Introduction
It is our pleasure to debate this important and clinically relevant topic. Atrial fibrillation (AF) is a leading cause of hospitalization and death (Calkins et al. 2012), and the need to improve AF therapy is urgent and requires dramatic advances in our mechanistic understanding. Decades of research have not dented the incidence of AF, now at epidemic proportions (Calkins et al. 2012), nor reduced its morbidity and mortality with the laudable exception of antithrombotic therapy (Calkins et al. 2012).
Our position
AF is initiated by triggers from regions including the pulmonary veins (Calkins et al. 2012), then sustained by specific mechanisms. We will present substantial evidence that these sustaining mechanisms include localized rotors in many patients.
Our position that ‘rotors have been demonstrated to drive [human] AF’ summarizes a century of bench-to-bedside studies, culminating in recent multicentre clinical trials in which ablation of AF rotors has eliminated AF and improved patient outcomes. Conversely, there is a paucity of evidence supporting the opposing notion: that disorganized activity per se sustains AF without underlying driving mechanisms.
Many studies that propose disorganized mechanisms are limited to ‘long-standing persistent AF’. Conversely, we provide evidence for a comprehensive mechanistic framework in which rotors sustain AF across clinical ‘classes’. The terms paroxysmal AF (self-limiting), persistent and longstanding persistent AF (continuous for >7 days and >1 year, respectively) are highly dependent on how often ECGs are obtained, since AF is often asymptomatic (Charitos et al. 2012) and patients with persistent AF may spontaneously present in sinus rhythm (Calkins et al. 2012) and could thus be reclassified as paroxysmal AF (Jahangir et al. 2007).
Definitions
While AF appears disorganized on the ECG, the atria clearly exhibit regions of spatio-temporal organization and disorganization. The debate on whether disorganization in AF is caused (‘driven’) by sources (Pandit & Jalife, 2013) or whether disorganization per se sustains AF (Fig. 1) has been ongoing for a century (Jalife, 2011).
Figure 1.
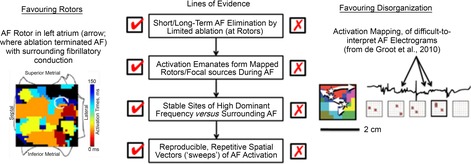
Lines of evidence favouring rotors over disorganized mechanisms for AF
Left human rotor (where ablation terminated AF) surrounded by fibrillatory conduction. Right, disorganized AF maps, but created from analysis of difficult-to-interpret AF electrograms (de Groot et al. 2010).
Spatially localized sources can be divided into rotors and focal sources. We define a ‘rotor’ as the phase singularity whose reverberations radiate ‘spiral waves’ at high speed into surrounding tissue (Pandit & Jalife, 2013). The spatial domain of 1:1 activation by a rotor depends on its frequency relative to the heterogeneity of local refractory periods. In the context of cardiac arrhythmias, rotors are highly localized drivers or organizing sources of reentrant tachycardia and fibrillation (Pandit & Jalife, 2013). Although not our primary topic, a focal source is an ectopic site from where activation spreads centrifugally based on regional refractoriness and conduction (Pandit & Jalife, 2013). Rotors have long been demonstrated in animal models of AF (Pandit & Jalife, 2013), and more recently in human AF where brief targeted ablation alone can terminate AF (Narayan et al. 2013b) and eliminate AF long term (Narayan et al. 2013a).
Conversely, it has been posited that AF depends on the disorganization of wavelets, itself due to longitudinal/transmural dissociation (de Groot et al. 2010). However, this is supported by mapping <10–20% of MRI-quantified atrial areas (Jadidi et al. 2013) in small numbers of patients without proof of causality (de Groot et al. 2010).
Evidence for our position
Many diverse observations are readily explained and in fact predicted by our position, yet unexplained by disorganization alone (Fig. 1). Human AF resistant even to electrical cardioversion can be terminated by very few or even a single (Herweg et al. 2003) ablation lesion(s) (Calkins et al. 2012; Narayan et al. 2012b), or by localized physical pressure (Tzou et al. 2011). Propagation vectors in AF repeat reproducibly over time (Gerstenfeld et al. 1992) and exhibit consistent spatio-temporal features (Sahadevan et al. 2004; Sanders et al. 2005; Atienza et al. 2009). These observations are difficult to reconcile with disorganized sustaining mechanisms, yet support spatially localized AF-sustaining regions in these patients.
Direct evidence for localized AF-sustaining mechanisms, including rotors, has been established in a variety of experimental systems. Optical mapping that utilizes voltage-sensitive dyes and contemporary signal-processing algorithms (Gray et al. 1998) has demonstrated AF rotors producing spatial gradients in the distribution of dominant atrial frequencies, the highest frequency lying at the rotor location (Fig. 1; Mansour et al. 2001). Clinical interventions show that ablation of high dominant frequency sites that eliminates left-to-right atrial frequency gradients predicts long-term freedom from AF (Atienza et al. 2009).
Direct clinical evidence for rotors now exists in patients with paroxysmal, persistent and longstanding persistent AF. Since human AF is spatially non-uniform, we used bi-atrial basket catheters (Narayan et al. 2013b) to simultaneously map much larger areas than in previous studies (de Groot et al. 2010) and then applied phase analysis to detect regions that activate sequentially but may be obscured by the fibrillatory milieu. Simultaneous analysis of wide areas increases the sensitivity to detect rotors that precess (show limited meander), and is essential to prove or disprove their existence. Yet, to the best of our knowledge, simultaneous analysis of wide areas is absent in studies supporting disorganized mechanisms (de Groot et al. 2010; Lee et al. 2014). Although higher spatial resolution would be welcome, we reasoned from minimum bi-atrial (Narayan et al. 2012a) repolarization (∼110 ms) and conduction velocity (∼40 cm s−1) that human atria can support 1:1 conduction from a rotor in circuit paths as short as ∼4–5 cm (Rensma et al. 1988). Clinical electrode spacing can map such reentry (Rappel & Narayan, 2013).
The CONFIRM trial (CONventional ablation for AF with or without Focal Impulse and Rotor Modulation, FIRM) used this approach in 92 patients at 107 procedures to reveal rotors (or focal sources) in 97% of patients with paroxysmal, persistent and longstanding persistent AF. Rotors were concurrent (2.1 ± 1.0 per patient), more prevalent with advanced AF (Narayan et al. 2012b), and precessed in ∼2–3 cm2 areas (Narayan et al. 2013b) causing irregular electrograms at the rotor tip (Zlochiver et al. 2008) and varying spiral waves from fibrillatory disorganization. Notably, causality was proven by the ability of brief targeted ablation to eliminate AF acutely and on long-term follow-up, with (Narayan et al. 2012b) or without (Narayan et al. 2013a) other ablation. These results are now independently validated (Shivkumar et al. 2012; Miller et al. 2013), and laboratories worldwide have identified rotors in human AF using diverse methods, including phase mapping (Ghoraani et al. 2013; Haissaguerre et al. 2013; Lin et al. 2013; Lee et al. 2014). Studies are defining how localized ablation eliminates AF, possibly analogous to the proven elimination of micro-reentrant atrial tachycardia by focal ablation.
Limitations of the opposing position
While it is often assumed that Maze surgery supports disorganized mechanisms (Moe et al. 1964), the numbers of lesions (Cox et al. 1991) are insufficient to constrain dozens or hundreds of the wavelets proposed (de Groot et al. 2010). Since rotors need ‘elbow room’ (typically 2× the centre of rotation or ∼5–10 mm), Maze may in fact simply prevent rotors from sustaining. Indeed, one original motivation for Maze was to intersect relatively large reentrant drivers (Cox et al. 1991).
Notably, studies supporting AF disorganization involved few patients (n = 49) (de Groot et al. 2010), and mapped only 10–21 cm2 or <10–20% of MRI-defined left atrial areas (100–138 cm2) (Jadidi et al. 2013). It is unclear why this work (de Groot et al. 2010) consistently fails to show any rotational activity in human AF, since others now show rotations of varying duration depending on the size of the mapping plaque and technique (Ghoraani et al. 2013; Haissaguerre et al. 2013; Lin et al. 2013; Narayan et al. 2013b; Lee et al. 2014). It is possible that activation mapping of these difficult-to-interpret AF electrograms (Fig. 1; de Groot et al. 2010) obscures rotation evident in other approaches (e.g. phase mapping). Importantly, to the best of our knowledge, interventions have never been used to prove that disorganization is an AF-sustaining mechanism rather than just a bystander to another mechanism (e.g. rotors). Finally, the disorganized AF hypothesis is inconsistent with and cannot easily explain the wealth of clinical and experimental data supporting localized AF sources in at least some patients as discussed above.
Conclusions and future directions
Rotors have been proven to sustain AF in patients in all clinical subtypes and numerous animal models. These data come from various groups using diverse methods with interventions and long-term outcomes to prove causality. The opposing contention, that rotors do not exist but that AF is sustained by disorganization without driving sources, is supported only circumstantially by experiments with important technical limitations, in a small numbers of patients, and with no proof of causality. Mapping and therapy of AF rotors has already substantially improved clinical outcomes in multicentre trials. Future work should define how electrical, structural or neural remodelling contributes to the formation of AF sources and disorganization from them to remaining tissue.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief comment. Comments may be posted up to 6 weeks after publication of the article, at which point the discussion will close and authors will be invited to submit a ‘final word’. To submit a comment, go to http://jp.physoc.org/letters/submit/jphysiol;592/15/3163
Biographies
Sanjiv M. Narayan is Professor of Medicine at the University of California San Diego, where he treats patients with heart rhythm disorders. He was trained in software engineering and neuroscience in addition to cardiology and clinical electrophysiology. He directs an actively funded translational laboratory that uses bioengineering solutions to understand arrhythmia mechanisms, and has pioneered unique therapies for cardiac fibrillation. He has authored or co-authored over 152 original papers and review articles.

José Jalife is the Cyrus and Jane Farrehi Professor of Cardiovascular Research and Professor of Internal Medicine at the University of Michigan. He is a leader in the study of mechanisms of cardiac arrhythmias. His work has led to major advances toward elucidating the molecular and cellular bases of atrial fibrillation, ventricular fibrillation and sudden cardiac death. He has published more than 300 original papers and review articles, and has edited/authored 15 books, including the internationally acclaimed Cardiac Electrophysiology: From Cell to Bedside, now in its sixth edition.

Additional information
Competing interests
S. M. Narayan is co-author of intellectual property owned by the University of California Regents and licensed to Topera Inc. (San Diego, CA, USA). Topera does not sponsor any research, including that presented here. S. M. Narayan holds equity in Topera, and reports having received honoraria from Medtronic, St Jude Medical and Biotronik. J. Jalife serves on the Scientific Advisory Board of Topera, Inc.
Funding
The authors' work is supported by National Heart, Lung, and Blood Institute grants HL103800 and HL83559 (S.M.N.) and the Leducq Foundation (J.J.).
References
- Atienza F, Almendral J, Jalife J, Zlochiver S, Ploutz-Snyder R, Torrecilla EG, Arenal A, Kalifa J, Fernandez-Aviles F, Berenfeld O. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm. 2009;6:33–40. doi: 10.1016/j.hrthm.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, DiMarco J, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm. 2012;9:632–696. [Google Scholar]
- Charitos EI, Stierle U, Ziegler PD, Baldewig M, Robinson DR, Sievers HH, Hanke T. A comprehensive evaluation of rhythm monitoring strategies for the detection of atrial fibrillation recurrence: insights from 647 continuously monitored patients and implications for monitoring after therapeutic interventions. Circulation. 2012;126:806–814. doi: 10.1161/CIRCULATIONAHA.112.098079. [DOI] [PubMed] [Google Scholar]
- Cox JL, Canavan TE, Schuessler RB, Cain ME, Lindsay BD, Stone C, Smith PK, Corr PB, Boineau JP. The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg. 1991;101:406–426. [PubMed] [Google Scholar]
- de Groot NM, Houben RP, Smeets JL, Boersma E, Schotten U, Schalij MJ, Crijns H, Allessie MA. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation. 2010;122:1674–1682. doi: 10.1161/CIRCULATIONAHA.109.910901. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld E, Sahakian A, Swiryn S. Evidence for transient linking of atrial excitation during atrial fibrillation in humans. Circulation. 1992;86:375–382. doi: 10.1161/01.cir.86.2.375. [DOI] [PubMed] [Google Scholar]
- Ghoraani B, Dalvi R, Gizurarson S, Das M, Ha A, Suszko A, Krishnan S, Chauhan VS. Localized rotational activation in the left atrium during human atrial fibrillation: Relationship to complex fractionated atrial electrograms and low-voltage zones. Heart Rhythm. 2013;10:1830–1838. doi: 10.1016/j.hrthm.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998;392:75–78. doi: 10.1038/32164. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Hocini M, Shah AJ, Derval N, Sacher F, Jais P, Dubois R. Noninvasive panoramic mapping of human atrial fibrillation mechanisms: a feasibility report. J Cardiovasc Electrophysiol. 2013;24:711–717. doi: 10.1111/jce.12075. [DOI] [PubMed] [Google Scholar]
- Herweg B, Kowalski M, Steinberg JS. Termination of persistent atrial fibrillation resistant to cardioversion by a single radiofrequency application. Pacing Clin Electrophysiol. 2003;26:1420–1423. doi: 10.1046/j.1460-9592.2003.t01-1-00203.x. [DOI] [PubMed] [Google Scholar]
- Jadidi AS, Cochet H, Shah AJ, Kim SJ, Duncan E, Miyazaki S, Sermesant M, Lehrmann H, Lederlin M, Linton N, Forclaz A, Nault I, Rivard L, Wright M, Liu X, Scherr D, Wilton SB, Roten L, Pascale P, Derval N, Sacher F, Knecht S, Keyl C, Hocini M, Montaudon M, Laurent F, Haïssaguerre M, Jaïs P. Inverse relationship between fractionated electrograms and atrial fibrosis in persistent atrial fibrillation: combined magnetic resonance imaging and high-density mapping. J Am Coll Cardiol. 2013;62:802–812. doi: 10.1016/j.jacc.2013.03.081. [DOI] [PubMed] [Google Scholar]
- Jahangir A, Lee V, Friedman PA, Trusty J, Hodge D, Kopecky S, Packer DL, Hammill SC, Shen W, Gersh BJ. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation. 2007;115:3050–3056. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- Jalife J. Déjà vu in the theories of atrial fibrillation dynamics. Cardiovasc Res. 2011;89:766–775. doi: 10.1093/cvr/cvq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Kumar S, Teh A, Madry A, Spence S, Larobina M, Goldblatt J, Brown R, Atkinson V, Moten S, Morton JB, Kistler PM, Sanders P, Kalman JM. Epicardial wave mapping in human long-lasting persistent atrial fibrillation: transient rotational circuits, complex wavefronts, and disorganized activity. Eur Heart J. 2014;35:86–97. doi: 10.1093/eurheartj/eht267. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Lo MT, Lin C, Chang SL, Lo LW, Hu YF, Hsieh WH, Chang HY, Lin WY, Chung FP, Liao JN, Chen YY, Hanafy D, Huang NE, Chen SA. Prevalence, characteristics, mapping, and catheter ablation of potential rotors in nonparoxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2013;6:851–858. doi: 10.1161/CIRCEP.113.000318. [DOI] [PubMed] [Google Scholar]
- Mansour M, Mandapati R, Berenfeld O, Chen J, Samie FH, Jalife J. Left-to-right gradient of atrial frequencies during acute atrial fibrillation in the isolated sheep heart. Circulation. 2001;103:2631–2636. doi: 10.1161/01.cir.103.21.2631. [DOI] [PubMed] [Google Scholar]
- Miller JM, Krummen DE, Clopton P, Day JD, Daubert JP, Ellenbogen KA, Hummel JD, Kowal RC, Mansour MC, Reddy VY, Shivkumar K, Steinberg JS, Swarup V, Wheelan KR, Narayan SM. Ablation of Atrial Fibrillation Rotors and Focal Sources Improves Outcome Over Conventional Ablation Alone in Independent Laboratories: Multicenter Validation of Focal Impulse And Rotor Modulation (FIRM) Circulation. 2013;128:A16890. [Google Scholar]
- Moe GK, Rheinboldt W, Abildskov J. A computer model of atrial fibrillation. Am Heart J. 1964;67:200–220. doi: 10.1016/0002-8703(64)90371-0. [DOI] [PubMed] [Google Scholar]
- Narayan SM, Krummen DE, Donsky A, Swarup V, Miller JM. Precise rotor elimination without concomitant pulmonary vein isolation for the successful elimination of paroxysmal atrial fibrillation. PRECISE-PAF. Heart Rhythm. 2013a;10:LBCT4. [Google Scholar]
- Narayan SM, Krummen DE, Rappel W-J. Clinical mapping approach to identify rotors and focal beats in human atrial fibrillation. J Cardiovasc Electrophysiol. 2012a;23:447–454. doi: 10.1111/j.1540-8167.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel W-J, Miller J. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation) trial. J Am Coll Cardiol. 2012b;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan SM, Shivkumar K, Krummen DE, Miller JM, Rappel W-J. Panoramic electrophysiological mapping but not electrogram morphology identifies stable sources for human atrial fibrillation. Stable atrial fibrillation rotors and focal sources relate poorly to fractionated electrograms. Circ Arrhythm Electrophysiol. 2013b;6:58–67. doi: 10.1161/CIRCEP.111.977264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit SV, Jalife J. Rotors and the dynamics of cardiac fibrillation. Circ Res. 2013;112:849–862. doi: 10.1161/CIRCRESAHA.111.300158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappel W-J, Narayan SM. Theoretical considerations for mapping activation in human cardiac fibrillation. Chaos. 2013;23:023113. doi: 10.1063/1.4807098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensma P, Allessie M, Lammers W, Bonke F, Schalij M. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ Res. 1988;62:395–410. doi: 10.1161/01.res.62.2.395. [DOI] [PubMed] [Google Scholar]
- Sahadevan J, Ryu K, Peltz L, Khrestian CM, Stewart RW, Markowitz AH, Waldo AL. Epicardial mapping of chronic atrial fibrillation in patients: preliminary observations. Circulation. 2004;110:3293–3299. doi: 10.1161/01.CIR.0000147781.02738.13. [DOI] [PubMed] [Google Scholar]
- Sanders P, Berenfeld O, Hocini M, Jaïs P, Vaidyanathan R, Hsu LF, Garrigue S, Takahashi Y, Rotter M, Sacher F, Scavée C, Ploutz-Snyder R, Jalife J, Haïssaguerre M. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- Shivkumar K, Ellenbogen KA, Hummel JD, Miller JM, Steinberg JS. Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: first multicenter experience of focal impulse and rotor modulation (FIRM) ablation. J Cardiovasc Electrophysiol. 2012;23:1277–1285. doi: 10.1111/jce.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzou WS, Saghy L, Lin D. Termination of persistent atrial fibrillation during left atrial mapping. J Cardiovasc Electrophysiol. 2011;22:1171–1173. doi: 10.1111/j.1540-8167.2011.02079.x. [DOI] [PubMed] [Google Scholar]
- Zlochiver S, Yamazaki M, Kalifa J, Berenfeld O. Rotor meandering contributes to irregularity in electrograms during atrial fibrillation. Heart Rhythm. 2008;5:846–854. doi: 10.1016/j.hrthm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


