Abstract
Neuronal nitric oxide synthase (nNOS or NOS1) is the major endogenous source of myocardial nitric oxide (NO), which facilitates cardiac relaxation and modulates contraction. In the healthy heart it regulates intracellular Ca2+, signalling pathways and oxidative homeostasis and is upregulated from early phases upon pathogenic insult. nNOS plays pivotal roles in protecting the myocardium from increased oxidative stress, systolic/diastolic dysfunction, adverse structural remodelling and arrhythmias in the failing heart. Here, we show that the downstream target proteins of nNOS and underlying post-transcriptional modifications are shifted during disease progression from Ca2+-handling proteins [e.g. PKA-dependent phospholamban phosphorylation (PLN-Ser16)] in the healthy heart to cGMP/PKG-dependent PLN-Ser16 with acute angiotensin II (Ang II) treatment. In early hypertension, nNOS-derived NO is involved in increases of cGMP/PKG-dependent troponin I (TnI-Ser23/24) and cardiac myosin binding protein C (cMBP-C-Ser273). However, nNOS-derived NO is shown to increase S-nitrosylation of various Ca2+-handling proteins in failing myocardium. The spatial compartmentation of nNOS and its translocation for diverse binding partners in the diseased heart or various nNOS splicing variants and regulation in response to pathological stress may be responsible for varied underlying mechanisms and functions. In this review, we endeavour to outline recent advances in knowledge of the molecular mechanisms mediating the functions of nNOS in the myocardium in both normal and diseased hearts. Insights into nNOS gene regulation in various tissues are discussed. Overall, nNOS is an important cardiac protector in the diseased heart. The dynamic localization and various mediating mechanisms of nNOS ensure that it is able to regulate functions effectively in the heart under stress.
Introduction
Nitric oxide (NO) has been identified as an important signalling molecule involved in a broad range of biological functions since the late 1970s (Arnold et al. 1977; Gruetter et al. 1979; Furchgott & Zawadzki, 1980), a discovery that led Robert F. Furchgott, Louis J. Ignarro and Ferid Murad to win the Nobel Prize in Physiology or Medicine in 1998. It is now clear that NO-producing enzymes, NO synthases (NOSs), are specified by three genes: neuronal NOS (nNOS or NOS1); inducible NOS (iNOS or NOS2), and endothelial NOS (eNOS or NOS3). Until recently, eNOS has been considered the only isoform of NOS in the myocardium and its regulation of a variety of functions in the heart is well documented (Shah & MacCarthy, 2000; Massion et al. 2003). In 1999, Xu et al. (1999) demonstrated that nNOS is constitutively expressed in the sarcoplasmic reticulum (SR) of cardiac myocytes and regulates sarcoplasmic Ca2+ ATPase (SERCA) reuptake of intracellular Ca2+. nNOS is also the only NOS isoform that is expressed in intrinsic cardiac neurons from autonomic nerves and ganglions (Mohan et al. 2000; Choate et al. 2001; Danson et al. 2005) and controls parasympathetic and sympathetic regulation of cardiac rhythm and contractility. Furthermore, nNOS is expressed in human coronary artery smooth muscle cells (Han et al. 2007) and maintains the basal blood flow (Seddon et al. 2009). Taken together, these findings confirm that nNOS is an important NOS in the heart that is expressed in all the vital fractions and plays important roles in regulating the rhythm, contractility and microcirculation of the heart (Fig. 1A).
Figure 1.
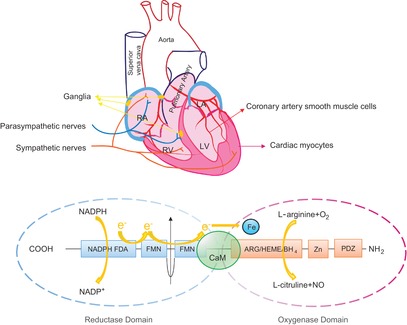
Distribution and structure of neuronal nitric oxide synthase (nNOS) protein in the heart
A, expression of nNOS protein in cardiac myocytes, coronary artery smooth muscle cells, cardiac ganglia, and sympathetic and parasympathetic nerves. B, structure of nNOS. Each monomer of nNOS contains an oxygenase domain (–COOH terminal) and a reductase domain (–NH2 terminal). Electrons from NADPH transfer from the reductase domain [via flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN)] to the oxygenase domain (heme iron), enable nNOS to catalyse the oxidation of l-arginine to l-citrulline and release NO. These two domains are linked and the enzyme is activated by Ca2+–CaM.
Neuronal NOS shares similarities with eNOS in structure and the logistics of NO production. Active nNOS is a homodimer in which each monomer is composed of an N-terminal oxygenase domain and a C-terminal reductase domain (Fig. 1B). NO production takes place in the oxygenase domain by catalysing l-arginine to l-citruline [endorsed by O2 as the other substrate in the presence of cofactor tetrahydrobiopterine (BH4) and electron transfer from nicotinamide adenine dinucleotide phosphate (NADPH) via the flavin adenine dinucleotide (FAD)–flavin mononucleotide (FMN) axis to the heme iron in the oxygenase domain of the other monomer]. Ca2+-calmodulin is essential in linking FMN and heme iron to ensure efficient electron transfer by facilitating FMN donation of electrons and the alignment of the FMN and heme domains (Masters et al. 1996; Feng et al. 2014) (Fig. 1B). In cardiac myocytes, nNOS differs from eNOS in localization and translocation upon stimulation, post-transcriptional modification and the mechanisms mediating physiological and pathological functions in the cardiovascular system (Alderton et al. 2001; Massion et al. 2003; Belge et al. 2005; Zhang & Casadei, 2012). For example, eNOS is primarily located in caveolae (Feron et al. 1996) and mediates mechanical stress stimulation of intracellular Ca2+ release from ryanodine receptors (RyR) (Petroff et al. 2001). nNOS is located in the SR (Xu et al. 1999; Bendall et al. 2004) and in plasma membrane (Ueda et al. 2008) and regulates Na+ and Ca2+ homeostasis in cardiac myocytes. nNOS-derived NO modulates myocyte contraction and facilitates relaxation by targeting key elements of excitation–contraction coupling [e.g. phospholamban (PLN) or L-type Ca2+ channels (LTCC)]. This is understandable because NO has a short half-life (∼10 s) and limited diffusion area (due to abundant NO scavenging myoglobin and NO-interacting biochemicals and proteins); thus the compartmentation of nNOS or the translocation of nNOS to the vicinity of target proteins seem essential to its competent effects. In essence, the spatial confinement of nNOS and its dynamic translocation in response to stimuli ensure that it is able to regulate diverse intracellular signalling pathways and myocardial functions in normal and diseased hearts.
The function and mechanisms of nNOS regulation in the heart
nNOS in the healthy heart
Using gene knockout of nNOS (Barouch et al. 2002; Sears et al. 2003; Dawson et al. 2005; Saraiva et al. 2005) and cardiomyocyte-specific nNOS overexpression models (Burkard et al. 2007; Loyer et al. 2008; Burkard et al. 2010) in conjunction with nNOS inhibitors [e.g. S-methyl-l-thiocitrulline (SMTC), N5-(1-imino-3-butenyl)-l-ornithine (l-VNIO) or 7-nitroindazole (7-NI)] (Xu et al. 1999; Sears et al. 2003; Bendall et al. 2004; Seddon et al. 2009), it has been established that NO produced from constitutive nNOS provides an intrinsic regulatory mechanism of myocardial contraction and relaxation in both the healthy and the diseased heart. In the healthy heart, nNOS-derived NO attenuates basal cardiac inotropy by modulating the activities of LTCC in the plasma membrane to reduce the amplitude of intracellular Ca2+ transients (Sears et al. 2003) through S-nitrosylation- or cGMP-dependent mechanisms. By contrast, the activity of the cardiac Na+–Ca2+ exchanger is unaffected (Sears et al. 2003). In the SR, nNOS-derived NO facilitates myocyte relaxation by promoting SERCA reuptake of intracellular Ca2+ by increasing PKA-dependent (but cGMP/PKG-independent) PLN phosphorylation at serine16 (PLN-Ser16) and Ca2+-calmodulin-dependent kinase II (CaMKII)-dependent PLN phosphorylation at threonine17 (PLN-Thr17), subsequent to the inhibition of cytosolic protein phosphatase 2A (PP2A) and protein phosphatase 1 (PP1) activities by nNOS-derived NO (Zhang et al. 2008). nNOS-derived NO may activate SERCA either directly through S-nitrosylation (Burger et al. 2009) or indirectly through peroxynitrite-dependent S-glutathionylation (Adachi et al. 2004). The role of nNOS-derived NO in RyR activity is controversial because nNOS gene deletion has been associated with both increased RyR leak (Gonzalez et al. 2007) and decreased open probability of RyR (Wang et al. 2010) (Fig. 2). In addition, nNOS forms a macrocomplex with the plasma membrane Ca2+ pump (PMCA4b) and cardiac Na+ channel (SCN5A) linked by α-syntrophin (SNTA1) and is tonically inhibited by PMCA4b. Dissociation of nNOS with PMCA4b in an SNTA1 mutation liberates nNOS from inactivation and subsequently increases S-nitrosylation of SCN5A and late Na+ current. Under these conditions, nNOS is associated with SCN5A-dependent long QT syndrome (Ueda et al. 2008) (Fig. 2).
Figure 2.
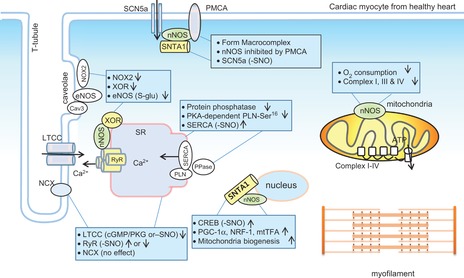
Neuronal nitric oxide synthase (nNOS) protein expression, compartmentation and function in healthy cardiac myocytes
The diagram illustrates the localization of nNOS and target proteins and regulation. nNOS is located in the sarcoplasmic reticulum (SR), mitochondria, plasma membrane and possibly nucleus. nNOS interacts with ryanodine receptor (RyR), xanthine oxidoreductase (XOR), α-syntrophin [forming a macrocomplex with Na+ channel (SCN5a) and plasma membrane Ca2+-ATPase (PMCA4b)]. nNOS modifies the activities of various proteins either through S-nitrosylation (-SNO) or through PKA-dependent, cGMP/PKG-dependent phosphorylations of downstream proteins.
Accumulating evidence shows that nNOS-derived NO regulates cardiac function by targeting proteins other than Ca2+-handling elements. For example, results reported by our group and others show that nNOS tonically controls the activities of constitutive cardiac oxidases such as xanthine oxidoreductase (Kinugawa et al. 2005; Idigo et al. 2012) and NADPH oxidase (Zhang et al. 2009; Jin et al. 2012) and moderates the levels of intracellular superoxide and reactive oxygen species (ROS). As ROS (e.g. hydrogen peroxide) and reactive nitrogen species (RNS) (e.g. peroxynitrite) target protein kinases/phosphatases (Brennan et al. 2006; Burgoyne et al. 2007; Kohr et al. 2009), nNOS regulation of ion channel activity and intracellular Ca2+-handling proteins may be mediated by an array of post-transcriptional modifications such as NO-dependent S-nitrosylation, ROS-dependent oxidation and kinase/phosphatase-dependent phosphorylation. In essence, the genres of the target proteins of nNOS-derived NO determine the downstream post-transcriptional modifications. Furthermore, nNOS may affect myocardial function by regulating mitochondrial proteins. Indeed, nNOS-derived NO was reported to inhibit the mitochondria respiration chain, including complexes I, III and IV (Torres et al. 1995; Welter et al. 1996; Chouchani et al. 2013) and reduces mitochondrial oxygen consumption and affects cardiac metabolism (Fig. 2). Whether such regulation is detrimental or beneficial in myocardial function in the healthy or diseased heart remains to be determined. Recently, nNOS was shown to be recruited by α-syntrophin to the nucleus membrane of murine skeletal muscle C2C12 cells and human HeLa cervix carcinoma cells and to be involved in mitochondrial biosynthesis by increasing the respiratory chain complexes through the promotion of cAMP response element-binding (CREB) protein S-nitrosylation-dependent activation of peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) and its downstream oxidative phosphorylation genes PGC-1α, NRF-1 and mtTFA (Aquilano et al. 2013). Whether similar mechanisms apply to the myocardium remains unknown. Overall, current evidence shows that nNOS-derived NO exerts cardiac functions by targeting essential proteins in various organelles in the myocardium.
nNOS in the diseased heart
Importantly, nNOS protein expression and activity are increased in the myocardium in the diseased heart, such as in ischaemia–reperfusion injury (Sun et al. 2006; Aragon et al. 2011), infarct (Takimoto et al. 2002; Bendall et al. 2004; Dawson et al. 2005; Burger et al. 2009), hypertrophy and heart failure (Damy et al. 2003, 2004; Niu et al. 2012), and exert manifold beneficial effects: nNOS-derived NO prevents diastolic dysfunction and increases β-adrenergic reserve, reduces left ventricular hypertrophy/dilatation/infarct size and protects the myocardium from arrhythmogenesis. In fact, recent findings by our group demonstrate that nNOS upregulation is an early event following pathogenic insult and during disease progression. We have shown that acute angiotensin II (Ang II, 1 μm) treatment to isolated left ventricular (LV) myocytes in vitro significantly increases mRNA/protein expressions and the activity of nNOS (Jin et al. 2012). In turn, nNOS-derived NO reduced NADPH oxidase production of superoxide and facilitated LV myocyte relaxation through cGMP/PKG-dependent (and PKA-independent) phosphorylation of PLN-Ser16 (Jin et al. 2012) (Fig. 3). Similarly, nNOS protein expression and activity were enhanced in LV myocytes from Ang II-induced early hypertensive rats (this is because Ang II infusion in vivo for 4 weeks did not develop hypertrophy and systolic/diastolic dysfunction detected using echocardiography). nNOS did not change contractility or LTCC activity, but did facilitate LV myocyte relaxation via myofilament Ca2+ desensitization mediated by cGMP/PKG-dependent phosphorylations of TnI-Ser23/24 and cMyBP-C-Ser273 (Jin et al. 2013) (Fig. 3). Interestingly, PLN-Ser16 was increased and the kinetics of Ca2+ transient decay (Ca2+ reuptake via SERCA) were faster in LV myocytes from hypertensive rats; however, these changes were independent of nNOS or cGMP/PKG-dependent signalling (Jin et al. 2013). In line with these findings, nNOS protein expression was increased in the myocardium after 3 days and 6 days of Ang II infusion in rats (Tambascia et al. 2001; Moreno et al. 2002). These results demonstrate that nNOS upregulation is an early event during disease progression and nNOS plays protective roles in the heart under stress. In addition, the downstream targets of nNOS-derived NO are dynamic in order to facilitate effective function.
Figure 3.
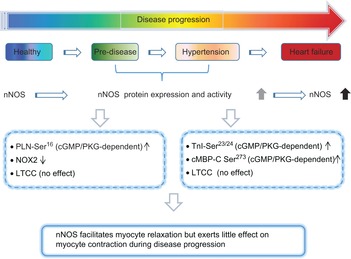
Cardiac neuronal nitric oxide synthase (nNOS) during disease progression
nNOS is functionally expressed in healthy myocardium. From the early stages of disease progression (pre-disease) to hypertension, cardiac nNOS is upregulated and facilitates myocyte relaxation. The mechanisms mediating nNOS shift from PKA-dependent PLN-Ser16 to PKG-dependent PLN-Ser16 at pre-disease and PKG-dependent cMBP-C Ser273 and cTnI Ser23/24 at hypertension. Myocyte contraction is not affected by nNOS.
In stellate ganglia neurons, the protein expression and activity of nNOS are reduced and intracellular [Ca2+]i are increased in pre-hypertensive (young spontaneously hypertensive) rats. Conversely, targeted nNOS transfer into sympathetic neurons using a novel noradrenergic cell-specific vector reduced [Ca2+]i (Li et al. 2013). As nNOS is important in modulating sympathetic neural transmission (Paton et al. 2002), these results suggest that nNOS in the autonomic nervous system and contracting myocardium may coordinate to facilitate cardiac protective effects (e.g. the positive lusitropic effect) in early hypertension.
In human dilated cardiomyopathy and in the murine heart after myocardial infarction, nNOS upregulation is associated with reduced interaction with RyR and increased binding with caveolin-3 (Cav3) in plasma membrane of cardiac myocytes (Bendall et al. 2004; Sun et al. 2006). This location may potentiate its modulation of Ca2+ flux in plasma membrane or NADPH oxidase inhibition. Indeed, nNOS association with Cav3 is enhanced following ischaemia–reperfusion injury and nNOS attenuates β-adrenergic stimulation of ICa (by increasing S-nitrosylation of LTCC), and reduces the Ca2+ transient and SR Ca2+ contents, particularly in female mice (Sun et al. 2006). It has also been shown that nNOS protects the heart from ventricular arrhythmias after left coronary artery ligation by increasing S-nitrosylation and moderating the activities of key Ca2+-handling proteins (e.g. LTCCα1c, SERCA2 and RyR2) and reducing Ca2+ influx, and diastolic and systolic Ca2+ transient amplitudes (Burger et al. 2009) (Fig. 4). Furthermore, nNOS exerts anti-arrhythmic effects by potentiating the peripheral vagal-induced modulation of heart rate (Choate et al. 2001; Heaton et al. 2005). In fact, stimulation of the vagal nerve increased nNOS production of NO in the left ventricle and was anti-arrhythmic (Brack et al. 2009, 2011). Given that systemic sympathetic nervous activity is elevated in the diseased heart (Coote, 2005), vagal control of cardiac rhythm by nNOS appears to exhibit significant clinical relevance in the attenuation of fatal ventricular arrhythmias (Herring & Paterson, 2009).
Figure 4.
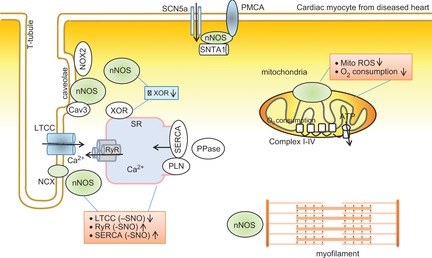
Neuronal nitric oxide synthase (nNOS) compartmentation, function and mechanism in failing cardiac myocyte
nNOS protein expression and activity are increased in failing myocardium. nNOS translocates to plasma membrane and interacts with caveolin-3 (Cav3) but dissociates from ryanodine receptors (RyR). nNOS-derived NO changes the activities of Ca2+ handling proteins via S-nitrosylation. Cardiac-specific overexpression of nNOS is associated with increased nNOS localization in mitochondria and moderates mitochondria activity.
The role of nNOS-dependent signalling in the mitochondria or myofilament in the diseased heart is not clear. In mice conditionally overexpressing nNOS, protein expression of nNOS is increased in mitochondria and myocardial availability of NO is increased. Consequently, nNOS reduced oxidative stress by attenuating mitochondria ROS and xanthine oxidoreductase (XOR) (Fig. 3) and attenuated infarct size following ischaemia–reperfusion (Burkard et al. 2010). Interestingly, depending on nNOS localization, downstream target proteins, mechanisms and functions, contrasting results occur in similar mouse models conditionally overexpressing nNOS (Burkard et al. 2007; Loyer et al. 2008). For example, overexpressed nNOS that is located predominantly in the SR increased LTCC activity and PLN-Ser16 and resulted in faster decay kinetics of [Ca2+]i (Loyer et al. 2008). By contrast, overexpressed nNOS in the plasma membrane increased its interaction with LTCC, reduced LTCC activity and exerted little effect on PLN-Ser16 and, therefore, slowed the decay kinetics of [Ca2+]i (Burkard et al. 2007). These results further strengthen the importance of spatial compartmentation of nNOS and its downstream target proteins in determining the phenotypes of diseased myocardium.
The nNOS gene, transcription and regulation
nNOS gene, multiple promoters/transcription and nNOS splice variants
The human nNOS or NOS1 gene is located on chromosome 12 (12q24.2) and the locus is dispersed over a region of 240 kb. The nucleotide sequence corresponding to the mRNA transcript is encoded by 29 exons and translates into a protein of 1434 amino acids with a predicted molecular weight of ≈160 kDa (Hall et al. 1994; Wang & Marsden, 1995). The nNOS gene has nine unique exon 1 variants for transcript initiation in different tissues and each transcript is expressed from a unique 5′-flanking region (Wang et al. 1999b); this makes the transcriptional/expressional regulation of nNOS extremely complex. In essence, diverse and structurally different nNOS mRNAs are initiated by a variety of transcriptional units via mechanisms including discrete promoters, alternative splicing, cassette exon deletions or insertions, and the usage of alternative polyadenylation signals (Wang et al. 1999a, b1999b). In neurons and in muscle, nNOS promoters are clustered in genomic regions upstream from exon 2. Heterogeneous mRNAs from these promoters encoding the same nNOS proteins differ in both enzymatic characteristics and structural features (Nakane et al. 1993; Xie et al. 1995; Young et al. 1995). The activation of promoters downstream of exon 2 results in the expression of transcripts that utilize alternative translation initiation sites and produce truncated forms of the protein (e.g. nNOSβ or nNOSγ); nNOSβ (but not nNOSγ) retains enzymatic activity but lacks a major PDZ protein–protein interaction domain, which is responsible for targeting nNOS to plasma or nucleus membranes (Eliasson et al. 1997). Different transcriptional initiation, processing and translational efficiency, stability and localization make nNOS transcripts susceptible to various stimuli and produce diverse isoforms. So far, five splice variants of nNOS are described (nNOSα, nNOSβ, nNOSγ, nNOSμ and nNOS2); however, little is known about the variants’ specificity, functional relevance and mechanisms in cardiac myocytes. nNOSα and nNOSμ are known to contain PDZ domain and localize in plasma membrane linked with α-syntrophin. With a 34 amino acid insertion, nNOSμ differs from nNOSα in electron transfer rates, modulation of electron flow by CaM, and heme–nitrosyl complex formation (Panda et al. 2013). nNOSβ and nNOSγ do not contain PDZ domains and may be located in the cytosol.
nNOS gene regulation in tissues other than the myocardium
The genomic regulation (transcriptional and translational mechanisms) of cardiac nNOS is sparse, despite increased expression of nNOS mRNA and protein in LV or in atria following various pathophysiological insults (Takimoto et al. 2002; Damy et al. 2004; Danson et al. 2004; Ganzinelli et al. 2007). Understanding the transcriptional control of nNOS is important in defining the transcription/translation of specific nNOS splice variants in cardiovascular normal physiology and diseases. In neurons, skeletal muscle or vascular or gastrointestinal smooth muscle, nNOS transcription is regulated by transcriptional factors such as CREB (Sasaki et al. 2000), SP/ZNF families (Saur et al. 2002) or NF-kB (Li et al. 2007). CREB regulates the transcription of sirtuin in neuronal cells by binding to Sirt-1 chromatin; Sirt-1, in turn, is recruited by CREB to DNA and promotes CREB-dependent expression of nNOS (Fusco et al. 2012). Sequence analysis of 5′-flanking regions of the human nNOS gene revealed potential binding sites for AP-2, TEF-1/MCBF, CREB/ATF/cFOS, Ets, NF-1 and NF-kB-like sequences (Hall et al. 1994). All of the transcription factors are present in cardiac myocytes and therefore this information provides useful clues to the mechanism(s) involved in affecting nNOS gene regulation in cardiac myocytes.
Similarly, evidence of nNOS in tissues other than the heart may enhance understanding of nNOS splice variants and the functions in the myocardium. For example, it is well known that nNOSμ is upregulated after exercise in skeletal muscle in both animal models (Vassilakopoulos et al. 2003) and in humans (McConell et al. 2007). Expressions of nNOSμ mRNA and protein levels are correlated to angiogenesis after exercise (Huber-Abel et al. 2012). Recently, nNOSβ has been found to target Golgi and to play a pivotal role in maintaining the structure of murine skeletal muscle and post-exercise muscle strength, as well as in increasing resistance to muscle fatigue (Percival et al. 2010). These results suggest that the functional significance of nNOS splice variants refers to the regulation of diverse skeletal muscle functions. However, whether nNOS variants (nNOSα, nNOSβ and nNOSμ) play distinctive roles in cardiac myocytes is not yet clear. In the rat kidney cortex during late pregnancy, nNOSβ protein expression was increased, whereas nNOSα was reduced; consequently, antioxidant capacity was increased so that the renal cortex was protected from oxidative stress (Cunningham et al. 2013). Additionally, a high salt diet (from 0.4% to 4%) increased expression of nNOSβ protein to a greater extent than that of nNOSα in the inner medullary collecting duct of rat kidney (Hyndman et al. 2013). Similar changes in nNOSβ occur after right kidney removal and left kidney two-thirds ablation/infarction, and in kidney transplant models (Tain et al. 2008, 2011). By the same token, relaxin was found to increase nNOSβ expression in mouse proximal colon neurons, but to decrease nNOSα expression in smooth muscle cells, resulting in reduced muscle tone and increasing the amplitude of spontaneous muscle contraction (Baccari et al. 2012). The rise in nNOSβ was selectively suppressed by the β-adrenergic antagonist, nebivolol, and the angiotensin II type 1 receptor antagonist, olmesartan (Sasser et al. 2012), further suggesting the distinctive regulation of nNOS splice variants in response to pathophysiological stimulus.
nNOS compartmentation, binding partners and regulation in cardiac and non-cardiac tissues
Other than the essential components that assemble in stoichiometry to form the oxygenase and reductase domains (including co-factors and substrates), growing numbers of proteins are shown to represent ‘binding partners’ of nNOS. nNOSα and nNOSμ interact with proteins containing PDZ-domain through direct PDZ–PDZ binding or C-terminal–PDZ interactions. These interactions constrain nNOS localization to specialized cell compartments and to specific signal transduction pathways. Despite the importance of such interaction elsewhere, only a few proteins have been confirmed to interact with nNOS in cardiac myocytes and thus much of the current information is derived from findings in the brain or skeletal muscle. To date, nNOS binding proteins in cardiac myocytes are: (i) α-syntrophin; (ii) CAPON, and (iii) Cav3. The first of these, α-syntrophin, binds to nNOS via C-terminal–PDZ domains (in the β-hairpin finger structure region) and docks nNOS to the plasma membrane to form a macromolecular complex with PMCA4b and SCN5a (Ueda et al. 2008). CAPON is an adapter/regulator of nNOS in the brain. The C-terminal binding motif of CAPON interacts with the N-terminal PDZ domain of nNOS to activate nNOS (Jaffrey et al. 1998). In neurons, CAPON activation of nNOS is important in mediating neuronal excitotoxicity (Zhou & Zhu, 2009) and also facilitates iron uptake and neurotoxicity (Cheah et al. 2006). nNOS activation leads to S-nitrosylation of dexras1, which binds to benzodiazepine receptor-associated protein (PAP7) to bring dexras1 to divalent metal transporter (DMT1), an iron import channel, and induce iron influx (Cheah et al. 2006). Genome-wide analysis in patients with an abnormal QT interval has identified that the polymorphism of a common variant (rs10494366) of the NOS1 regulator, the NOS1AP gene (encoding CAPON) is strongly associated with variation in the cardiac QT interval (Arking et al. 2006); interestingly, the correlation is more significant in women than in men (Arking et al. 2006; Tobin et al. 2008). In fact, nNOS activated by CAPON has been implicated in the inhibition of LTCC, subsequently leading to shorter cardiac action potentials (Chang et al. 2008). nNOS increases its association with Cav3 in failing myocardium, but the functional relevance of this interaction remains unidentified. Cav3 may inhibit nNOS activity by preventing Ca-CaM binding to nNOS (Garcia-Cardena et al. 1997) and attenuate nNOS-derived cellular responses.
A number of nNOS binding proteins are reported in tissues other than myocardium. Representative proteins include: protein inhibitor of nNOS (PIN); a Ca2+-dependent protease, calpain; phosphofructokinase (PFK); various heat shock proteins (HSP90 and HSP70); nitric oxide synthase interacting protein (NOSIP); PSD95; NIDD, and carboxyl-terminal-binding protein (CtBP), etc. PIN physically interacts with nNOS and functions as an endogenous inhibitor of nNOS by destabilizing nNOS dimerization (Jaffrey et al. 1996); by binding to CBP, a fusion protein, CBP–PIN also reduces catalytic activity of nNOS without affecting its dimerization (Xia et al. 2006). Calpain binding leads to the acceleration of nNOS degradation, which determines the short lifetimes of nNOSα and nNOSμ (∼12 min and ∼50 min, respectively) (Laine & de Montellano, 1998). PSD95 links nNOS to N-methyl-d-aspartate receptor (NMDAR); NMDAR stimulation activates nNOS, which is critical to the postsynaptic activity of nNOS (Brenman et al. 1996; Doucet et al. 2012). Interestingly, CAPON is shown to compete with PSD-95 and PSD-93 for binding to nNOS, which results in the inactivation and cytosol localization of nNOS (Jaffrey et al. 1998). In nNOS-expressing synaptic vesicles, nNOS interacts with PFK and the product of PFK, fructose-1, 6-bisphosphate, may exert a neuroprotective effect (Firestein & Bredt, 1999). Furthermore, nNOS interacts with a transcription factor, CtBP, to target CtBP in cytosol and prevents its localization in the nucleus. By restricting CtBP from interacting with nuclear proteins (i.e. histone deacetylase and transcription factors), nNOS may maintain homeostasis of transcriptional repression and activation (Riefler et al. 2001). HSP90 binds to nNOS and enhances its Ca2+–CaM binding and activity (Song et al. 2001), and prevents nNOS aggregation and ubiquitination to uphold its stability and location (Corso-Diaz & Krukoff, 2010; Peng et al. 2012), all of which mediate protein–protein interaction and receptor–ligand interaction of nNOS to regulate cell function. By contrast, HSP70 promotes nNOS ubiquitination (Peng et al. 2012) and plays a contrasting role to HSP90. The identification of nNOS binding proteins and their functional regulations in special compartments are important in pinpointing the roles of nNOS in healthy and diseased myocardium.
Future perspectives: nNOS implications in the diseased heart
Cardiac nNOS regulates basal myocardial contraction and relaxation in the healthy heart. This is an important mechanism that protects against the progression and development of fatal heart diseases. Novel post-translational modifications of nNOS regulation are emerging and enhance our understandings of the important roles played by nNOS in cardiovascular disease. Aspects of nNOS gene regulation in response to pathophysiological stimuli, specific nNOS splice variants, and respective compartment/binding partners are known for various physiological systems, but cardiac-specific knowledge of nNOS and its downstream pathways in different heart diseases is required for the development of effective therapeutic strategies.
Acknowledgments
None.
Glossary
- eNOS
endothelial nitric oxide synthase
- FAD
flavin adenine dinucleotide
- FMN
flavin mononucleotide
- iNOS
inducible nitric oxide synthase
- LTCC
L-type Ca2+ channel
- l-VNIO
N5-(1-imino-3-butenyl)-l-ornithine
- 7-NI
7-nitroindazole
- NMDAR
N-methyl-d-aspartate receptor
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
- NOSIP
nitric oxide synthase interacting protein
- PFK
phosphofructokinase
- PIN
protein inhibitor of nNOS
- PLN
phospholamban
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RYR
ryanodine receptors
- SERCA
sarcoplasmic Ca2+ ATPase
- SMTC
S-methyl-l-thiocitrulline
- SR
sarcoplasmic reticulum
Biography
Dr. Yin Hua Zhang is Associate Professor at Seoul National University, College of Medicine, where she was trained as a Cardiac Electrophysiologist with Professor Yung E. Earm. She received further training in Bristol University with Professor Jules Hancox and in Oxford University with Professor Barbara Casadei. Her current research projects involve molecular mechanisms mediating cardiac protection from failing during disease progression (hypertension & metabolic syndrome). In addition, her team aims to reveal novel targets of nitric oxide and reactive oxygen species in regulating myocyte Ca2+ homeostasis, myofilament proteins, metabolism and contraction in the myocardium of cardiac arrhythmias and heart failure.

Additional information
Competing interests
None declared.
Author contributions
YHZ contributed to the writing of the paper and presented the research at the XXXVII Congress of the International Union of Physiological Sciences (IUPS) in Birmingham, UK. CZJ, JHJ and YW contributed to the work in YHZ's laboratory.
Funding
The present study was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Education, Science and Technology, South Korea (no. 2013R1A2A2A01068067), by the Brain Korea 21 Graduate Programme of the Korean Ministry of Education, Science and Technology, Seoul National University Hospital, the Korean Society of Hypertension (2013) and by the SK Telecom Research Fund (no. 3420130290).
References
- Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schöneich C, Cohen RA. S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquilano K, Baldelli S, Ciriolo MR. Nuclear recruitment of neuronal nitric-oxide synthase by α-syntrophin is crucial for the induction of mitochondrial biogenesis. J Biol Chem. 2013;289:365–378. doi: 10.1074/jbc.M113.506733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragón JP, Condit ME, Bhushan S, Predmore BL, Patel SS, Grinsfelder DB, Gundewar S, Jha S, Calvert JW, Barouch LA, Lavu M, Wright HM, Lefer DJ. β3-adrenoreceptor stimulation ameliorates myocardial ischemia–reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation. J Am Coll Cardiol. 2011;58:2683–2691. doi: 10.1016/j.jacc.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking DE, Pfeufer A, Post W, Kao WH, Newton-Cheh C, Ikeda M, West K, Kashuk C, Akyol M, Perz S, Jalilzadeh S, Illig T, Gieger C, Guo CY, Larson MG, Wichmann HE, Marbán E, O'Donnell CJ, Hirschhorn JN, Kääb S, Spooner PM, Meitinger T, Chakravarti A. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat Genet. 2006;38:644–651. doi: 10.1038/ng1790. [DOI] [PubMed] [Google Scholar]
- Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccari MC, Traini C, Garella R, Cipriani G, Vannucchi MG. Relaxin exerts two opposite effects on mechanical activity and nitric oxide synthase expression in the mouse colon. Am J Physiol Endocrinol Metab. 2012;303:E1142–E1150. doi: 10.1152/ajpendo.00260.2012. [DOI] [PubMed] [Google Scholar]
- Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O'Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- Belge C, Massion PB, Pelat M, Balligand JL. Nitric oxide and the heart: update on new paradigms. Ann N Y Acad Sci. 2005;1047:173–182. doi: 10.1196/annals.1341.016. [DOI] [PubMed] [Google Scholar]
- Bendall JK, Damy T, Ratajczak P, Loyer X, Monceau V, Marty I, Milliez P, Robidel E, Marotte F, Samuel JL, Heymes C. Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in β-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation. 2004;110:2368–2375. doi: 10.1161/01.CIR.0000145160.04084.AC. [DOI] [PubMed] [Google Scholar]
- Brack KE, Coote JH, Ng GA. Vagus nerve stimulation protects against ventricular fibrillation independent of muscarinic receptor activation. Cardiovasc Res. 2011;91:437–446. doi: 10.1093/cvr/cvr105. [DOI] [PubMed] [Google Scholar]
- Brack KE, Patel VH, Mantravardi R, Coote JH, Ng GA. Direct evidence of nitric oxide release from neuronal nitric oxide synthase activation in the left ventricle as a result of cervical vagus nerve stimulation. J Physiol. 2009;587:3045–3054. doi: 10.1113/jphysiol.2009.169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Brennan JP, Bardswell SC, Burgoyne JR, Fuller W, Schröder E, Wait R, Begum S, Kentish JC, Eaton P. Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J Biol Chem. 2006;281:21827–21836. doi: 10.1074/jbc.M603952200. [DOI] [PubMed] [Google Scholar]
- Burger DE, Lu X, Lei M, Xiang FL, Hammoud L, Jiang M, Wang H, Jones DL, Sims SM, Feng Q. Neuronal nitric oxide synthase protects against myocardial infarction-induced ventricular arrhythmia and mortality in mice. Circulation. 2009;120:1345–1354. doi: 10.1161/CIRCULATIONAHA.108.846402. [DOI] [PubMed] [Google Scholar]
- Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schröder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- Burkard N, Rokita AG, Kaufmann SG, Hallhuber M, Wu R, Hu K, Hofmann U, Bonz A, Frantz S, Cartwright EJ, Neyses L, Maier LS, Maier SK, Renné T, Schuh K, Ritter O. Conditional neuronal nitric oxide synthase overexpression impairs myocardial contractility. Circ Res. 2007;100:e32–44. doi: 10.1161/01.RES.0000259042.04576.6a. [DOI] [PubMed] [Google Scholar]
- Burkard N, Williams T, Czolbe M, Blömer N, Panther F, Link M, Fraccarollo D, Widder JD, Hu K, Han H, Hofmann U, Frantz S, Nordbeck P, Bulla J, Schuh K, Ritter O. Conditional overexpression of neuronal nitric oxide synthase is cardioprotective in ischemia/reperfusion. Circulation. 2010;122:1588–1603. doi: 10.1161/CIRCULATIONAHA.109.933630. [DOI] [PubMed] [Google Scholar]
- Chang KC, Barth AS, Sasano T, Kizana E, Kashiwakura Y, Zhang Y, Foster DB, Marbán E. CAPON modulates cardiac repolarization via neuronal nitric oxide synthase signalling in the heart. Proc Natl Acad Sci U S A. 2008;105:4477–4482. doi: 10.1073/pnas.0709118105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah JH, Kim SF, Hester LD, Clancy KW, Patterson SE, 3rd, Papadopoulos V, Snyder SH. NMDA receptor–nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron. 2006;51:431–440. doi: 10.1016/j.neuron.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choate JK, Danson EJ, Morris JF, Paterson DJ. Peripheral vagal control of heart rate is impaired in neuronal NOS knockout mice. Am J Physiol Heart Circ Physiol. 2001;281:H2310–H2317. doi: 10.1152/ajpheart.2001.281.6.H2310. [DOI] [PubMed] [Google Scholar]
- Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, James AM, Cochemé HM, Reinhold J, Lilley KS, Partridge L, Fearnley IM, Robinson AJ, Hartley RC, Smith RA, Krieg T, Brookes PS, Murphy MP. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med. 2013;19:753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol. 2005;90:169–173. doi: 10.1113/expphysiol.2004.029041. [DOI] [PubMed] [Google Scholar]
- Corso-Diaz X, Krukoff TL. nNOSα and nNOSβ localization to aggresome-like inclusions is dependent on HSP90 activity. J Neurochem. 2010;114:864–872. doi: 10.1111/j.1471-4159.2010.06813.x. [DOI] [PubMed] [Google Scholar]
- Cunningham MW, Jr, Sasser JM, West CA, Baylis C. Renal redox response to normal pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol. 2013;304:R443–R449. doi: 10.1152/ajpregu.00496.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damy T, Ratajczak P, Robidel E, Bendall JK, Oliviéro P, Boczkowski J, Ebrahimian T, Marotte F, Samuel JL, Heymes C. Up-regulation of cardiac nitric oxide synthase 1-derived nitric oxide after myocardial infarction in senescent rats. FASEB J. 2003;17:1934–1936. doi: 10.1096/fj.02-1208fje. [DOI] [PubMed] [Google Scholar]
- Damy T, Ratajczak P, Shah AM, Camors E, Marty I, Hasenfuss G, Marotte F, Samuel JL, Heymes C. Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet. 2004;363:1365–1367. doi: 10.1016/S0140-6736(04)16048-0. [DOI] [PubMed] [Google Scholar]
- Danson EJ, Mankia KS, Golding S, Dawson T, Everatt L, Cai S, Channon KM, Paterson DJ. Impaired regulation of neuronal nitric oxide synthase and heart rate during exercise in mice lacking one nNOS allele. J Physiol. 2004;558:963–974. doi: 10.1113/jphysiol.2004.062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danson EJ, Choate JK, Paterson DJ. Cardiac nitric oxide: emerging role for nNOS in regulating physiological function. Pharmacol Ther. 2005;106:57–74. doi: 10.1016/j.pharmthera.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Dawson D, Lygate CA, Zhang MH, Hulbert K, Neubauer S, Casadei B. nNOS gene deletion exacerbates pathological left ventricular remodeling and functional deterioration after myocardial infarction. Circulation. 2005;112:3729–3737. doi: 10.1161/CIRCULATIONAHA.105.539437. [DOI] [PubMed] [Google Scholar]
- Doucet MV, Harkin A, Dev KK. The PSD-95/nNOS complex: new drugs for depression. Pharmacol Ther. 2012;133:218–229. doi: 10.1016/j.pharmthera.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Eliasson MJ, Blackshaw S, Schell MJ, Snyder SH. Neuronal nitric oxide synthase alternatively spliced forms: prominent functional localizations in the brain. Proc Natl Acad Sci U S A. 1997;94:3396–3401. doi: 10.1073/pnas.94.7.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Chen L, Li W, Elmore BO, Fan W, Sun X. Dissecting regulation mechanism of the FMN to heme interdomain electron transfer in nitric oxide synthases. J Inorg Biochem. 2014;130:130–140. doi: 10.1016/j.jinorgbio.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- Firestein BL, Bredt DS. Interaction of neuronal nitric-oxide synthase and phosphofructokinase-M. J Biol Chem. 1999;274:10545–10550. doi: 10.1074/jbc.274.15.10545. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Fusco S, Ripoli C, Podda MV, Ranieri SC, Leone L, Toietta G, McBurney MW, Schütz G, Riccio A, Grassi C, Galeotti T, Pani G. A role for neuronal cAMP responsive-element binding (CREB)-1 in brain responses to calorie restriction. Proc Natl Acad Sci U S A. 2012;109:621–626. doi: 10.1073/pnas.1109237109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzinelli S, Joensen L, Borda E, Bernabeo G, Sterin-Borda L. Mechanisms involved in the regulation of mRNA for M2 muscarinic acetylcholine receptors and endothelial and neuronal NO synthases in rat atria. Br J Pharmacol. 2007;151:175–185. doi: 10.1038/sj.bjp.0707180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cardeña G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the NOS caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci U S A. 2007;104:20612–20617. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter CA, Barry BK, McNamara DB, Gruetter DY, Kadowitz PJ, Ignarro L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res. 1979;5:211–224. [PubMed] [Google Scholar]
- Hall AV, Antoniou H, Wang Y, Cheung AH, Arbus AM, Olson SL, Lu WC, Kau CL, Marsden PA. Structural organization of the human neuronal nitric oxide synthase gene (NOS1. J Biol Chem. 1994;269:33082–33090. [PubMed] [Google Scholar]
- Han G, Ma H, Chintala R, Miyake K, Fulton DJ, Barman SA, White RE. Nongenomic, endothelium-independent effects of estrogen on human coronary smooth muscle are mediated by type I (neuronal) NOS and PI3-kinase-Akt signalling. Am J Physiol Heart Circ Physiol. 2007;293:H314–H321. doi: 10.1152/ajpheart.01342.2006. [DOI] [PubMed] [Google Scholar]
- Heaton DA, Golding S, Bradley CP, Dawson TA, Cai S, Channon KM, Paterson DJ. Targeted nNOS gene transfer into the cardiac vagus rapidly increases parasympathetic function in the pig. J Mol Cell Cardiol. 2005;39:159–164. doi: 10.1016/j.yjmcc.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Herring N, Paterson DJ. Neuromodulators of peripheral cardiac sympatho-vagal balance. Exp Physiol. 2009;94:46–53. doi: 10.1113/expphysiol.2008.044776. [DOI] [PubMed] [Google Scholar]
- Huber-Abel FA, Gerber M, Hoppeler H, Baum O. Exercise-induced angiogenesis correlates with the up-regulated expression of neuronal nitric oxide synthase (nNOS) in human skeletal muscle. Eur J Appl Physiol. 2012;112:155–162. doi: 10.1007/s00421-011-1960-x. [DOI] [PubMed] [Google Scholar]
- Hyndman KA, Xue J, MacDonell A, Speed JS, Jin C, Pollock JS. Distinct regulation of inner medullary collecting duct nitric oxide production from mice and rats. Clin Exp Pharmacol Physiol. 2013;40:233–239. doi: 10.1111/1440-1681.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idigo WO, Reilly S, Zhang MH, Zhang YH, Jayaram R, Carnicer R, Crabtree MJ, Balligand JL, Casadei B. Regulation of endothelial nitric-oxide synthase (NOS) S-glutathionylation by neuronal NOS: evidence of a functional interaction between myocardial constitutive NOS isoforms. J Biol Chem. 2012;287:43665–43673. doi: 10.1074/jbc.M112.412031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffrey SR, Snowman AM, Eliasson MJ, Cohen NA, Snyder SH. CAPON: a protein associated with neuronal nitric oxide synthase that regulates its interactions with PSD95. Neuron. 1998;20:115–124. doi: 10.1016/s0896-6273(00)80439-0. [DOI] [PubMed] [Google Scholar]
- Jaffrey SR, Snyder SH. PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science. 1996;274:774–777. doi: 10.1126/science.274.5288.774. [DOI] [PubMed] [Google Scholar]
- Jin CZ, Jang JH, Kim HJ, Wang Y, Hwang IC, Sadayappan S, Park BM, Kim SH, Jin ZH, Seo EY, Kim KH, Kim YJ, Kim SJ, Zhang YH. Myofilament Ca2+ desensitization mediates positive lusitropic effect of neuronal nitric oxide synthase in left ventricular myocytes from murine hypertensive heart. J Mol Cell Cardiol. 2013;60:107–115. doi: 10.1016/j.yjmcc.2013.04.017. [DOI] [PubMed] [Google Scholar]
- Jin CZ, Jang JH, Wang Y, Kim JG, Bae YM, Shi J, Che CR, Kim SJ, Zhang YH. Neuronal nitric oxide synthase is up-regulated by angiotensin II and attenuates NADPH oxidase activity and facilitates relaxation in murine left ventricular myocytes. J Mol Cell Cardiol. 2012;52:1274–1281. doi: 10.1016/j.yjmcc.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Kinugawa S, Huang H, Wang Z, Kaminski PM, Wolin MS, Hintze TH. A defect of neuronal nitric oxide synthase increases xanthine oxidase-derived superoxide anion and attenuates the control of myocardial oxygen consumption by nitric oxide derived from endothelial nitric oxide synthase. Circ Res. 2005;96:355–362. doi: 10.1161/01.RES.0000155331.09458.A7. [DOI] [PubMed] [Google Scholar]
- Kohr MJ, Davis JP, Ziolo MT. Peroxynitrite increases protein phosphatase activity and promotes the interaction of phospholamban with protein phosphatase 2a in the myocardium. Nitric Oxide. 2009;20:217–221. doi: 10.1016/j.niox.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine R, de Montellano PR. Neuronal nitric oxide synthase isoforms α and μ are closely related calpain-sensitive proteins. Mol Pharmacol. 1998;54:305–312. doi: 10.1124/mol.54.2.305. [DOI] [PubMed] [Google Scholar]
- Li D, Nikiforova N, Lu CJ, Wannop K, McMenamin M, Lee CW, Buckler KJ, Paterson DJ. Targeted neuronal nitric oxide synthase transgene delivery into stellate neurons reverses impaired intracellular calcium transients in prehypertensive rats. Hypertension. 2013;61:202–207. doi: 10.1161/HYPERTENSIONAHA.111.00105. [DOI] [PubMed] [Google Scholar]
- Li Y, Li G, Li C, Zhao Y. Identification of nuclear factor-κB responsive element within the neuronal nitric oxide synthase exon 1f-specific promoter. Acta Biochim Biophys Sin (Shanghai) 2007;39:247–254. doi: 10.1111/j.1745-7270.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- Loyer X, Gómez AM, Milliez P, Fernandez-Velasco M, Vangheluwe P, Vinet L, Charue D, Vaudin E, Zhang W, Sainte-Marie Y, Robidel E, Marty I, Mayer B, Jaisser F, Mercadier JJ, Richard S, Shah AM, Bénitah JP, Samuel JL, Heymes C. Cardiomyocyte overexpression of neuronal nitric oxide synthase delays transition toward heart failure in response to pressure overload by preserving calcium cycling. Circulation. 2008;117:3187–3198. doi: 10.1161/CIRCULATIONAHA.107.741702. [DOI] [PubMed] [Google Scholar]
- Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res. 2003;93:388–398. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- Masters BS, McMillan K, Sheta EA, Nishimura JS, Roman LJ, Martasek P. Neuronal nitric oxide synthase, a modular enzyme formed by convergent evolution: structure studies of a cysteine thiolate-liganded heme protein that hydroxylates l-arginine to produce NO as a cellular signal. FASEB J. 1996;10:552–558. doi: 10.1096/fasebj.10.5.8621055. [DOI] [PubMed] [Google Scholar]
- McConell GK, Bradley SJ, Stephens TJ, Canny BJ, Kingwell BA, Lee-Young RS. Skeletal muscle nNOSμ protein content is increased by exercise training in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293:R821–R828. doi: 10.1152/ajpregu.00796.2006. [DOI] [PubMed] [Google Scholar]
- Mohan RM, Choate JK, Golding S, Herring N, Casadei B, Paterson DJ. Peripheral pre-synaptic pathway reduces the heart rate response to sympathetic activation following exercise training: role of NO. Cardiovasc Res. 2000;47:90–98. doi: 10.1016/s0008-6363(00)00066-3. [DOI] [PubMed] [Google Scholar]
- Moreno C, López A, Llinás MT, Rodríguez F, López-Farré A, Nava E, Salazar FJ. Changes in NOS activity and protein expression during acute and prolonged ANG II administration. Am J Physiol Regul Integr Comp Physiol. 2002;282:R31–R37. doi: 10.1152/ajpregu.2002.282.1.R31. [DOI] [PubMed] [Google Scholar]
- Nakane M, Schmidt HH, Pollock JS, Forstermann U, Murad F. Cloned human brain nitric oxide synthase is highly expressed in skeletal muscle. FEBS Lett. 1993;316:175–180. doi: 10.1016/0014-5793(93)81210-q. [DOI] [PubMed] [Google Scholar]
- Niu X, Watts VL, Cingolani OH, Sivakumaran V, Leyton-Mange JS, Ellis CL, Miller KL, Vandegaer K, Bedja D, Gabrielson KL, Paolocci N, Kass DA, Barouch LA. Cardioprotective effect of β-3 adrenergic receptor agonism: role of neuronal nitric oxide synthase. J Am Coll Cardiol. 2012;59:1979–1987. doi: 10.1016/j.jacc.2011.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda SP, Li W, Venkatakrishnan P, Chen L, Astashkin AV, Masters BS, Feng C, Roman LJ. Differential calmodulin-modulatory and electron transfer properties of neuronal nitric oxide synthase μ compared to the α variant. FEBS Lett. 2013;587:3973–3978. doi: 10.1016/j.febslet.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF, Kasparov S, Paterson DJ. Nitric oxide and autonomic control of heart rate: a question of specificity. Trends Neurosci. 2002;25:626–631. doi: 10.1016/s0166-2236(02)02261-0. [DOI] [PubMed] [Google Scholar]
- Peng HM, Morishima Y, Pratt WB, Osawa Y. Modulation of heme/substrate binding cleft of neuronal nitric-oxide synthase (nNOS) regulates binding of Hsp90 and Hsp70 proteins and nNOS ubiquitination. J Biol Chem. 2012;287:1556–1565. doi: 10.1074/jbc.M111.323295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival JM, Anderson KN, Huang P, Adams ME, Froehner SC. Golgi and sarcolemmal neuronal NOS differentially regulate contraction-induced fatigue and vasoconstriction in exercising mouse skeletal muscle. J Clin Invest. 2010;120:816–826. doi: 10.1172/JCI40736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff MG, Kim SH, Pepe S, Dessy C, Marbán E, Balligand JL, Sollott SJ. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol. 2001;3:867–873. doi: 10.1038/ncb1001-867. [DOI] [PubMed] [Google Scholar]
- Riefler GM, Firestein BL. Binding of neuronal nitric-oxide synthase (nNOS) to carboxyl-terminal-binding protein (CtBP) changes the localization of CtBP from the nucleus to the cytosol: a novel function for targeting by the PDZ domain of nNOS. J Biol Chem. 2001;276:48262–48268. doi: 10.1074/jbc.M106503200. [DOI] [PubMed] [Google Scholar]
- Saraiva RM, Minhas KM, Raju SV, Barouch LA, Pitz E, Schuleri KH, Vandegaer K, Li D, Hare JM. Deficiency of neuronal nitric oxide synthase increases mortality and cardiac remodeling after myocardial infarction: role of nitroso–redox equilibrium. Circulation. 2005;112:3415–3422. doi: 10.1161/CIRCULATIONAHA.105.557892. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Gonzalez-Zulueta M, Huang H, Herring WJ, Ahn S, Ginty DD, Dawson VL, Dawson TM. Dynamic regulation of neuronal NO synthase transcription by calcium influx through a CREB family transcription factor-dependent mechanism. Proc Natl Acad Sci U S A. 2000;97:8617–8622. doi: 10.1073/pnas.97.15.8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasser JM, Moningka NC, Tsarova T, Baylis C. Nebivolol does not protect against 5/6 ablation/infarction induced chronic kidney disease in rats – comparison with angiotensin II receptor blockade. Life Sci. 2012;91:54–63. doi: 10.1016/j.lfs.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Seidler B, Paehge H, Schusdziarra V, Allescher HD. Complex regulation of human neuronal nitric-oxide synthase exon 1c gene transcription. Essential role of Sp and ZNF family members of transcription factors. J Biol Chem. 2002;277:25798–25814. doi: 10.1074/jbc.M109802200. [DOI] [PubMed] [Google Scholar]
- Sears CE, Bryant SM, Ashley EA, Lygate CA, Rakovic S, Wallis HL, Neubauer S, Terrar DA, Casadei B. Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ Res. 2003;92:e52–e59. doi: 10.1161/01.RES.0000064585.95749.6D. [DOI] [PubMed] [Google Scholar]
- Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P, Shah AM. Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation. 2009;119:2656–2662. doi: 10.1161/CIRCULATIONAHA.108.822205. [DOI] [PubMed] [Google Scholar]
- Shah AM, MacCarthy PA. Paracrine and autocrine effects of nitric oxide on myocardial function. Pharmacol Ther. 2000;86:49–86. doi: 10.1016/s0163-7258(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Song Y, Zweier JL, Xia Y. Heat-shock protein 90 augments neuronal nitric oxide synthase activity by enhancing Ca2+/calmodulin binding. Biochem J. 2001;355:357–360. doi: 10.1042/0264-6021:3550357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel α1 subunit and reduced ischemia/reperfusion injury. Circ Res. 2006;98:403–411. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- Tain YL, Ghosh S, Krieg RJ, Baylis C. Reciprocal changes of renal neuronal nitric oxide synthase-α and -β associated with renal progression in a neonatal 5/6 nephrectomized rat model. Pediatr Neonatol. 2011;52:66–72. doi: 10.1016/j.pedneo.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tain YL, Muller V, Szabo AJ, Erdely A, Smith C, Baylis C. Renal cortex neuronal nitric oxide synthase in response to rapamycin in kidney transplantation. Nitric Oxide. 2008;18:80–86. doi: 10.1016/j.niox.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto Y, Aoyama T, Tanaka K, Keyamura R, Yui Y, Sasayama S. Augmented expression of neuronal nitric oxide synthase in the atria parasympathetically decreases heart rate during acute myocardial infarction in rats. Circulation. 2002;105:490–496. doi: 10.1161/hc0402.102662. [DOI] [PubMed] [Google Scholar]
- Tambascia RC, Fonseca PM, Corat PD, Moreno H, Jr, Saad MJ, Franchini KG. Expression and distribution of NOS1 and NOS3 in the myocardium of angiotensin II-infused rats. Hypertension. 2001;37:1423–1428. doi: 10.1161/01.hyp.37.6.1423. [DOI] [PubMed] [Google Scholar]
- Tobin MD, Kähönen M, Braund P, Nieminen T, Hajat C, Tomaszewski M, Viik J, Lehtinen R, Ng GA, Macfarlane PW, Burton PR, Lehtimäki T, Samani NJ. Gender and effects of a common genetic variant in the NOS1 regulator NOS1AP on cardiac repolarization in 3761 individuals from two independent populations. Int J Epidemiol. 2008;37:1132–1141. doi: 10.1093/ije/dyn091. [DOI] [PubMed] [Google Scholar]
- Torres J, Darley-Usmar V, Wilson MT. Inhibition of cytochrome c oxidase in turnover by nitric oxide: mechanism and implications for control of respiration. Biochem J. 1995;312:169–173. doi: 10.1042/bj3120169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K, Valdivia C, Medeiros-Domingo A, Tester DJ, Vatta M, Farrugia G, Ackerman MJ, Makielski JC. Syntrophin mutation associated with long QT syndrome through activation of the nNOS–SCN5A macromolecular complex. Proc Natl Acad Sci U S A. 2008;105:9355–9360. doi: 10.1073/pnas.0801294105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilakopoulos T, Deckman G, Kebbewar M, Rallis G, Harfouche R, Hussain SN. Regulation of nitric oxide production in limb and ventilatory muscles during chronic exercise training. Am J Physiol Lung Cell Mol Physiol. 2003;284:L452–L457. doi: 10.1152/ajplung.00270.2002. [DOI] [PubMed] [Google Scholar]
- Wang H, Viatchenko-Karpinski S, Sun J, Györke I, Benkusky NA, Kohr MJ, Valdivia HH, Murphy E, Györke S, Ziolo MT. Regulation of myocyte contraction via neuronal nitric oxide synthase: role of ryanodine receptor S-nitrosylation. J Physiol. 2010;588:2905–2917. doi: 10.1113/jphysiol.2010.192617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Marsden PA. Nitric oxide synthases: gene structure and regulation. Adv Pharmacol. 1995;34:71–90. doi: 10.1016/s1054-3589(08)61081-9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Newton DC, Marsden PA. Neuronal NOS: gene structure, mRNA diversity, and functional relevance. Crit Rev Neurobiol. 1999a;13:21–43. doi: 10.1615/critrevneurobiol.v13.i1.20. [DOI] [PubMed] [Google Scholar]
- Wang Y, Newton DC, Robb GB, Kau CL, Miller TL, Cheung AH, Hall AV, VanDamme S, Wilcox JN, Marsden PA. RNA diversity has profound effects on the translation of neuronal nitric oxide synthase. Proc Natl Acad Sci U S A. 1999b;96:12150–12155. doi: 10.1073/pnas.96.21.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter R, Yu L, Yu CA. The effects of nitric oxide on electron transport complexes. Arch Biochem Biophys. 1996;331:9–14. doi: 10.1006/abbi.1996.0276. [DOI] [PubMed] [Google Scholar]
- Xia Y, Berlowitz CO, Zweier JL. PIN inhibits nitric oxide and superoxide production from purified neuronal nitric oxide synthase. Biochim Biophys Acta. 2006;1760:1445–1449. doi: 10.1016/j.bbagen.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Xie J, Roddy P, Rife TK, Murad F, Young AP. Two closely linked but separable promoters for human neuronal nitric oxide synthase gene transcription. Proc Natl Acad Sci U S A. 1995;92:1242–1246. doi: 10.1073/pnas.92.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1999;96:657–662. doi: 10.1073/pnas.96.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AP, Murad F, Vaessin H, Xie J, Rife TK. Transcription of the human neuronal nitric oxide synthase gene in the central nervous system is mediated by multiple promoters. Adv Pharmacol. 1995;34:91–112. doi: 10.1016/s1054-3589(08)61082-0. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Casadei B. Sub-cellular targeting of constitutive NOS in health and disease. J Mol Cell Cardiol. 2012;52:341–350. doi: 10.1016/j.yjmcc.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Dingle L, Hall R, Casadei B. The role of nitric oxide and reactive oxygen species in the positive inotropic response to mechanical stretch in the mammalian myocardium. Biochim Biophys Acta. 2009;1787:811–817. doi: 10.1016/j.bbabio.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Zhang MH, Sears CE, Emanuel K, Redwood C, El-Armouche A, Kranias EG, Casadei B. Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res. 2008;102:242–249. doi: 10.1161/CIRCRESAHA.107.164798. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 2009;20:223–230. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]


