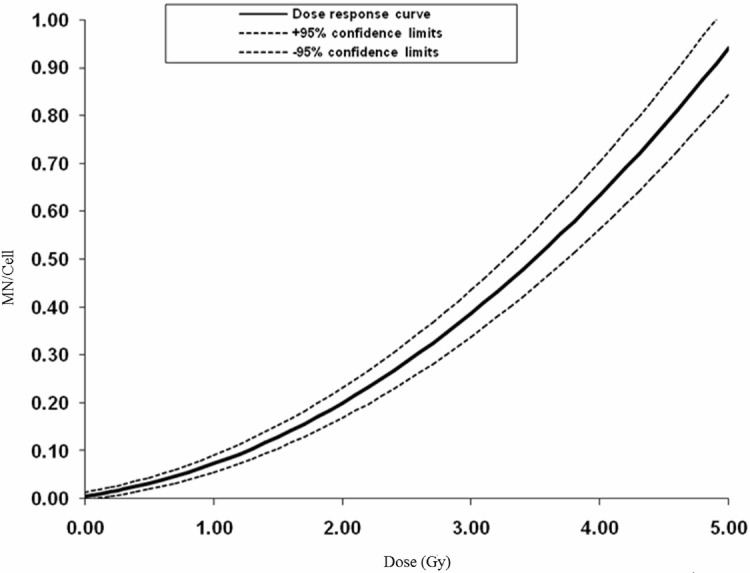
To reduce the scorer bias, the stained slides were coded by a person and the scorer was unaware of the doses. After completing the scoring the doses were disclosed. The background MN frequency obtained among the three donors was 0.004, 0.014 and 0.018 respectively; since there is no significant difference in the background frequencies and the induced MN, the data was pooled from three donors. Total number of cells scored, MN yield and its distribution pattern obtained are given in Table 1 and the dose-response curve in Fig. 1. The u value ranges from −3.75 to +12.45 and it includes over dispersion (OD) and under dispersion (UD) (Table 1). The obtained coefficients (y=0.036D+0.030D2+0.006) for the dose-response curve were comparable with the earlier published reports. The α/β values, which describe the curvature of a cell survival curve calculated for the present study was 1.2.
TABLE 1.
Distribution and frequencies of MN obtained from PBL exposed to the 60Co (γ-rays) radiation.
| Dose (Gy) | BN cells scored | MN Distribution
|
MN | MN/Cell ± SE | u | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |||||
| 0.00 | 5214 | 5179 | 34 | 1 | 0 | 0 | 0 | 0 | 36 | 0.007±0.0004 | 2.53 |
| 0.10 | 3064 | 3037 | 24 | 2 | 1 | 0 | 0 | 0 | 31 | 0.010±0.002 | 12.45 |
| 0.25 | 3123 | 3078 | 43 | 2 | 0 | 0 | 0 | 0 | 47 | 0.015±0.002 | 2.81 |
| 0.50 | 3004 | 2920 | 75 | 8 | 1 | 0 | 0 | 0 | 94 | 0.031±0.003 | 7.91 |
| 0.75 | 3095 | 2955 | 129 | 10 | 1 | 0 | 0 | 0 | 152 | 0.049±0.004 | 4.83 |
| 1.00 | 3114 | 2890 | 203 | 16 | 2 | 2 | 1 | 0 | 248 | 0.079±0.005 | 10.5 |
| 2.00 | 3011 | 2483 | 454 | 67 | 5 | 2 | 0 | 0 | 620 | 0.206±0.008 | 4.08 |
| 3.00 | 3042 | 2136 | 727 | 147 | 24 | 5 | 3 | 1 | 1132 | 0.372±0.011 | 5.70 |
| 4.00 | 3019 | 1575 | 992 | 352 | 77 | 18 | 5 | 0 | 2021 | 0.669±0.015 | 2.41 |
| 5.00 | 3000 | 1111 | 1202 | 500 | 155 | 29 | 3 | 0 | 2796 | 0.932±0.018 | −3.75 |
u-dispersion index.
FIGURE 1.
The fitted dose response curve obtained from the human PBL exposed to various doses of 60Co radiation. Pooled data of MN yields with the 95% confidence interval (CI); the 95% CI based on the raw data of the 3 donors (Table 1). Fitted curves for the 95% lower confidence limit (95% LCL) and the 95% upper confidence limit (95% UCL) were added (grey, dashed lines).
3.2. MN frequency obtained from the PBL exposed to gamma irradiation and cultured six and twenty four hours post-irradiation
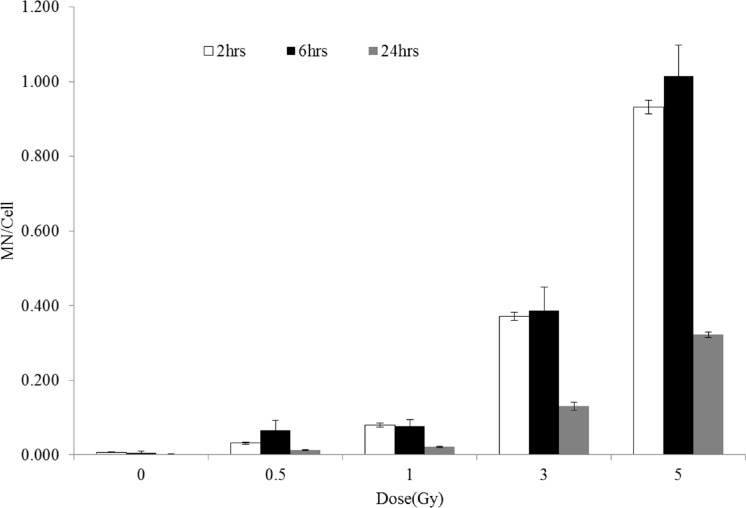
In keeping the view of delayed blood sampling or time needed to transport blood in case of accidental radiation exposure the study was extended to investigate the yield of MN in delayed mitogenic stimulated sample. The MN frequency was quantified from the PBL exposed to irradiation (0, 0.5, 1, 3 and 5Gy), incubated at 37°C for six and twenty four hours post-irradiation and cultured for MN assay. Fig. 2 shows frequencies of MN obtained from the PBL cultured after two, six and twenty four hours of post-irradiation respectively. The results shows that while, the frequencies of MN obtained from PBL cultured at two and six hours of post-irradiation did not show significant difference (p>0.05), whereas at twenty four hours, the MN frequency shows a significant decrease (p<0.05) at all the doses studied.
FIGURE 2.
The MN frequency obtained from the blood samples irradiated to 60Co gamma rays (0.5, 1, 3 and 5Gy) and culture initiated after two, six and twenty four hours. Each bar represents the mean ± SE of the MN for two independent experiments (N=2). Significance levels of twenty four hours MN yield showed P < 0.05 (*) were indicated in the test bars compared to two and six hours.
3.3. Nuclear division index in PBL exposed to gamma radiation
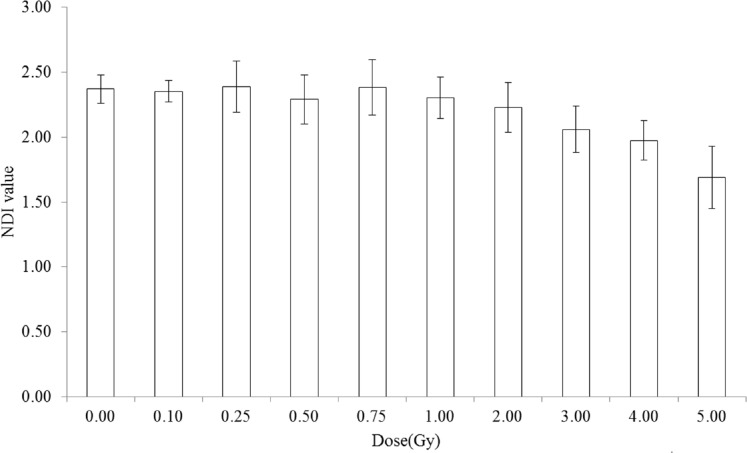
The in vitro reference dose-response curve for the MN frequency was constructed from the pooled data of yield obtained from three donors, and there was no significant difference in the yield of MN frequency at any given dose. Further to investigate if any difference on cell cycle proliferation among the donors upon exposure to radiation the nuclear division index was calculated. This NDI value was used to study the cell cycle progression of delayed mitotic stimulation after the exposures to radiation. Fig. 3 shows the averaged data of three donors and the value up to 0.75Gy no difference in the NDI value whereas from 1Gy NDI value decreases as dose increases.
FIGURE 3.
Analysis of the NDI value obtained from the blood samples irradiated to 60Co gamma radiation. Each bar represents the mean ± SE of the MN for three independent experiments (N=3).
4. DISCUSSION
As in-consistent reports are available on the yield of chromosomal aberrations and MN in delayed mitogenic stimulation of blood lymphocytes after exposure to ionizing radiations, an attempt was made to investigate the yield of MN in blood samples exposed to gamma radiation, held at 37°C for six and twenty four hours then stimulated with mitogen the PHA. To compare the yield of MN observed in delayed mitogen stimulated samples, the MN frequency was also calculated from the in vitro exposed blood samples and stimulated with PHA from the same donors two hours of post-irradiation for ten dose points. A dose-response curve for the data was constructed for biological dosimetry applications; the observed yield of radiation induced MN, dose-response curve and their co-efficient values are comparable with the published reports (Silva et al 1994; Sreedevi and Rao 1994; Koksal et al 1996; Solomon et al 1997). It is appears to be a consistent increase in the variance to mean ratio (> 1), resulted in the positive u value. The distribution pattern of MN as per u test indicates over-dispersion in nine out of ten doses showed higher value of ±1.96. As the MN distribution obtained at various doses, deviate from the Poisson distribution, it cannot be used for the discrimination of partial body exposure from that of whole body exposures unlike DC and the α/β ratio also is comparable to the published data.
In the case of radiation accidents or un-intentional exposures, chances of exposures to large population are high. Such a mass casualties dose assessment is paramount important in medical management. Due to lack of arrangement or inadequate capacity, samples might not be collected under the controlled temperatures and sample processing may be delayed, in particular, during the transport of collected blood to far-away biodosimetry laboratories for dose assessment. Keeping the above technical difficulties in view, the present study was designed to examine the MN frequency in blood lymphocytes incubated at 37°C for six and twenty four hours post exposure without adding PHA and then stimulated to enter into cell cycle and processed for the MN assay. Our results suggest that there was no significant change in the frequency of MN, in irradiated blood samples, incubated at 37°C and cultured for either two hours or six hours post-irradiation. However, in the samples cultured twenty four hours post-irradiation, the MN frequency showed a significant reduction (Fig. 2). The results are different from the report of Lee et al (1999), in which 2Gy of gamma radiation exposed blood lymphocytes stored at 5°C or 22°C even for 96 and 120 hours and then stimulated with PHA did not show any differences in their yield of MN. Hoffmann et al (2002), Krishnaja and Sharma (2006) have been reported that the yield of DC and MN is increasing by the prolonged culture of lymphocytes with PHA at different time intervals. Similar results have been reported for chromosomal aberrations, in which delayed stimulation of lymphocytes followed by radiation exposures showed elevated levels of aberration than that of lymphocytes stimulated immediately (Wilkins et al 2008). Furthermore, irradiated blood sample stored at 4°C after irradiation significantly (p < 0.0001) increased the yield of DC as compare to that of stored at 37°C have been documented earlier (Moroni et al 2008). Even though there exists a lack of consistency on the yield of radiation induced chromosomal aberrations in samples stored at different temperatures, our results strongly supports the view that storage of blood samples for 24 hours and delayed mitogenic stimulation (24 hours post-irradiation stored at 37°C) reduced the MN frequency.
A significant reduction in the MN frequency in twenty hour post-irradiation stimulated lymphocytes, when compared to that of two and six hours post-irradiation stimulated lymphocytes, might be due the checkpoint activation and cell-cycle arrest or repair of radiation induced DNA damages. It has been demonstrated that the DNA damage could induce cell cycle arrest by activating many signalling molecules and pathways. Of which G1 checkpoint could have played a dominant role as the circulating blood lymphocytes are in the G0/G1 stage of cell cycle in ATM- and P53-dependent manner (Lavin and Khanna 1999). In the absence of growth factor or in response to DNA damage, D cyclins are rapidly get destroyed (Diehl et al 1998); their degradation causes the release of p21/WAF1 molecules from cdk4/6 complexes to arrest progression in G1, by inhibiting cdk2 activity (Agami and Bernards 2002). Cell cycle progression is mediated by sequential activation of cyclins and cdk, with the increase in cyclin D expression being among the initial steps in the proliferative process. In association with their binding partners, cdk 4 and 6, D-type cyclins promote G0/G1 to S-phase transition by activating phosphorylation of pRb, thereby helping to cancel its growth-repressive function (Matsushime et al 1992). Ample evidence supports the role of cyclin D in mitogenic signalling: for example mitogenic pathways (RAS and ERK1/2) positively regulate expression of D-type cyclins (Lavoie et al 1996). Indeed, the checkpoint activation and slow progression of cells was evidenced in the present study that at higher doses their NDI decreased significantly when compared to that of lower doses (Fig. 3).
Alternatively, the highly damaged cells fail to enter into cell cycle progression and get eliminated by cell death. It has been shown that radiation induces interphase and reproductive cell death in exposed population (Chang and Little 1992). Loss of lymphocytes viability also was observed at refrigerated conditions (IAEA 2001). In contrast, it has been reported that the blood lymphocytes stored at 4°C in the presence of PHA increased the cell viability and proliferation (mitotic index) of the cell (Belloni et al 2008). On the other hand, blood alone is transferred in blood storage vials during blood transportation to other lab and pre-addition of PHA may not be possible as it requires aseptic conditions and it is not feasible to add all the collected blood samples during the mass radiation casualties. In this present study we have kept temperature (37°C) as a constant by varying the time points for the all exposed samples. Furthermore, the exposed blood samples were maintained without culture media in serum-free environment. All the required reagents were added by the corresponding time points and observed a reduction in the MN yield in blood samples twenty four hours of delayed mitogen stimulation.
CONCLUSION
It is clearly observed from the results obtained earlier that a delayed mitogenic stimulation of whole blood culture would increase the chromosomal aberrations. The time and temperature is most concern for biodosimetry application because the estimation of unknown (whole or partial body exposure) dose is important for the triage medical management. Besides there is a constant need for improvement of biodosimetry tool for the accurate dose estimation and prevention of long term effects in suspected exposed individuals. If the blood received after twenty four hours after exposure, the estimation of dose from the MN frequency would lead to the under estimation of the dose exposed. Further studies are required in this area to improve clear understanding in the assessment or estimation of the unknown dose with precision in delayed blood sampling.
Acknowledgments
The authors express their thanks to Ms. Anjali for the irradiation of blood samples.
Footnotes
FUNDING INFORMATION
The authors sincerely acknowledge the financial assistance provided from the INMAS, DRDO, Government of India (project No: ITC/2519/INM-08/2011).
CONFLICT OF INTEREST
The authors state that there are no conflicts of interest.
REFERENCES
- Agami R, Bernards R. Convergence of mitogenic and DNA damage signaling in the G1 phase of the cell cycle. Cancer Lett. 2002;177:111–118. doi: 10.1016/s0304-3835(01)00785-6. [DOI] [PubMed] [Google Scholar]
- Beinke C, Oestreicher U, Riecke A, Kulka U, Meineke V, Romm H. Inter-laboratory comparison to validate the dicentric assay as a cytogenetic triage tool for medical management of radiation accidents. Radiat Meas. 2011;46:929–935. [Google Scholar]
- Belloni P, Meschini R, Palitti F. Effects of storage conditions of human whole blood on the viability of lymphocytes. Int J Radiat Biol. 2008;84:613–619. doi: 10.1080/09553000802203630. [DOI] [PubMed] [Google Scholar]
- Bryce SM, Avlasevich SL, Bemis JC, Lukamowicz M, Elhajouji A, Van Goethem F, De Boeck M, Beerens D, Aerts H, Van Gompel J, Collins JE, Ellis PC, White AT, Lynch AM, Dertinger SD. Interlaboratory evaluation of a flow cytometric, high contentin vitro micronucleus assay. Mutat Res. 2008;650:181–195. doi: 10.1016/j.mrgentox.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WP, Little JB. Delayed reproductive death as a dominant phenotype in cell clones surviving. X-irradiation. Carcinogenesis. 1992;13:923–928. doi: 10.1093/carcin/13.6.923. [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AA, Dennis JA. Polyfit-A computer program for fitting a specified degree of polynomial to data points. NRPB-M. 1973;11:1–18. [Google Scholar]
- Fenech M. Cytokinesis-block micronucleus cytome assay. Nature Protocols. 2007;2(5):1084–2005. doi: 10.1038/nprot.2007.77. [DOI] [PubMed] [Google Scholar]
- Fenech M, Bonassi S, Turner J, Lando C, Ceppi M, Chang WP, Holland N, Kirsch-Volders M, Zeiger E, Paola Bigattig M, Bolognesi C, Cao J, De Luca G, Di Giorgiok M, Ferguson LR, Fucic A, Garcia Lima O, Hadjidekova V, Hrelia P, Jaworska A, Joksic G, Krishnaja AP, Lee TK, Martelli A, McKay MJ, Migliore L, Mirkova E, Müller WU, Odagiri Y, Orsiere T, Scarfi MR, Silva MJ, Sofuni T, Suralles J, Trenta G, Vorobtsova I, Vral A, Zijno A. Intra- and inter-laboratory variation in the scoring of micronuclei and nucleoplasmic bridges in binucleated human lymphocytes Results of an international slide-scoring exercise by the HUMN project. Mutat Res. 2003;534:45–64. doi: 10.1016/s1383-5718(02)00248-6. [DOI] [PubMed] [Google Scholar]
- Garcia OF, Ramalho AT, Di Giorgio M, Mir SS, Espinoza ME, Manzano J, Nasazzi N, Lopez I. Inter-comparison in cytogenetic Dosimetry among five laboratories from Latin America. Mutat Res. 1995;327:33–39. doi: 10.1016/0027-5107(94)00066-e. [DOI] [PubMed] [Google Scholar]
- Hayashi M, MacGregor JT, Gatehouse DG, Adler ID, Blakey DH, Dertinger SD, Krishna G, Morita T, Russo A, Sutou S. In vivo rodent erythrocyte micronucleus assay: Aspects of protocol design including repeated treatments, integration with toxicity testing, and automated scoring. Environ Mol Mutagen. 2000;35:234–252. [PubMed] [Google Scholar]
- Hoffmann GR, Sayer AM, Littlefield LG. Higher frequency of chromosome aberrations in late-arising first-division metaphases than in early-arising metaphases after exposure of human lymphocytes to X-rays in Go. Int J Radiat Biol. 2002;78:765–772. doi: 10.1080/09553000210152962. [DOI] [PubMed] [Google Scholar]
- Holmberg K, Meijer AE, Harms-Ringdahl M, Lambert B. Chromosomal instabilty in human lymphocytes after low dose rate γ-irradiation and delayed mitogen stimulation. Int. J. Radiat. Biol. 1998;73:21–34. doi: 10.1080/095530098142671. [DOI] [PubMed] [Google Scholar]
- IAEA (International Atomic Energy Agency) Cytogenetic analysis for radiation dose assessment. 2001. IAEA Technical Report Series, No. 405.
- IAEA (International Atomic Energy Agency) Cytogenetic Dosimetry: Applications in Preparedness for and Response to Radiation Emergencies 2011.
- Koksal G, OnerDalci D, Sibel Pala F. Micronuclei in human lymphocytes: the Co-60 gamma-ray dose-response. Mutat Res. 1996;359:151–157. doi: 10.1016/s0165-1161(96)90261-7. [DOI] [PubMed] [Google Scholar]
- Krishnaja AP, Sharma NK. Differential radiation effects in smokers-culture time dependence of the yield of gamma ray-induced chromosome damage in first division metaphases. Int J Radiat Biol. 2006;82:363–377. doi: 10.1080/09553000600774097. [DOI] [PubMed] [Google Scholar]
- Lavin MF, Khanna KK. ATM: the protein encoded by the gene mutated in the radiosensitive syndrome ataxia-telangiectasia. Int J Radiat Biol. 1999;75:1201–1214. doi: 10.1080/095530099139359. [DOI] [PubMed] [Google Scholar]
- Lavoie JN, Allemain GL, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- Lee TK, O’Brien K, Eaves GS, Christie KI, Varga L. Effect of blood storage on radiation-induced micronuclei in human lymphocytes. Mutat Res. 1999;444:201–206. doi: 10.1016/s1383-5718(99)00078-9. [DOI] [PubMed] [Google Scholar]
- Matsushime H, Ewen ME, Strom DK, Kato JY, Hanks SK, Roussel MF, Sherr CJ. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D-type G1 cyclins. Cell. 1992;71:323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- Moroni MM, Krasnopolsky K, Subramanian U, Martin PR, Doherty KM, Prasanna PGS. Does Cell Culture Type And Blood Transport Temperature Affect Dicentric Yield and Radiation Dose Assessment? J Med CBR Def. 2008;6 [Google Scholar]
- Paillole N, Voisin P. Is micronucleus yield variability a problem for overexposure dose assessment to ionizing radiation? Mutat Res. 1998;413:47–56. doi: 10.1016/s1383-5718(98)00015-1. [DOI] [PubMed] [Google Scholar]
- Savage JRK. Sites of Radiation induced chromosome exchanges. Curr. Top. Radiat. Res. 1970;6:129–194. [Google Scholar]
- Silva MJ, Carothers A, Dias A, Luis JH, Piper J, Boavida MG. Dose dependence of radiation-induced micronuclei in cytokinesis-blocked human lymphocytes. Mutat Res. 1994;322:117–128. doi: 10.1016/0165-1218(94)00019-0. [DOI] [PubMed] [Google Scholar]
- Solomon Paul FD, Venkatachalam P, Jeevanram RK. A comparative study of synchronised and conventional culture methods on the micronucleus dose–response curve. Mutat Res. 1997;391:91–98. doi: 10.1016/s0165-1218(97)00038-4. [DOI] [PubMed] [Google Scholar]
- Sreedevi B, Rao BS. Assay of Micronuclei in peripheral blood lymphocytes as a biological indicator of radiation dose. Radiat Prot Dosi. 1994;51:41–45. [Google Scholar]
- Virsik-Peuckert RP, Harder D. Temperature and the formation of radiation-induced chromosome aberrations: II. The temperature dependence of lesion repair and lesion interaction. Int J Radiat Biol. 1986;49:673–681. doi: 10.1080/09553008514552921. [DOI] [PubMed] [Google Scholar]
- Wilkins RC, Romm H, Kao TC, Awa AA, Yoshida MA, Livingston GK, Jenkins MS, Oestreicher U, Pellmar TC, Prasanna PGS. Interlaboratory comparison of the dicentric chromosome assay for radiation biodosimetry in mass casualty events. Radiat Res. 2008;169:551–560. doi: 10.1667/RR1272.1. [DOI] [PubMed] [Google Scholar]
- Willems P, August L, Slabbert J, Romm H, Oestreicher U, Thierens H, Vral A. Automated micronucleus (MN) scoring for population triage in case of large scale radiation events. Int. J. Radiat. Biol. 2010;86:2–11. doi: 10.3109/09553000903264481. [DOI] [PubMed] [Google Scholar]
- Yoshida MA, Hayata I, Tateno H, Tanaka K, Sonta S, Kodama S, Kodama Y, Sasaki MS. The chromosome network for biodosimetry in Japan. Radiat Meas. 2007;42:1125–1127. [Google Scholar]