Abstract
Cellular processes are exquisitely sensitive to H+ and Ca2+ ions because of powerful ionic interactions with proteins. By regulating the spatial and temporal distribution of intracellular [Ca2+] and [H+], cells such as cardiac myocytes can exercise control over their biological function. A well-established paradigm in cellular physiology is that ion concentrations are regulated by specialized, membrane-embedded transporter proteins. Many of these couple the movement of two or more ionic species per transport cycle, thereby linking ion concentrations among neighbouring compartments. Here, we compare and contrast canonical membrane transport with a novel type of Ca2+–H+ coupling within cytoplasm, which produces uphill Ca2+ transport energized by spatial H+ ion gradients, and can result in the cytoplasmic compartmentalization of Ca2+ without requiring a partitioning membrane. The mechanism, demonstrated in mammalian myocytes, relies on diffusible cytoplasmic buffers, such as carnosine, homocarnosine and ATP, to which Ca2+ and H+ ions bind in an apparently competitive manner. These buffer molecules can actively recruit Ca2+ to acidic microdomains, in exchange for the movement of H+ ions. The resulting Ca2+ microdomains thus have the potential to regulate function locally. Spatial cytoplasmic Ca2+–H+ exchange (cCHX) acts like a ‘pump’ without a membrane and may be operational in many cell types.
The signalling ion calcium is highly compartmentalized in cardiac cells
Calcium (Ca2+) ions regulate a wide variety of intracellular signalling cascades (Clapham, 2007), which, in the heart, include electrical excitation, contraction, growth and development (Bers, 2002). The chemical basis for these interactions is the ability of Ca2+ ions to bind to proteins and modulate their function. Cells, however, cannot exercise control over Ca2+ signals by means of synthesis or break-down, as is the case for many other signalling agents such as cyclic nucleotides (Hardman et al. 1971). Instead, Ca2+ signalling operates on the principle of sub-cellular compartmentalization by ion transport (Berridge et al. 2000). Microdomains of elevated [Ca2+] may modulate biological function locally, or could be the source of Ca2+ for release into adjacent regions for dynamic signalling (Cheng et al. 1993).
The size and stability of [Ca2+] non-uniformity is limited by Ca2+ dissipation down electrochemical gradients. The lipid matrix of membranes is a barrier that reduces the magnitude of dissipative Ca2+ fluxes and allows large [Ca2+] gradients to be formed across plasmalemmal, sarcoplasmic reticulum (SR) and lysosomal membranes (Fig. 1). Within aqueous compartments, the magnitude and longevity of [Ca2+] gradients depend on the ion's diffusivity. Cytoplasmic Ca2+ diffusivity is reduced by volume exclusion due to macromolecules (which increase diffusion path length) and by reversible binding to buffer molecules (e.g. troponin, calmodulin or ATP) (Kushmerick & Podolsky, 1969; Baylor & Hollingworth, 1998). Typically, only one in a hundred Ca2+ ions is free to diffuse, and the diffusivity of the remaining calcium depends on the mobility of the Ca2+–buffer complex (Berlin et al. 1994; Baylor & Hollingworth, 1998). These phenomena reduce cytoplasmic Ca2+ diffusivity to a level that supports, albeit transiently, spatially confined signalling modalities such as Ca2+ sparks (Cheng et al. 1993).
Figure 1.
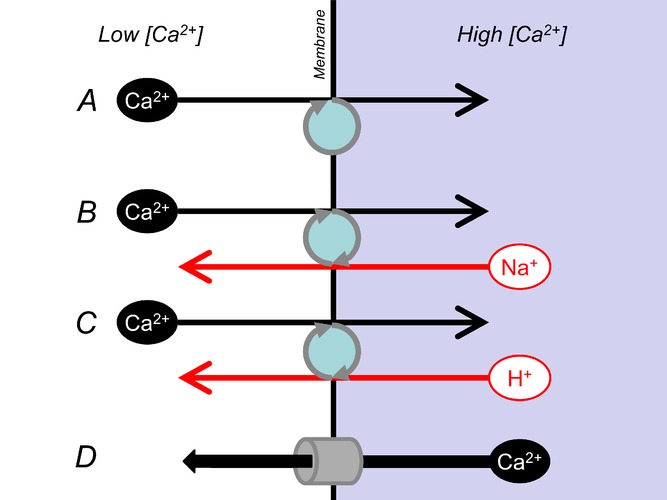
Membrane transport can compartmentalize calcium ions within cardiac ventricular myocytes
Calcium ions cannot be synthesized or degraded, but transport can produce local elevations in their concentration. Spatial non-uniformity of [Ca2+] within cells can lead to local modulation of biological function and provide the driving force for Ca2+ transfer between adjacent regions for dynamic signalling. It is generally accepted that stable compartmentalization of Ca2+ is achieved by transporters that pump Ca2+ across membranes (e.g. SERCA, A). In some cases, pumping of Ca2+ is coupled to the movement of other ionic species (e.g. Na+–Ca2+ exchange, B, or plasmalemmal Ca2+–H+-ATPase, C). Compartments of high [Ca2+] can be discharged by increasing the ‘leak’ across membranes, e.g. by activating Ca2+ channels (such as ryanodine receptors, IP3 receptors or voltage-gated Ca2+ channels, D).
Restricted Ca2+ diffusivity in aqueous compartments, and low Ca2+ permeability across lipid bilayers are not absolute barriers to Ca2+ back-flux. Nonetheless, steep [Ca2+] gradients can be maintained across membranes by active transport, which compensates for Ca2+ leakage. Active transport involves membrane-embedded proteins that couple uphill translocation of Ca2+ with hydrolysis of ATP (primary active transport) or downhill movement of an ion other than Ca2+ (secondary active transport). In cardiac cells, the primary active Ca2+ transporters are plasmalemmal Ca2+–H+-ATPase pumps (PMCA) and sarco–endoplasmic reticulum Ca2+-ATPase pumps (SERCA), while secondary active transport is by Na+–Ca2+ exchangers (NCX) (Bassani et al. 1994; Bers, 2002). SERCA sequesters Ca2+ into the SR and produces a steep (up to 104-fold) [Ca2+] gradient that remains stable during diastole. The activity of NCX and PMCA keeps cytoplasmic [Ca2+] considerably lower (up to 104-fold) than extracellular [Ca2+] (Bassani et al. 1994; Bers, 2002). These transporter proteins are able to produce a net flux because they are anchored in a specific orientation in the lipid matrix of membranes. Since cytoplasm cannot similarly restrict the orientation of soluble proteins, it has commonly been accepted that the aqueous compartments cannot support stable [Ca2+] gradients. Our recent findings challenge this notion by demonstrating that a class of soluble molecules can mediate uphill Ca2+ transport driven by a cytoplasmic [H+] ([H+]i) gradient but without a membrane (Swietach et al. 2013). The basis for this novel paradigm in ion exchange is the mechanism of H+ ion transport in cytoplasm.
Cytoplasmic H+ transport involves the exchange of protonated and unprotonated molecules
H+ ions, like Ca2+ ions, bind avidly to intracellular buffer molecules. However, there are many more cytoplasmic binding sites for H+ compared to Ca2+, such that only a negligible fraction (∼1:500,000 in cardiac myoplasm) of H+ ions is free to diffuse (Vaughan-Jones et al. 2009). These buffer sites include titratable residues on large (essentially immobile) proteins. If buffering capacity were attributable exclusively to these immobile sites, H+ diffusivity in cytoplasm would be reduced ∼500,000-fold (Junge & McLaughlin, 1987; Irving et al. 1990). To test this, the apparent H+ diffusion coefficient can be measured experimentally by injecting H+ ions into a small region of cytoplasm (either via acid-filled patch pipette or by local photolytic H+ uncaging) and recording the pH response in downstream regions of cytoplasm (Vaughan-Jones et al. 2002; Zaniboni et al. 2003; Swietach et al. 2007b). In myocyte cytoplasm, H+ ion diffusivity is considerably slower than in pure water, yet much faster than expected from immobile buffering alone (Fig. 2Aa). These findings suggest that a sub-population of H+-binding buffers is mobile, i.e. of low molecular weight. The apparent H+ diffusion coefficient was also found to increase with intracellular pH (pHi), which is consistent with an increase in the ratio of mobile-to-fixed buffering capacity (Fig. 2Ab) and argues for chemically distinct populations of mobile and fixed buffers (Swietach et al. 2007b).
Figure 2.
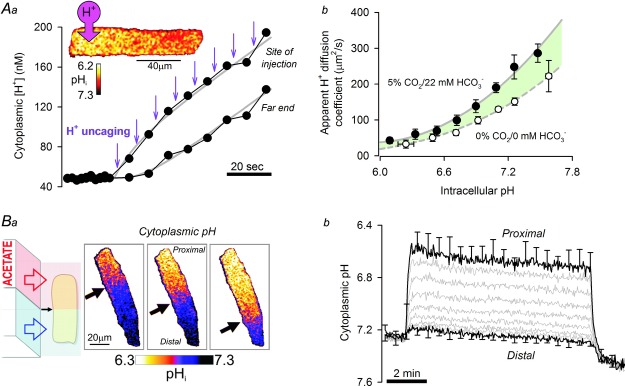
Cytoplasmic transport of H+ ions requires mobile H+ buffers
A, H+ ions are heavily buffered in cytoplasm, to the extent that free H+ ion diffusion is negligible. Cytoplasmic H+ ion mobility is therefore determined by the diffusivity of H+-binding buffers. Aa, photolytic uncaging of H+ ions from the caged H+-donor 2-nitrobenzaldehyde in a small region of a rat ventricular myocyte produces an acidic microdomain that dissipates with relatively slow diffusivity (∼120 μm2 s–1), as reported by the pH-sensitive dye cSNARF1. Ab, cytoplasmic H+ diffusivity increases with cytoplasmic pH and is greater in the presence of physiological CO2–HCO3− buffer (shaded area). B, rat ventricular myocyte superfused with Na+-free, Ca2+-free solutions to block major transmembrane Ca2+ and H+ fluxes. Ba, dual microperfusion device exposes the rat myocyte to two sharply separated microstreams of solution (black arrow indicates boundary position). Regional exposure to 80 mm acetate (proximal end) produces a local influx of acid (acetic acid entry) that is dissipated (acetic acid exit) in the unexposed (distal) region. H+-equivalent fluxes across membranes and along the cytoplasm achieve, at steady state, a large and stable pH gradient (measured with cSNARF1). Bb, one-half of myocyte exposed to acetate. Cytoplasmic pH gradient remains stable for the duration of dual microperfusion because of the constant exchange between protonated and deprotonated mobile buffer molecules.
Carbonic (CO2–HCO3−) buffer is an important and highly mobile contributor to cytoplasmic H+ buffering, particularly at alkaline pHi. Excluding CO2–HCO3− from experimental solutions removes this component of buffering from the cytoplasm (Leem et al. 1999), but only reduces H+ diffusivity by a third (Swietach et al. 2007b). These findings indicate that intrinsic mobile buffers must be present in myocyte cytoplasm. Biochemical assays have identified a family of histidyl dipeptides (HDPs), including carnosine (histidine coupled with β-alanine), homocarnosine (histidine with γ-amino butyric acid, GABA) anserine, (methylhistidine with β-alanine) and their acetylated derivatives, present collectively in cardiac myoplasm at 10–20 mm (O'Dowd et al. 1988; House et al. 1989). The small size (∼240 Da) and the presence of a titratable imidazole group (pKa ∼7), make HDPs suitable for mediating the transport of H+ ions in cytoplasm (Vaughan-Jones et al. 2002). Interestingly, neonatal heart cells have lower HDP levels and slower cytoplasmic H+ diffusion (Swietach et al. 2010). Other small molecules present in cytoplasm, such as inorganic phosphate, ATP and phosphocreatine, provide an additional degree of mobile buffering, but this only amounts to about half of that accounted for by HDPs (Vaughan-Jones et al. 2002).
The passive shuttling of H+ ions aboard mobile buffers, from regions of low pH to high pH, necessitates the return of deprotonated buffer molecules to the source of H+ ions. Diffusive exchange of these protonated and deprotonated molecules can be studied at steady-state by introducing constant H+ influx and H+ efflux at opposite ends of a myocyte's cytoplasmic compartment. This is attainable experimentally by exposing one-half of a myocyte to the salt of a membrane-permeant weak acid, such as acetate, using a dual microperfusion system (Spitzer et al. 2000; Swietach et al. 2005; Fig. 2Ba). Transmembrane acetic acid entry at the acetate-exposed end of the myocyte and exit from the other end of the cell result in compartmentalized H+ influx and H+ efflux, which are coupled by cytoplasmic H+ diffusion. Since intracellular H+ diffusivity is low, a large cytoplasmic pH gradient is necessary to drive an H+ flux that matches the substantial transmembrane H+ fluxes at steady state (Fig. 2Bb). In the case of buffers with rapid protonation and deprotonation kinetics, such as imidazoles, the chemical reactions at the H+ ion source and sink are not rate-limiting relative to buffer-mediated H+ diffusion (Swietach et al. 2005). CO2–HCO3− is unusual because of its slow spontaneous chemical reactions (time constant ∼5 s). The degree to which this is accelerated by carbonic anhydrases is only modest in myocyte cytoplasm (∼3-fold; Leem & Vaughan-Jones, 1998; Schroeder et al. 2013). Thus, the ability of CO2–HCO3− to shuttle H+ ions spatially can be rate-limited by chemical reactions. This explains the observation that a relatively small fraction of diffusive H+ traffic is carried by CO2–HCO3−, despite this buffer being a major contributor to the total buffering capacity at equilibrium in myocytes (Swietach et al. 2005).
Aside from differences between buffer types with respect to size or kinetics, there are also important chemical differences between the protonated and deprotonated forms of each buffer. As explained below, the difference in electric charge gives rise to some surprising ionic interactions of physiological importance.
Preferential binding of Ca2+ to deprotonated mobile buffer can result in Ca2+ transport
According to the ping-pong model of membrane-embedded transporters, transfer of ions across a membrane arises from the translocation of an ion-bound state from one side of the membrane to the other. If the returning state were able to bind selectively to a different type of ion, the transport cycle would result in ion exchange, as is the case for NCX. Exchange of protonated and deprotonated buffer-molecules resembles the transport cycle of membrane-embedded proteins, except that it occurs in cytoplasm rather than across a membrane. Following this analogy, coupled ion exchange in cytoplasm would be possible if ions other than H+ were able to bind preferentially to the deprotonated buffer molecule.
The principal cytoplasmic monovalent ions (Na+, K+, Cl−) bind only very weakly, if at all, to most organic molecules. In contrast, the higher charge density of divalent ions (e.g. Ca2+, Mg2+) allows for stronger interactions. The mobile H+ buffer ATP binds Mg2+ and Ca2+ ions with micromolar affinity (Kushmerick, 1997; Baylor & Hollingworth, 1998). Interestingly, HDPs were originally described as biochemical anti-oxidants on the basis of their ability to chelate divalent cations such as copper(II) (Boldyrev, 1993; Pavlov et al. 1993; Baran, 2000). Carnosine, a representative HDP, also binds Ca2+ and Mg2+ with affinity ∼1 mm. Over the physiological concentration range of intracellular Ca2+ and Mg2+ (0.1–1.0 μm and 0.5–1.0 mm, respectively), Mg2+ occupancy exceeds Ca2+ occupancy by several orders of magnitude. Nonetheless, the amount of Ca2+ carried by HDPs (1–10 μm, over typical [Ca2+] range) is biologically meaningful because the total concentration of these buffers in cells is very high (10–20 mm).
Divalent ions are expected to bind more stably to deprotonated molecules, i.e. at more alkaline pH (Baran, 2000). This was tested in vitro by measuring the stability of Ca2+-bound buffers in response to acidification (Swietach et al. 2013). Buffer-containing solutions (in equilibrium with a fixed [Ca2+]) were set in agarose and subjected to photolytic H+ uncaging. In response to acidification, a substantial rise in free [Ca2+] was observed in the presence of carnosine and ATP (Fig. 3Aa), as expected for an apparent competition between Ca2+ and H+ ions.
Figure 3.
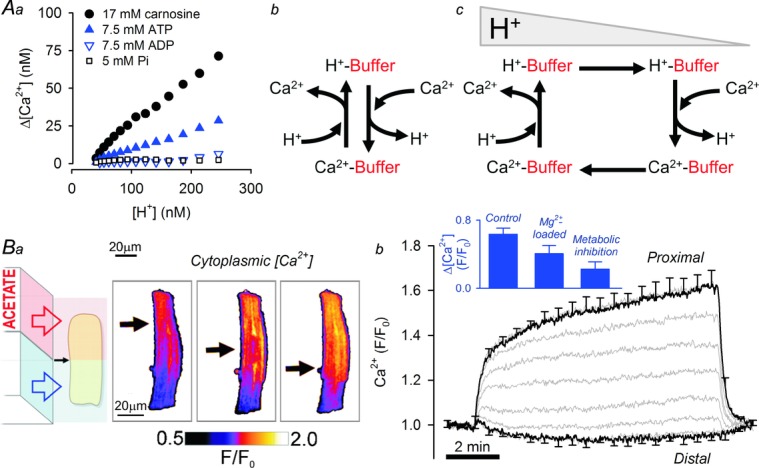
pH-sensitive binding of Ca2+ to mobile H+ buffers results in cytoplasmic Ca2+–H+ exchange
A, Ca2+ binds to many small molecules, including mobile buffers. Aa, stability of Ca2+ complexes of carnosine (a histidyl dipeptide mobile buffer), ATP, ADP and inorganic phosphate (Pi) during acidification (photolytic H+ uncaging), determined in vitro from rise in free [Ca2+] in agarose-set solutions. Ab, binding of Ca2+ and H+ ions to carnosine and ATP shows a degree of apparent competitiveness. Ac, a locally imposed [H+] gradient produces a spatial gradient of Ca2+-loaded mobile buffers. H+- and Ca2+-bound mobile buffers diffuse down their respective gradients, producing Ca2+–H+ exchange. B, rat ventricular myocyte superfused with Na+-free, Ca2+-free solutions. Ba, response of resting [Ca2+] (measured with Ca2+ dye Fluo3) during regional exposure to 80 mm acetate (arrow indicates boundary between acetate-containing and acetate-free microstreams). A gradient of [Ca2+] maps spatially onto the imposed gradient of [H+]. Bb, one-half of myocyte exposed to acetate. Rise of [Ca2+] at the proximal (acidic) end of myocyte does not dissipate towards distal end, indicating that an uphill Ca2+ flux is balancing the diffusive back-flux. Inset shows size of [Ca2+] gradient measured at 8 min of dual microperfusion under control conditions, following a protocol that raises cytoplasmic [Mg2+] fourfold (superfusion with 30 mm Mg2+-containing, Na+-free solution) and following metabolic inhibition with rotenone (10 μm), antimycin (10 μm) and deoxyglucose (5 mm). Raised [Mg2+] reduces the Ca2+-carrying capacity of histidyl dipeptides. Metabolic inhibition depletes ATP, a key mobile buffer, and raises cytoplasmic [Mg2+].
A global decrease of cytoplasmic pH will release divalent cations from mobile buffers, such as carnosine and ATP, because of a shift towards the protonated form. In the case of a local fall of cytoplasmic pH, the depletion of the deprotonated form would, additionally, drive a diffusive influx from adjacent cytoplasmic regions, in exchange for the protonated form. Indeed, this is the mechanism by which H+ ions diffuse out of the acidic microdomain. Since divalents bind preferentially to the deprotonated form, the exchange of protonated for deprotonated buffer results in net transport of divalents towards acid regions (Fig. 3Ac). This process resembles the transport cycle for canonical Ca2+–H+ exchangers, except that it does not require a partitioning membrane.
Acidic microdomains in cytoplasm recruit Ca2+ and generate stable Ca2+ gradients
H+-linked transport of divalents may produce microdomains of elevated [Ca2+] and [Mg2+], unless this is short-circuited by passive back-flux. The magnitude of divalent back-flux depends on cytoplasmic diffusivity, which is slow in the case of Ca2+ because of extensive buffering. The ability of a cytoplasmic pH gradient to produce an uphill Ca2+ flux in excess of dissipative back-flux was tested experimentally by imposing a standing gradient of pH in the cytoplasm of isolated ventricular myocytes, using dual microperfusion. The Ca2+-sensitive fluorophore Fluo3 reported a ∼80 nm rise of [Ca2+] at the acidic end of the cell (Fig. 3Ba). Importantly, the [Ca2+] gradient did not collapse over time.
These findings demonstrate that spatial gradients of Ca2+ can, in principle, be formed in cytoplasm over regions of pH non-uniformity. Paradoxically, a partitioning membrane is not required for compartmentalizing Ca2+ ions because Ca2+ back-flux (set by cytoplasmic Ca2+ diffusivity) is low and surmountable by an evoked uphill Ca2+ flux. However, it is important to note that adequate diffusivity of the H+-sensitive Ca2+ carrier is required to ensure that the uphill Ca2+ transport overcomes the dissipative back-flux. For this reason, immobile buffers, alone, cannot support this form of interaction, even if they exhibit competitive Ca2+–H+ binding. Similarly, organelles that exchange H+ ions for Ca2+ ions by means of a membrane-embedded transporter, such as Letm1 in mitochondria (Tsai et al. 2014), cannot, alone, support the observed standing [Ca2+] gradient. However, these sources of Ca2+ can provide the cargo for mobile buffer exchange.
For the experiment shown in Fig. 3B, the ratio of the rise in free [Ca2+] per rise in free [H+] is 1:2. This, is merely an apparent stoichiometry because the buffering capacities for H+ and Ca2+ ions are not equal. The apparent exchange ratio is also sensitive to the size of the underlying pH gradient because of the pH-dependence of buffering. Computational models predict that a 0.1 pH unit gradient would produce a 30 nm [Ca2+] gradient under resting conditions, i.e. an exchange ratio approaching ∼2Ca2+:1H+ (Swietach et al. 2013).
Uphill H+ transport by reverse-mode cytoplasmic Ca2+–H+ exchange requires large Ca2+ gradients
Cytoplasmic Ca2+–H+ exchange (cCHX) could, in principle, be reversed to produce uphill H+ transport powered by a [Ca2+] gradient. However, the apparent H+/Ca2+ stoichiometry of this process is very high and not a simple inverse of H+-driven Ca2+ transport. To explain this, it is important to consider the traffic of ions carried by buffers that are capable of binding H+ and Ca2+ ions competitively. An imposed pHi gradient drives the diffusive flux of buffers that, by and large, exhibit Ca2+/H+ competition. Due to the overwhelming extent of H+-buffering, no significant flux of free H+ ions occurs. In contrast, an imposed Ca2+ gradient evokes a sizeable flux of free Ca2+ ions (which, by definition, cannot be coupled to H+ ions) plus movement of Ca2+ aboard buffers, many of which are not significant H+ buffers. Consequently, a steep [Ca2+] gradient is necessary to drive a sufficient flux of H+-carrying buffers, capable of establishing a meaningful pH gradient.
Ca2+-driven uphill transport of H+ ions has been demonstrated experimentally in the cytoplasm of ventricular myocytes by activating NCX transport in opposite directions on either end of a cell using dual microperfusion (Swietach et al. 2013). However, the underlying cause of this [Ca2+] gradient is not physiological. A more plausible source of [Ca2+] non-uniformity is the SR Ca2+ release event. At the peak of a Ca2+ transient, bulk cytoplasmic [Ca2+] can approach 1 μm, whereas near the SR release sites at dyadic spaces, it may reach 70 μm (Cannell & Kong, 2012). This magnitude of [Ca2+] non-uniformity is predicted to be sufficient for evoking uphill H+ transport by reverse-mode cCHX. Although the Ca2+ release event is transient, its cyclical and regular pattern will result in a time-averaged [Ca2+] gradient that may be sufficient to generate an acidic nanodomain at the dyadic space. A substantial accumulation of dyadic H+ ions may feed-back negatively on the release process through the pHi sensitivity of ryanodine receptor (RyR) channels (Xu et al. 1996; Balnave & Vaughan-Jones, 2000). Furthermore, this interaction may show rate dependence, as the time-averaged Ca2+ gradient is likely to become steeper at faster heart rates.
The cell's energetic status can affect cytoplasmic Ca2+–H+ exchange
Mathematical simulations predict that HDPs are the major contributors to cCHX because of their high concentration, an acid-dissociation constant that is near resting cytoplasmic pH (pK ∼6.8, ensuring comparable concentrations of protonated and deprotonated forms) and adequate Ca2+ binding (Swietach et al. 2013). In contrast, the contribution of ATP to cCHX is small, despite a high affinity for Ca2+ (10−4.6 m) and abundance (∼7.5 mm in cardiac myocytes), because the low acid-dissociation constant (pKa 6.5) makes it a weaker H+ buffer than HDPs. Nonetheless, the unique biological properties of ATP, stemming from its role as an energy source, result in important effects on cCHX.
Compromised ATP production or uncompensated raised demand will reduce the magnitude of cCHX and result in smaller cytoplasmic Ca2+ gradients (i.e. a lower Ca2+–H+ exchange ratio). This has been observed experimentally by measuring the size of [Ca2+] gradients evoked by an acidic microdomain in metabolically inhibited ventricular myocytes. The degree of inhibition of cCHX, however, was larger than expected from the depletion of intracellular ATP alone (Fig. 3Bb). The additional decrease in the Ca2+–H+ exchange ratio can be explained in terms of the rise in [Mg2+] caused by ATP hydrolysis to ADP, a nucleotide with much lower divalent affinity (Kushmerick, 1997). The additional Mg2+ released into the cytoplasm from net ATP hydrolysis is buffered by HDPs, at the expense of reducing their Ca2+-carrying capacity (i.e. lower Ca2+–H+ exchange ratio). This effect was confirmed experimentally in metabolically normal myocytes, after cytoplasmic [Mg2+] had been raised globally by driving whole-cell transmembrane Na+–Mg2+ exchange in the outward current mode. The observed end-to-end [Ca2+] gradient in response to a longitudinal standing pHi gradient was now reduced (Fig. 3Bb). Thus, the inhibitory effect of raising intracellular [Mg2+] on cCHX is comparable to the effect of competitive antagonists on membrane-embedded transporter-proteins. cCHX is therefore exquisitely sensitive to ATP levels (i.e. the cell's metabolic status) but in a unique manner compared to primary active transporters.
The fact that ATP is a highly labile chemical component of cells means that gradients of cytoplasmic [ATP] can be produced rapidly as a result of regional differences in energy demand and supply. The diffusive exchange of ATP and ADP between such regions can evoke uphill Ca2+ transport – even without an underlying pH gradient – because of preferential divalent binding to ATP (Swietach et al. 2013). Since net ATP hydrolysis yields H+ ions, non-uniformity in the ATP demand/supply ratio will generate pH and Ca2+ gradients concurrently.
Comparing cytoplasmic and transmembrane uphill transport of Ca2+
Until recently, transporter proteins embedded in membranes have been recognized as the only means of producing uphill transport of ions, leading to ionic compartmentalization. Although a transient and localized increase in ionic concentration in cytoplasm, such as during a Ca2+ spark, may be considered a form of compartmentalization, the cytoplasm has not generally been believed to have the intrinsic means to ‘pump’ ions. The cytoplasmic Ca2+–H+ exchanger is thus a novel paradigm in ionic transport (Figure 4). Like secondary active transporters (e.g. NCX), cCHX is driven by an underlying solute gradient (in this case, an H+ gradient). Also, in common with membrane-embedded transporters, the cytoplasmic Ca2+–H+ exchanger can be ‘antagonized’ (by Mg2+) and described kinetically using the Michaelis–Menten formalism (affinity and maximal velocity).
Figure 4.

Cytoplasmic Ca2+–H+ exchange by the mobile buffer shuttle is a new paradigm in spatial Ca2+–H+ interactions
Diffusive exchange of H+-bound mobile buffer (down a spatial [H+] gradient) for Ca2+-bound mobile buffer, produces uphill Ca2+ transport that can result in the establishment of a stable spatial [Ca2+] gradient. Non-uniformity of pH (resulting from gradients in metabolic output, regional exposure to membrane-permeant weak acids/bases or compartmentalized acid–base membrane transport) will automatically produce a microdomain of Ca2+ that can locally regulate function.
Despite a number of qualitative similarities with canonical membrane-embedded transporters, major differences are apparent. Firstly, the back-flux that short-circuits cCHX is comparably much higher than membrane ‘leakage’ in the case of transmembrane transport. This limits the size of [Ca2+] gradients and the distance over which they spread. Considering that cytoplasmic pH gradients are unlikely to exceed one unit in magnitude, cCHX could, at most, generate [Ca2+] in the sub-micromolar range, spanning distances in the micrometre range. In contrast, membrane-embedded transporters are capable of generating much larger [Ca2+] gradients of up to 104-fold across a 10 nm-wide membrane. Whereas membrane-embedded transporters can support sharply demarcated boundaries between regions of dramatically different degrees of Ca2+ activation, cytoplasmic transport produces a more graded distribution of Ca2+ activation. The latter may be optimal for fine-tuning cellular physiology spatially across several sarcomeres. In contrast, only membrane transport would have the capacity to create Ca2+ stores necessary for dynamic signalling, such as Ca2+ transients.
A second difference is the scope for regulating the transport process. Membrane-transporters can be regulated at multiple levels, from gene expression through to allosteric modulation, and very often in a highly targeted manner. In contrast, cCHX operates on the basis of small molecules that are not coded directly by genes and are not subject to the same degree of regulation as transporter proteins. The key buffers responsible for cCHX serve additional roles, and this restricts the scope of targeting the Ca2+–H+ exchange process selectively for regulation. ATP is the cell's energy store and, unsurprisingly, ATP levels are controlled principally by energetic cues. HDPs are anti-oxidants, major pH buffers, and possibly important metabolic regulators. Proteins involved in the synthesis (carnosine synthase CARNS1; Drozak et al. 2010), break-down (cytosolic carnosinase CN2; Teufel et al. 2003) and uptake (peptide transport PepT1 and 2; Yamashita et al. 1997; Vistoli et al. 2012) of carnosine have been described (Boldyrev et al. 2013), but the precise mechanisms by which these are co-ordinated to meet the cell's demand for HDPs remains unclear.
Cytoplasmic Ca2+–H+ exchange is physiologically important
Cytoplasmic Ca2+–H+ exchange is driven automatically between regions with different concentrations of protonated and unprotonated forms of buffers like HDPs and ATP pH. Gradients of pHi occur physiologically in the heart myoplasm as a result of the extensive system of acid–base transporters at membranes, regional differences in blood perfusion and metabolic rate.
In ventricular myocytes, membrane acid extrusion on Na+/H+ exchanger 1 (NHE1) produces spatial gradients of pHi of up to 0.1 units (with subsarcolemmal regions more alkaline than bulk cytoplasm), which can last for several tens of seconds (Swietach & Vaughan-Jones, 2005; Garciarena et al. 2013). By evoking cCHX, such H+ microdomains will drive uphill Ca2+ transport towards the more acidic regions of the cell. A prompt buffer-mediated rise of resting [Ca2+]i during acidosis is likely to provide physiological compensation for H+ interference with Ca2+-activated processes that share common Ca2+/H+ binding sites. By diverting Ca2+ towards acidic regions, cytoplasmic Ca2+–H+ exchange may help spatially to unify Ca2+ responses during periods of local pHi non-uniformity.
Intracellular acidity has a direct inhibitory effect on the contractile apparatus, but an up-regulation in Ca2+ signalling can correct for this, and even produce positive inotropy (Bountra & Vaughan-Jones, 1989; Choi et al. 2000). This compensatory mechanism involves the activation of Na+ influx via NHE1 at low pHi, which then leads to greater cellular retention of Ca2+ by decreasing the Na+-driving force for Ca2+ extrusion via NCX. The retained Ca2+ is sequestered by SERCA into the SR to increase SR Ca2+ load and hence Ca2+ transient amplitude (Vaughan-Jones et al. 2009; Garciarena et al. 2013). However, many of the Ca2+-handling proteins involved in the functional coupling between NHE1 and SERCA are inhibited by H+ ions. Cytoplasmic Ca2+–H+ exchange may help to rescue this coupling in acidic microdomains by locally raising diastolic Ca2+ to overcome H+ inhibition.
Slow cytoplasmic Ca2+ diffusivity may limit the rate of Ca2+ uptake into organellar stores, such as the SR and mitochondria. The additional, uphill Ca2+ flux evoked by an [H+] gradient could facilitate Ca2+ handling by improving diffusive coupling. H+ extrusion by NHE1 will drive Ca2+ diffusion away from the sarcolemma, passing the SR en route to the more acidic myocyte core. This Ca2+ delivery pipeline may improve the functional coupling between Na+-driven pHi regulators and SR Ca2+ load. In another example of H+ extrusion, the electron transport chain of the inner mitochondrial membrane acidifies the inter-membrane space, relative to the bulk cytoplasm (Xiong et al. 2010; Schroeder et al. 2013). As the outer mitochondrial membrane is freely permeable to mobile buffers, this pH gradient would be expected to facilitate Ca2+ delivery to mitochondria.
During periods of spatially heterogeneous blood flow, as may occur in myocardial ischaemia, impaired washout of metabolites produces gradients of CO2 and lactic acid in the heart, particularly at so-called ischaemic border zones (Case et al. 1979; Cascio et al. 1992). Such partitioning of extracellular membrane-permeant weak acids will give rise to local intracellular acidosis, accompanied by gradients of pHi extending out towards the normal myocardium (Spitzer et al. 2000; Swietach et al. 2005). Since HDPs can permeate gap junctional channels and because junctional channels remain open at relatively low pHi values (Swietach et al. 2007a), cCHX is likely to be evoked among myocytes coupled over much larger spatial scales than just the single cell. Spatial Ca2+–H+ exchange is predicted to respond to pHi non-uniformity by diverting Ca2+ from normal myocardium into acidic regions. Ca2+ delivery may be beneficial as part of a compensatory reaction, but in excess, it may contribute to the injurious and arrhythmogenic phenomenon of intracellular Ca2+ overload during regional ischaemia in the heart.
Cytoplasmic Ca2+–H+ exchange is not unique to the cardiac myocyte. ATP is ubiquitous in living cells and high levels of HDPs have been measured in many cells, including skeletal muscle, neurons and glia (O'Dowd et al. 1988, 1990). Large pHi gradients have been demonstrated in neurons (Schwiening & Willoughby, 2002; Willoughby & Schwiening, 2002), and these may exploit cCHX for Ca2+ delivery and local control of signalling.
Buffer-mediated cytoplasmic Ca2+–H+ exchange adds a new paradigm to our understanding of ion transport and mechanisms of local signalling by Ca2+ and H+ ions.
Glossary
- cCHX
cytoplasmic Ca2+–H+ exchange
- HDP
histidyl dipeptide
- NCX
Na+–Ca2+ exchanger
- PMCA
Ca2+–H+-ATPase pump
- SERCA
sarco–endoplasmic reticulum Ca2+-ATPase pump
- SR
sarcoplasmic reticulum
Biographies
Pawel Swietach is a Royal Society University Research Fellow and University Lecturer at the Department of Physiology, Anatomy and Genetics, Oxford University, UK. From Poland, he completed his BA in Physiological Sciences at Oxford and later a doctorate on pH regulation in the heart in the lab of Richard Vaughan-Jones. Pawel obtained further research training in electrophysiology and Ca2+ signalling with Kenneth Spitzer (Salt Lake City, USA), and in cancer biology with Adrian Harris (Oxford).

Richard Vaughan-Jones is Professor of Cellular Physiology at Oxford, Joint Director of the Burdon Sanderson Cardiac Centre, and Tutorial Fellow at Exeter College. His first degree (Physiology) and PhD were from Bristol University. He was a Medical Research Council Senior Fellow at Oxford, before appointment to the Department of Physiology. Collaborative work with Kenneth Spitzer (University of Utah, USA) dates from 1997. He is currently Deputy President of the Physiological Society.

Additional information
Competing interests
None declared.
Author contributions
All authors contributed to writing the paper. PS presented the work at the XXXVII Congress of the International Union of Physiological Sciences (IUPS) in Birmingham, UK.
Funding
This work was supported by the British Heart Foundation (RG/08/016 to RDVJ), Royal Society (PS) and National Institutes of Health (R37HL042873-24 to KWS)
References
- Balnave CD, Vaughan-Jones RD. Effect of intracellular pH on spontaneous Ca2+ sparks in rat ventricular myocytes. J Physiol. 2000;528:25–37. doi: 10.1111/j.1469-7793.2000.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran EJ. Metal complexes of carnosine. Biochemistry (Mosc) 2000;65:789–797. [PubMed] [Google Scholar]
- Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor SM, Hollingworth S. Model of sarcomeric Ca2+ movements, including ATP Ca2+ binding and diffusion, during activation of frog skeletal muscle. J Gen Physiol. 1998;112:297–316. doi: 10.1085/jgp.112.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin JR, Bassani JW, Bers DM. Intrinsic cytosolic calcium buffering properties of single rat cardiac myocytes. Biophys J. 1994;67:1775–1787. doi: 10.1016/S0006-3495(94)80652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Boldyrev AA. Does carnosine possess direct antioxidant activity. Int J Biochem. 1993;25:1101–1107. doi: 10.1016/0020-711x(93)90587-5. [DOI] [PubMed] [Google Scholar]
- Boldyrev AA, Aldini G, Derave W. Physiology and pathophysiology of carnosine. Physiol Rev. 2013;93:1803–1845. doi: 10.1152/physrev.00039.2012. [DOI] [PubMed] [Google Scholar]
- Bountra C, Vaughan-Jones RD. Effect of intracellular and extracellular pH on contraction in isolated, mammalian cardiac muscle. J Physiol. 1989;418:163–187. doi: 10.1113/jphysiol.1989.sp017833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell MB, Kong CH. Local control in cardiac E–C coupling. J Mol Cell Cardiol. 2012;52:298–303. doi: 10.1016/j.yjmcc.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Cascio WE, Yan GX, Kleber AG. Early changes in extracellular potassium in ischemic rabbit myocardium. The role of extracellular carbon dioxide accumulation and diffusion. Circ Res. 1992;70:409–422. doi: 10.1161/01.res.70.2.409. [DOI] [PubMed] [Google Scholar]
- Case RB, Felix A, Castellana FS. Rate of rise of myocardial PCO2 during early myocardial ischemia in the dog. Circ Res. 1979;45:324–330. doi: 10.1161/01.res.45.3.324. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation–contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Choi HS, Trafford AW, Orchard CH, Eisner DA. The effect of acidosis on systolic Ca2+ and sarcoplasmic reticulum calcium content in isolated rat ventricular myocytes. J Physiol. 2000;529:661–668. doi: 10.1111/j.1469-7793.2000.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Drozak J, Veiga-da-Cunha M, Vertommen D, Stroobant V, Van Schaftingen E. Molecular identification of carnosine synthase as ATP-grasp domain-containing protein 1 (ATPGD1) J Biol Chem. 2010;285:9346–9356. doi: 10.1074/jbc.M109.095505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciarena CD, Youm JB, Swietach P, Vaughan-Jones RD. H+-activated Na+ influx in the ventricular myocyte couples Ca2+-signalling to intracellular pH. J Mol Cell Cardiol. 2013;61:51–59. doi: 10.1016/j.yjmcc.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Hardman JG, Robison GA, Sutherland EW. Cyclic nucleotides. Annu Rev Physiol. 1971;33:311–336. doi: 10.1146/annurev.ph.33.030171.001523. [DOI] [PubMed] [Google Scholar]
- House JR, Miller DJ, O'Dowd JJ. Differences in the distribution of the imidazoles of rat heart between atria and ventricles. J Physiol. 1989;417:162P. [Google Scholar]
- Irving M, Maylie J, Sizto NL, Chandler WK. Intracellular diffusion in the presence of mobile buffers. Application to proton movement in muscle. Biophys J. 1990;57:717–721. doi: 10.1016/S0006-3495(90)82592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge W, McLaughlin S. The role of fixed and mobile buffers in the kinetics of proton movement. Biochim Biophys Acta. 1987;890:1–5. doi: 10.1016/0005-2728(87)90061-2. [DOI] [PubMed] [Google Scholar]
- Kushmerick MJ. Multiple equilibria of cations with metabolites in muscle bioenergetics. Am J Physiol Cell Physiol. 1997;272:C1739–C1747. doi: 10.1152/ajpcell.1997.272.5.C1739. [DOI] [PubMed] [Google Scholar]
- Kushmerick MJ, Podolsky RJ. Ionic mobility in muscle cells. Science. 1969;166:1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Leem CH, Lagadic-Gossmann D, Vaughan-Jones RD. Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. J Physiol. 1999;517:159–180. doi: 10.1111/j.1469-7793.1999.0159z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem CH, Vaughan-Jones RD. Out-of-equilibrium pH transients in the guinea-pig ventricular myocyte. J Physiol. 1998;509:471–485. doi: 10.1111/j.1469-7793.1998.471bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dowd JJ, Cairns MT, Trainor M, Robins DJ, Miller DJ. Analysis of carnosine, homocarnosine, and other histidyl derivatives in rat brain. J Neurochem. 1990;55:446–452. doi: 10.1111/j.1471-4159.1990.tb04156.x. [DOI] [PubMed] [Google Scholar]
- O'Dowd JJ, Robins DJ, Miller DJ. Detection, characterisation, and quantification of carnosine and other histidyl derivatives in cardiac and skeletal muscle. Biochim Biophys Acta. 1988;967:241–249. doi: 10.1016/0304-4165(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Pavlov AR, Revina AA, Dupin AM, Boldyrev AA, Yaropolov AI. The mechanism of interaction of carnosine with superoxide radicals in water solutions. Biochim Biophys Acta. 1993;1157:304–312. doi: 10.1016/0304-4165(93)90114-n. [DOI] [PubMed] [Google Scholar]
- Schroeder MA, Ali MA, Hulikova A, Supuran CT, Clarke K, Vaughan-Jones RD, Tyler DJ, Swietach P. Extramitochondrial domain rich in carbonic anhydrase activity improves myocardial energetics. Proc Natl Acad Sci U S A. 2013;110:E958–967. doi: 10.1073/pnas.1213471110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiening CJ, Willoughby D. Depolarization-induced pH microdomains and their relationship to calcium transients in isolated snail neurones. J Physiol. 2002;538:371–382. doi: 10.1113/jphysiol.2001.013055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer KW, Ershler PR, Skolnick RL, Vaughan-Jones RD. Generation of intracellular pH gradients in single cardiac myocytes with a microperfusion system. Am J Physiol Heart Circ Physiol. 2000;278:H1371–H1382. doi: 10.1152/ajpheart.2000.278.4.H1371. [DOI] [PubMed] [Google Scholar]
- Swietach P, Camelliti P, Hulikova A, Kohl P, Vaughan-Jones RD. Spatial regulation of intracellular pH in multicellular strands of neonatal rat cardiomyocytes. Cardiovasc Res. 2010;85:729–738. doi: 10.1093/cvr/cvp343. [DOI] [PubMed] [Google Scholar]
- Swietach P, Leem CH, Spitzer KW, Vaughan-Jones RD. Experimental generation and computational modeling of intracellular pH gradients in cardiac myocytes. Biophys J. 2005;88:3018–3037. doi: 10.1529/biophysj.104.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swietach P, Rossini A, Spitzer KW, Vaughan-Jones RD. H+ ion activation and inactivation of the ventricular gap junction: a basis for spatial regulation of intracellular pH. Circ Res. 2007a;100:1045–1054. doi: 10.1161/01.RES.0000264071.11619.47. [DOI] [PubMed] [Google Scholar]
- Swietach P, Spitzer KW, Vaughan-Jones RD. pH-Dependence of extrinsic and intrinsic H+-ion mobility in the rat ventricular myocyte, investigated using flash photolysis of a caged-H+ compound. Biophys J. 2007b;92:641–653. doi: 10.1529/biophysj.106.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swietach P, Vaughan-Jones RD. Spatial regulation of intracellular pH in the ventricular myocyte. Ann N Y Acad Sci. 2005;1047:271–282. doi: 10.1196/annals.1341.024. [DOI] [PubMed] [Google Scholar]
- Swietach P, Youm JB, Saegusa N, Leem CH, Spitzer KW, Vaughan-Jones RD. Coupled Ca2+/H+ transport by cytoplasmic buffers regulates local Ca2+ and H+ ion signaling. Proc Natl Acad Sci U S A. 2013;110:E2064–2073. doi: 10.1073/pnas.1222433110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufel M, Saudek V, Ledig JP, Bernhardt A, Boularand S, Carreau A, Cairns NJ, Carter C, Cowley DJ, Duverger D, Ganzhorn AJ, Guenet C, Heintzelmann B, Laucher V, Sauvage C, Smirnova T. Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J Biol Chem. 2003;278:6521–6531. doi: 10.1074/jbc.M209764200. [DOI] [PubMed] [Google Scholar]
- Tsai MF, Jiang D, Zhao L, Clapham D, Miller C. Functional reconstitution of the mitochondrial Ca2+/H+ antiporter Letm1. J Gen Physiol. 2014;143:67–73. doi: 10.1085/jgp.201311096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones RD, Peercy BE, Keener JP, Spitzer KW. Intrinsic H+ ion mobility in the rabbit ventricular myocyte. J Physiol. 2002;541:139–158. doi: 10.1113/jphysiol.2001.013267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones RD, Spitzer KW, Swietach P. Intracellular pH regulation in heart. J Mol Cell Cardiol. 2009;46:318–331. doi: 10.1016/j.yjmcc.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Vistoli G, Carini M, Aldini G. Transforming dietary peptides in promising lead compounds: the case of bioavailable carnosine analogs. Amino Acids. 2012;43:111–126. doi: 10.1007/s00726-012-1224-z. [DOI] [PubMed] [Google Scholar]
- Willoughby D, Schwiening CJ. Electrically evoked dendritic pH transients in rat cerebellar Purkinje cells. J Physiol. 2002;544:487–499. doi: 10.1113/jphysiol.2002.027508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong JW, Zhu L, Jiao X, Liu SS. Evidence for DeltapH surface component (DeltapH(S)) of proton motive force in ATP synthesis of mitochondria. Biochim Biophys Acta. 2010;1800:213–222. doi: 10.1016/j.bbagen.2009.07.032. [DOI] [PubMed] [Google Scholar]
- Xu L, Mann G, Meissner G. Regulation of cardiac Ca2+ release channel (ryanodine receptor) by Ca2+, H+, Mg2+, and adenine nucleotides under normal and simulated ischemic conditions. Circ Res. 1996;79:1100–1109. doi: 10.1161/01.res.79.6.1100. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Shimada S, Guo W, Sato K, Kohmura E, Hayakawa T, Takagi T, Tohyama M. Cloning and functional expression of a brain peptide/histidine transporter. J Biol Chem. 1997;272:10205–10211. doi: 10.1074/jbc.272.15.10205. [DOI] [PubMed] [Google Scholar]
- Zaniboni M, Swietach P, Rossini A, Yamamoto T, Spitzer KW, Vaughan-Jones RD. Intracellular proton mobility and buffering power in cardiac ventricular myocytes from rat, rabbit, and guinea pig. Am J Physiol Heart Circ Physiol. 2003;285:H1236–H1246. doi: 10.1152/ajpheart.00277.2003. [DOI] [PubMed] [Google Scholar]


