Abstract
Here we describe the ability of a high-density diamond microelectrode array targeted to resolve multi-site detection of fast exocytotic events from single cells. The array consists of nine boron-doped nanocrystalline diamond ultra-microelectrodes (9-Ch NCD-UMEA) radially distributed within a circular area of the dimensions of a single cell. The device can be operated in voltammetric or chronoamperometric configuration. Sensitivity to catecholamines, tested by dose–response calibrations, set the lowest detectable concentration of adrenaline to ∼5 μm. Catecholamine release from bovine or mouse chromaffin cells could be triggered by electrical stimulation or external KCl-enriched solutions. Spikes detected from the cell apex using carbon fibre microelectrodes showed an excellent correspondence with events measured at the bottom of the cell by the 9-Ch NCD-UMEA, confirming the ability of the array to resolve single quantal secretory events. Subcellular localization of exocytosis was provided by assigning each quantal event to one of the nine channels based on its location. The resulting mapping highlights the heterogeneous distribution of secretory activity in cell microdomains of 12–27 μm2. In bovine chromaffin cells, secretion was highly heterogeneous with zones of high and medium activity in 54% of the cell surface and zones of low or no activity in the remainder. The ‘non-active’ (‘silent’) zones covered 24% of the total and persisted for 6–8 min, indicating stable location. The 9-Ch NCD-UMEA therefore appears suitable for investigating the microdomain organization of neurosecretion with high spatial resolution.
Key points
A planar nanocrystalline diamond array with nine ultra-microelectrodes (9-Ch NCD-UMEA) has been designed for high spatial resolution of amperometric recordings in single chromaffin cells.
The 9-Ch NCD-UMEA operates in voltammetric and amperometric mode to reveal low doses of adrenaline, dopamine and serotonin. The lowest detectable concentration of adrenaline is ∼5 μm.
Using mouse and bovine chromaffin cells, single quantal exocytotic events are recorded from nine microareas of 12–27 μm2. We found an excellent correspondence with recordings from the cell apex using carbon fibre electrodes.
In the bovine, secretion is heterogeneous. There are areas of high and medium activity covering 54% of the cell surface and areas of low and no activity covering the remainder. The ‘non-active zones’ (silent) cover 24% of the cell surface and persist for minutes as the ‘active zones’.
The 9-Ch NCD-UMEA brings new insights into the spatial mapping of secretory sites in chromaffin cells.
Introduction
Exocytosis is an essential step for neurotransmission, allowing the vesicle content to be released into the extracellular space. Focusing on oxidizable neurotransmitters, the quantal nature of exocytosis can be uncovered by amperometric recordings. Properly polarized electrodes are placed next to the secreting cell and the electrochemically active surface of the electrode gives rise to a transient oxidation current (spike), which reflects the time course of vesicle fusion and release of oxidizable neurotransmitter molecules (Wightman et al. 1991, 1995; Chen et al. 1994; Xin & Wightman, 1998). The main features of this technique are: (i) the sub-millisecond temporal resolution, (ii) the high signal-to-noise ratio, which allows estimation of the quantity of released molecules and the fusion pore formation, and (iii) the ability to provide a direct measure of exocytosis, independent of vesicle retrieval (endocytosis) (Haller et al. 1998).
Amperometric spikes using carbon fibre electrodes (CFEs) are routinely detected on catecholamine-secreting chromaffin cells containing large dense-core vesicles (Wightman et al. 1991), but they can also be associated with the fusion of small synaptic vesicles in midbrain dopaminergic neurons (Staal et al. 2004). CFEs are easily shaped by a scalpel blade but have two main disadvantages: (1) their usage is limited to single-cell experiments, and (2) the large electrode active surface (∼45 μm2) severely hinders the recording from different microareas of the same cell. This latter issue can be partially overcome by using small-sized CFEs (∼2 μm diameter) carefully placed in different areas of the cell to reveal discontinuity of secretory events (Schroeder et al. 1994). This approach, however, is time consuming and does not furnish a quantitative view of the regional diversities of chromaffin cell secretion.
To overcome these drawbacks several attempts have been made to produce either planar multielectrode arrays for simultaneous recordings from different cells (Ayers et al. 2010; Barizuddin et al. 2010; Carabelli et al. 2010; Kim et al. 2012) or multi-site arrays for electrochemical detection from a single cell, to improve the spatial resolution of secretory events (Dias et al. 2002; Hafez et al. 2005; Zhang et al. 2008; Kisler et al. 2012; Lin et al. 2012). To fulfil the second task, two CFE prototypes have been recently assembled, one made of seven tightly packed carbon fibres (Zhang et al. 2008) and one with 8–15 carbon micro-rings assembled on a single tip (Lin et al. 2012). Despite the increased spatial resolutions these microelectrodes possesses the same advantages and drawbacks of classical CFEs. Concerning the planar multielectrode arrays with high spatial resolution, only 4-channel planar arrays have been so far realized based on different conducting materials. Platinum (Dias et al. 2002; Hafez et al. 2005), and more recently nitrogen-doped diamond-like carbon, thin gold films and indium tin oxide (ITO) have been employed (Kisler et al. 2012). All these arrays could detect fast amperometric spikes with high time resolution, apart from ITO which exhibited a significantly reduced sensitivity to catecholamines.
Given the importance of resolving the molecular events controlling the exocytotic machinery at higher-spatial resolution, we recently fabricated a high-density diamond ultra-microelectrode array with nine recording channels (9-Ch NCD-UMEA), whose overall area fits the size of a bovine chromaffin cell (Colombo et al. 2011). The microchip relies on the outstanding properties of boron-doped nanocrystalline diamond (Carabelli et al. 2010; Gao et al. 2011), i.e. biocompatibility, chemical inertness and stability, electrochemical sensitivity, optical transparency and wide potential window for water dissociation, thus allowing the electrochemical detection in both anodic and cathodic range (Pasquarelli et al. 2011).
Here we show that, when tested on bovine and mouse chromaffin cells, the 9-Ch NCD-UMEA possesses the same temporal resolution and catecholamine sensitivity of CFEs and is thus able to fully resolve amperometric spikes >8 pA arising from areas as small as 12 μm2. We found that the overall frequency of catecholamine release measured by all electrodes was significantly higher than CFEs, and proportional to the microelectrode dimension. Under these conditions, we show that the nine electrodes can independently reveal zones of variable secretory activity, ascribed uniquely to different frequency of release, while temporal and quantitative spike parameters are unmodified among the secreting zones. The 9-Ch NCD-UMEA uncovers zones of high and medium activity distributed over 52% of the total area while zones of low or no activity cover the remaining part and persisted for minutes. The ‘silent zones’ covered 24% of the total area and furnished a quantitative estimate of early observations by Schroeder et al. (1994) on the existence of stable ‘inactive release zones’ of secretion in bovine chromaffin cells (BCCs). The 9-Ch NCD-UMEA thus provides a user-friendly tool for resolving amperometric signals with high spatial resolution that can be employed to investigate the heterogeneous distribution of secretory sites on the chromaffin membrane surface (Schroeder et al. 1994; Robinson et al. 1995; Klingauf & Neher, 1997; Carabelli et al. 1998).
Methods
Ethical approval
All experimental protocols were approved by the University of Turin Animal Care and Use Committee (Turin, Italy) and were performed according to the National Guide for the Care and Use of Laboratory Animals adopted by the Italian Ministry of Health. Every effort was made to minimize animal suffering and the number of animals used. For removal of tissues, mice were deeply anaesthetized by means of CO2 inhalation and rapidly killed by cervical dislocation. Bovine adrenal glands were provided by the municipal slaughterhouse of Turin. They were isolated during the removal of the internal organs of the dead animal under veterinary supervision and with permission of the competent authorities.
Device fabrication and design
The 9-Ch NCD-UMEA is constructed by growing the nanocrystalline diamond (NCD) thin film on a double-sided polished sapphire wafer (Epistone, Longgang, Shenzhen, China, 2 inch DSP wafers), after an initial bias-enhanced nucleation process, which ensures strong covalent adhesion of the NCD film on the substrate (Colombo et al. 2011). A 200 nm NCD intrinsic layer is grown by means of hot filament chemical vapour deposition (HFCVD). Then, e-beam lithography and reactive ion etching (RIE) in argon/oxygen plasma were used to define the nine microelectrode geometry. The etching process was performed down to the sapphire substrate, to achieve the electrical insulation between the ultra-microelectrodes (>200 GΩ). A ∼250 nm boron-doped NCD layer was deposited onto the intrinsic NCD tracks by microwave plasma chemical vapour deposition (CVD), then the surface was treated with chromosulphuric acid in order to remove possible graphitic content and to obtain an oxygen surface termination. Notice that the bias-enhanced nucleation process involves the deposition of a thin silicon interlayer, which reduces the optical transparency of the 9-Ch NCD-UMEA, thus limiting its employment for fluorescence measurements.
Ohmic contacts were achieved by Ti/Au deposition and optical lithography; a Si3N4 passivation layer (1 μm) was deposited by plasma enhanced CVD because of its mechanical and chemical resistance. In the passivation layer, we opened a central hole (22 μm in diameter), corresponding to the same position of the nine microelectrodes, by optical lithography and RIE. The chip was assembled underneath a carrier board by flip-chip bonding with conductive epoxy and finally a glass ring was glued on the device to provide a perfusion chamber (150 μl) for the cells. Figure 1 shows the chip assembled onto the carrier board (panel A), an enlarged view of the perfusion chamber (panel B) and the details of the nine ultra-microelectrodes array in the round central hole (panel C) with a mouse chromaffin cell positioned on top of the electrodes by means of a glass patch-clamp pipette as indicated.
Figure 1.
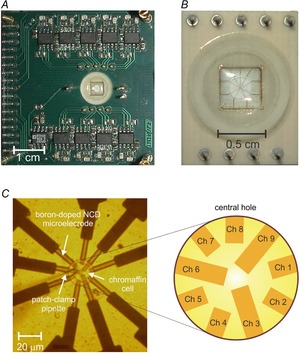
The 9-Ch NCD MEA geometry
A, chip assembly into the carrier board. B, detail of connecting carrier and the perfusion chamber. C, geometry of the electrode array: the whole surface is passivated with the exception of the circular central area, in which the nine electrodes contacting the gold strips (dark tracks) form a radial pattern. On the left a patch-clamp pipette has been used to place a mouse chromaffin cell on the electrodes.
Isolation and culture of mouse chromaffin cells
Chromaffin cells of the adrenal medulla were obtained from young (1–3 months) C57BL/6N mice, killed by cervical dislocation, and cultured as previously described (Marcantoni et al. 2009). Medulla digestion was achieved for 28 min at 37°C in a Dulbecco's modified Eagle's medium (DMEM) containing (in mm): 1.5 l-cysteine, 1 CaCl2, 0.5 EDTA and 20 U ml−1 of papain (Worthington Biochemical Corp., Lakewood, NJ, USA) and washing two times with a Locke solution containing 1 mm CaCl2 and 10 mg ml−1 bovine serum albumin (BSA; Sigma Chemical Co., St Louis, MO, USA). Cells were then resuspended in 2 ml DMEM supplemented with 15% fetal calf serum (Invitrogen, Grand Island, NY, USA) and plated in four-well plastic dishes previously treated with 5% BSA in order to avoid adhesion. After 1 h, 1.8 ml of DMEM supplemented with 15% fetal calf serum, 50 IU ml−1 penicillin, and 50 μg ml−1 streptomycin (Invitrogen) was added. Cells were then incubated at 37°C in a water-saturated atmosphere with 5% CO2 and used within 2–4 days after plating.
Isolation and culture of bovine chromaffin cells
Bovine chromaffin cells were isolated as previously described (Carabelli et al. 1998). Adrenal glands from 6- to 18-month-old cows, were rapidly transported to the culture unit in Locke buffer containing (mm): 154 NaCl, 5.6 KCl, 3.5 NaHCO3, 5.6 glucose, and 10 Hepes (pH 7.3 with NaOH). After controlling the integrity of the gland, the medulla digestion was started by injecting 3 ml of Locke solution containing 0.2% collagenase (Sigma), 1.7% hyaluronidase (Sigma), 0.5% BSA (Sigma) and 0.15% DNase I (Sigma). This was repeated three times at 30 min intervals while glands were kept at 37°C. Separation of the medulla from the cortex was performed manually with a disposable blade. Digestion solution was washed, and the tissue suspension was filtered with a nylon mesh (217 μm pore) and centrifuged at 71., . 5 g for 12 min at room temperature.
The supernatant and the upper pellet of erythrocytes were removed, while the pellet of chromaffin was resuspended in DMEM (GIBCO, Grand Island, NY, USA) and filtered with a nylon mesh (80 μm pore).
Cells were plated at a density of 1.5 × 104 ml–1 in BSA-treated plastic dishes, to avoid cell adhesion, and incubated at 37°C in a water-saturated 5% CO2 atmosphere. The culture medium contained: DMEM, fetal calf serum (15%; GIBCO), penicillin (50 IU ml−1) and streptomycin (50 μg ml−1; GIBCO). Cells were used within 2–4 days after plating.
Amperometric recordings with 9-Ch NCD-UMEA and CFEs
In order to detect amperometric spikes due to the oxidation of secreted catecholamines, mouse (or bovine) chromaffin cells were removed from non-adherent BSA-treated dishes and centrifuged for 4 min at 71., . 5 g. Then the pellet was suspended in a physiological solution containing (mm): 128 NaCl, 2 MgCl2, 10 Hepes, 10 glucose, 10 CaCl2, 4 KCl. Finally 50 μl of the cell suspension was placed in the perfusion chamber of the device. After waiting a few seconds for the cells to deposit on the bottom, we used a piezo-electric-driven micromanipulator to position one cell on the central hole, where the nine ultra-microelectrodes emerge. We used two different strategies to elicit catecholamine release: either an electrical stimulation of ±2 V square pulses of 50 ms duration, provided through the nine planar recording electrodes, or a KCl-enriched solution containing (mm): 100 NaCl, 2 MgCl2, 10 Hepes, 10 glucose, 30 KCl, 10 CaCl2. Settings of the electrical stimulation protocol were adjusted to obtain secretory responses comparable to those evoked by extracellular KCl-enriched solution. To oxidize released catecholamines, both NCD-UMEA electrodes and CFEs were polarized at +800 mV in amperometric mode and signals were monitored along with 2 min recordings. All potentials are measured vs. the Ag–AgCl reference electrode. Amperometric recordings were analysed by means of Igor macros, as previously described (Carabelli et al. 2007).
National Instruments hardware and software were employed for data acquisition and extracellular stimulation. Signal acquisition was performed at 16 bit resolution with a sampling rate of 4 kHz per channel and filtered at 1 kHz with a 6th order Bessel low-pass filter.
The electrical noise of each microelectrode was determined in spike-free trace segments of 10 s duration. The noise level was evaluated and then averaged over the nine electrodes, leading to a mean background noise amplitude of 2.1 ± 0.3 pA (r.m.s.), in good agreement with previous devices (Berberian et al. 2009). Following this, in the software routine the amperometric spikes were considered fully resolved if their amplitude was >8 pA. It is worth noticing that the noise level of the recording system is set by the dynamic range of the acquisition parameters (16 bit resolution and ±10 V input range) and not by the NCD electrodes or the front-end electronics (transimpedance amplifiers with 100 MΩ feedback resistor). In fact by reducing the dynamic range, the noise level scales down linearly by more than one decade. The reason for using a large dynamic range was to shorten the time the amplifiers recover their linear operation after they saturate during the electrical stimulation. Since the system cannot detect signals until recovery of linear operation, we have chosen the configuration with a shorter off-operation time in order to maximize the collection of secretory events immediately after each stimulus using a large dynamic range, even if it increases the background noise. The same conditions were also adopted when using the chemical stimulation.
Unless otherwise indicated, data are given as mean ± S.E.M.
Subcellular spatial mapping of exocytosis
To map the spatial distribution of exocytotic events on the chromaffin cell surfaces, we developed a Mathworks, Natick, MA UNITED STATES (MATLAB) routine which assigned each detected amperometric spike to one of the nine microelectrodes. To provide a graphical representation of the exocytotic events in distinct subcellular microdomains, the number of spikes revealed by each microelectrode was converted to a colour scale and the resulting colour assigned to the area of a 44 × 44 square grid occupied by the microelectrode (see Fig. 7). The routine also analysed the raster plot of the nine channel recordings to identify spikes simultaneously detected by neighbouring electrodes. For this purpose, we assumed that two or more spikes were simultaneous, and thus corresponding to the same exocytotic event, if their temporal shift was comparable with the time spent by catecholamines diffusing from the release site to the ultra-microelectrodes. Given that the cell was positioned on top of the electrodes and the distance between the cell (release site) and the ultra-microelectrodes is negligible compared to the average distance between ultra-microelectrodes (Δl ∼6 μm), the time required for the catecholamines to reach the electrodes is Δt = Δl2/6D (Schroeder et al. 1994), where D = 6 × 10–6 cm2 s–1 (Gerhardt & Adams, 1982) is the diffusion coefficient of catecholamines in aqueous solution. Thus, when the difference in time between the spike maxima was less than 10 ms, the recordings were identified as corresponding to a single exocytotic event and the routine assigned the spike to the microelectrode that detected it with larger amplitude.
Figure 7.
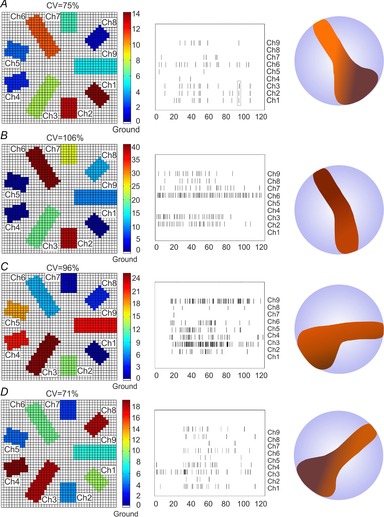
Spatial mapping of exocytosis in BCCs viewed by the 9-Ch NCD-UMEA
A–D, examples of the heterogeneous exocytosis recorded in four individual BCCs placed on top of the 9-Ch NCD-UMEA. The panels to the left illustrate the geometry of the 9 recording channels. The colours indicate the number of secretory events recorded by each electrode in 2 min. Data in A are derived from the recordings of Fig. 5A. The middle panels are the corresponding raster plots of the amperometric recordings which generate the colour to the left. For each example, the coefficient of variation (CV) of the recorded events is indicated. To the right are shown the manual reconstructions of the high-activity areas (brown) derived from the panels to the left as they would appear in a round BCC. The light-blue areas indicate the rest of the surface area which displays medium, low and no activity.
At variance with Hafez et al. (2005), who localized simultaneous events on the basis of random walk simulation and fitting the position of release events detected by more than two electrodes, we have chosen not to interpolate events detected from more than one electrode. This is because the spatial resolution of the device was about 27 μm2, corresponding to the area of the largest electrodes (channels 3, 6 and 9 in Fig. 1C), but the electrodes were separated by a similar average area (∼25 μm2), so the error was comparable to the resolution of the instrument. Finally, since three of the nine electrodes had a larger area (Fig. 1C), the number of events detected by each electrode was normalized to the electrode length.
Cleaning procedure of the array
The robustness and stability of the proposed device make the 9-Ch NCD-UMEA an extremely versatile tool for in vitro recordings from living cells. Thus the prototype could be easily cleaned and re-used many times without damage. The cleaning procedure started by washing the device with an enzymatic detergent (Tergazyme, Sigma), to remove organic residues due to the presence of cells in the perfusion chamber. The device was immersed in a solution containing 1% Tergazyme in distilled water for 4 h. The solution was heated (35°C) and stirred to facilitate the removal of the organic material deposited on the nine microelectrodes. After 4 h the 9-Ch NCD-UMEA was rinsed thoroughly with distilled water to remove residues of Tergazyme and then soaked in ethyl alcohol at 70% for 2 h to obtain the sterile conditions required for cell survival. Cleaning did not damage the 9-Ch NCD-UMEA, and could be thus repeated as needed, avoiding damage or performance degradation of the device.
Results
Calibration of the 9-Ch NCD-UMEA in voltammetric and amperometric operation mode
In a preliminary series of experiments we first assayed the redox sensitivity of the microelectrode array by cyclic voltammetry using voltage ramps from 0 to +1.2 V with a 20 mV s−1 scan rate. As shown in Fig. 2A for one representative electrode, in the presence of standard Tyrode saline solution, no redox activity was detected within the hydrolysis window (currents were <2 pA from 0 to +900 mV). Then, we tested the electrochemical response of the device in the presence of increasing concentrations of adrenaline ([A] = 10, 100, 1000 μm) (Fig. 2B–D). We compared traces from the same representative electrode and found that the catecholamine-induced oxidation current increased proportionally with [A] to reach maximal values at around 700–900 mV. Mean maximal currents increased from 61 ± 5 pA with 10 μm to 800 ± 20 pA with 1 mm [A]. The NCD-UMEA was also found to be comparably sensitive to dopamine (100 μm) and serotonin (30 μm) (Fig. 2E and F), broadening the physiological applications of the device. We obtained mean currents of 72 ± 8 pA when 100 μm dopamine was applied and 33 ± 5 pA with 30 μm serotonin.
Figure 2.

Voltammetric configuration: responses to catecholamines
Cyclic voltammetric recordings from 0 to +1.2 V are shown for one representative electrode in the presence of a Tyrode standard solution (A), and Tyrode solution plus: 10 μm adrenaline (B), 100 μm adrenaline (C), 1 mm adrenaline (D), 100 μm dopamine (E), and 30 μm serotonin (F). Insets in A and B show an enlarged view of CV limited to the range that is relevant for oxidation.
To quantify the sensitivity of the 9-Ch NCD-UMEA to adrenaline, we set the oxidation potential to +800 mV and tested the microelectrodes’ responsiveness in the amperometric mode. Figure 3A shows recordings of one representative electrode in response to 1–100 μm adrenaline ([A]). For each [A], data were acquired for 1 min and then the chip was washed with Tyrode solution and tested for the next concentration. In Fig. 3A the first second of each recording is displayed sequentially, without time gaps. The sensitivity of the nine microelectrodes is shown in Fig. 3B, where it is evident that all nine channels exhibited comparable currents, within an uncertainty of 2σ. Finally, averaged data points from Fig. 3B were fitted by linear regression (r = 0.98, slope 0.51 ± 0.02 pA μm−1), indicating a direct proportionality of the amperometric response to [A] for all nine microelectrodes (Fig. 3C). For all these experiments the current values were scaled to the perimeter of the electrodes and thus to the length, since the width was the same for all electrodes. It is worth recalling that in the case of disc-shaped microelectrodes the steady-state current (Iss) is proportional to the radius of the electrode (Wightman, 1981), while in general, Iss is proportional to the geometrical parameter that defines the boundary of the recording area (Sanderson & Anderson, 1985; Shea & Bard, 1987; Cohen & Kunz, 2000; Strutwolf & Williams, 2005). The general formula for calculating Iss is: Iss = nFDCΦ, where n is the number of electrons transferred, F is the Faraday constant, D is the diffusion coefficient, C is the concentration of the redox species, and Φ is the linear geometrical term defining the electrode. In our case, the geometrical parameter is the electrode length. Thus, the amperometric signals detected by the different microelectrodes were normalized to the major side of the rectangles shaping the electrodes.
Figure 3.
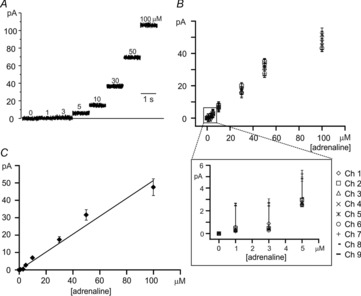
Amperometric configuration: sensitivity to adrenaline
A, amperometric responses to adrenaline at the indicated concentrations are shown for a representative electrode, polarized to +800 mV. Traces of one electrode over 120 s total recording have been selected. Only the first second of the recording is shown for each concentration. Consecutive recordings using increasing concentrations of adrenaline were separated by time breaks (not indicated in the figure), in order to properly remove the previous solution and wash the device. B, mean values and SD of amperometric response obtained from the nine channels at the concentrations indicated. Inset provides an enlarged view at low adrenaline concentrations. C, linear regression line through the averaged data taken from B (r = 0.98; see text).
Secretory events elicited in mouse chromaffin cells: the NCD-UMEA versus CFE
Exocytotic events from cultured mouse chromaffin cells (MCCs) were induced using either an electrical pulse generated by the stimulation circuitry included in the read-out electronics of the 9-Ch NCD-UMEA prototype or using KCl-enriched solutions, as previously reported using a similar boron-doped NCD-MEA (Carabelli et al. 2010) or carbon fibre electrodes (CFE) (Marcantoni et al. 2009; Gosso et al. 2011).
In both cases, MCCs were cultured on non-adherent dishes, as previously described (Carabelli et al. 2010) and then mechanically positioned on the array by means of a patch-clamp glass pipette. In this experimental configuration, the tight contact between cell and NCD electrodes strongly limits catecholamine dilution by diffusion. The electrical stimulation was applied through all nine electrodes and consisted of a ±2 V square pulse of 50 ms. Under these conditions the release of oxidizable molecules could occur by either a depolarization of the cell in contact with the stimulation electrode or by electroporation of the plasma membrane. To identify the type of mechanism we used Trypan Blue (0.4% Sigma) staining, which reveals the presence of plasma membrane electroporation during stimulation (Ghosh et al. 2013). We used a final 0.02% Trypan Blue solution to stain the MCCs and applied trains of ±2 V square pulses of 50 ms. In n = 7 trials the cells remained unstained with no visible uptake of the dye inside the cell, suggesting no evident irreversible plasma membrane electroporation during electrical stimulation, although we cannot exclude a transient mechanism of electroporation. Apart from this, it is thus likely that catecholamine release is generated by a capacitative current that depolarizes the cell (Dittami & Rabbitt, 2010) and induces Ca2+-driven exocytosis in the form of trains of amperometric spikes of comparable size to those evoked by chemical KCl stimulation (Carabelli et al. 2007, 2010; Marcantoni et al. 2009).
Representative amperometric spikes using electrical stimulation are shown in Fig. 4A and the corresponding spike parameters are compared with recordings using KCl stimulation (Fig. 4B).
Figure 4.
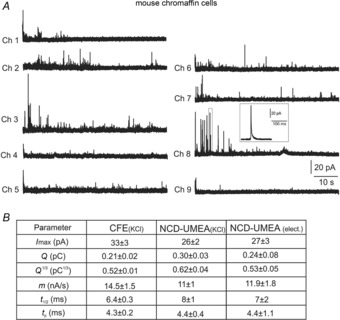
Detection of quantal events from mouse chromaffin cells
A, representative amperometric spikes from a MCC detected by the nine microelectrodes, indicated as Ch 1 to Ch 9. A single spike detected by electrode 8 (Ch 8) is shown on an enlarged time scale in the inset. B, mean values of spike parameters and related standard errors averaged from n = 21 MCCs for CFEs and n = 10 MCCs for 9-Ch NCD-UMEA for KCl and electrical stimulation. Data referring to the maximal current amplitude (Imax), quantal charge (Q) and its cubic root (Q1/3), rising phase slope (m), half-time width (t1/2) and time to peak (tp) are compared for CFEs and 9-Ch NCD-UMEA.
Analysis of amperometric spikes was performed using Igor macros (see Machado et al. 2001; Carabelli et al. 2007) and the spike-detection criteria described in the Methods. Briefly, we measured: (1) the maximum oxidation current (Imax) as the height of each spike, (2) the vesicular charge content (Q) as the time integral of the current, (3) the cubic root of Q (Q1/3), which is proportional to the vesicle diameter, (4) the rise-time of the spike 25–75% (m), (5) the width of the spike at half of its maximum height (t1/2) and (6) the time to the peak (tp). As reported in Fig. 4B, none of the six parameters obtained with electrical stimulation were statistically different from those obtained using KCl-enriched external solution (P > 0.05, ANOVA followed by Bonferroni post hoc comparison). All this suggests that, even in the case of a hypothetical transient electroporation, alternated electric pulses produce similar exocytotic responses to KCl-induced depolarization.
In order to validate the device sensitivity in the presence of chromaffin cells, amperometric spikes from n = 20 MCCs were then compared with those recorded by conventional CFEs (n = 21). We found that all spike parameters were not significantly different if measured by the 9-Ch NCD–UMEA or conventional CFEs (P > 0.05) regardless of the type of stimulation used (electrical or KCl), indicating comparable sensitivity and time resolution of the two devices.
Mapping of amperometric spikes in bovine chromaffin cells
The geometry of the 9-Ch NCD-UMEA was designed with the specific aim of detecting secretory events from different membrane microareas of a single cell. Spatial mapping of secretory sites was performed using bovine instead of mouse chromaffin cells, due to their larger diameter (18–22 μm). In this way, the area of the central hole containing the nine detecting microelectrodes (22 μm diameter; Fig. 1C) perfectly matched the size of a bovine chromaffin cell (BCC). Representative traces of amperometric spikes using KCl-stimulated BCCs are shown in Fig. 5. Mean values of spike parameters obtained from 12 cells were then compared with those recorded by CFEs from KCl-stimulated BCCs (n = 10). As shown in Fig. 5B, there was excellent agreement among the amplitude and total charge of the spikes detected by either one of the two devices (P > 0.05, calculated with Student's two-tailed t test), as well as in the kinetic parameters (t1/2, m and tp). We found that the mean Q1/3 value, estimated with the 9-Ch-NCD-UMEA, was also in good agreement with that measured by other groups using CFEs, ranging from 1.04 ± 0.41 to 1.09 ± 0.46 pC1/3 (Wightman et al. 1991; Finnegan et al. 1996; Graham et al. 2000).
Figure 5.
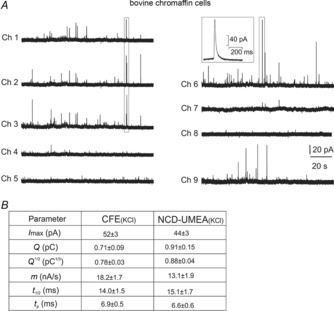
Detection of quantal events from bovine chromaffin cells
A, representative amperometric spikes from a BCC detected by the nine microelectrodes, indicated as Ch 1 to Ch 9. A single spike detected by electrode 6 (Ch 6) is shown on an enlarged time scale in the inset. Notice the low or no-release activity of Ch 4, 5 and 8. B, mean values of spike parameters averaged from n = 10 bovine chromaffin cells for CFEs and n = 12 bovine chromaffin cells for 9-Ch NCD-UMEA. Data referring to maximum current amplitude (Imax), quantal charge (Q) and its cubic root (Q1/3), rising phase slope (m), half-time width (t1/2), time to peak (tp) are compared for CFEs and 9-Ch NCD-UMEA.
As an additional confirmation, we found strong similarities among the distributions of Q1/3 and t1/2 obtained with CFEs and the 9-Ch NCD-UMEA. The double Gaussian functions required to fit the Q1/3 distribution, were centred at 0.73 and 1.00 pC1/3 (R2 = 0.99) for the NCD-UMEA, and at 0.64 and 0.89 pC1/3 (R2 = 0.98) for the CFE (thin curves in Fig. 6B). Thick curves represent the sum of the two single Gaussian curves. Notice that the mean charge associated with chromaffin granules fusion is approximately threefold greater than that estimated with MCCs (0.71 ± 0.09 pC versus 0.21 ± 0.02 pC), in good agreement with previous reports (Graham et al. 2000; Gosso et al. 2011). This confirms that the 9Ch NCD-UMEA has a nearly equal sensitivity to that of the CFE.
Figure 6.

Spike size and kinetics comparison: CFEs recordings vs. 9-Ch NCD-UMEA
A, representative amperometric spikes detected by one channel of the 9-Ch NCD-UMEA (left) and by a CFE (right) using bovine chromaffin cells. Notice the ‘foot’ anticipating the fast rising phase of the two amperometric spikes. B, cubic root charge histogram distribution fitted by a double Gaussian function derived from the 9-Ch NCD-UMEA (left) and CFE (right) spike recordings (see text for details of the fitting). C, half-time width histogram derived from the 9-Ch NCD-UMEA (left) and CFE (right) spike recordings.
It is worth noticing that the kinetic parameters of the spike are also in good agreement with those recorded by CFEs. Histograms of square root values of t1/2 are shown in Fig. 6C for spikes collected by the 9-Ch NCD-UMEA and CFEs. Data were collected from 12 and 10 cells, respectively, and ranged between 1.2 ms1/2 and 10.3 ms1/2 for CFE and between 1.4 ms1/2 and 13.7 ms1/2 with mean values of (3.7 ms)1/2 and (3.9 ms)1/2, suggesting comparable distributions with previously reported data in BCCs using CFEs (Schroeder et al. 1992; Jankowski et al. 1993). Finally, we considered the parameters of amperometric spikes detected by the nine electrodes and compared their mean values by one-way ANOVA followed by a Bonferroni post hoc analysis. We found no statistically significant differences in the spike parameters recorded with different electrodes (P > 0.05), confirming that each of the electrodes detects exocytotic events with the same sensitivity and resolution.
Spike frequencies revealed with the 9-Ch NCD-UMEA and CFEs are comparable
We next compared the frequency of exocytotic events from MCCs when using CFEs and the 9-Ch NCD-UMEA. We obtained 0.44 ± 0.07 Hz with CFEs (n = 21 cells) and either 0.82 ± 0.18 Hz (n = 10 cells) or 0.77 ± 0.16 Hz (n = 10 cells) with the 9-Ch NCD-UMEA when secretion was evoked by electrical stimulation or KCl-enriched solution, respectively. Because the detection areas of the two devices were considerably different, the frequency of the events was scaled to the active surface, 38.5 μm2 for CFE and 153 μm2 for 9-Ch NCD-UMEA. Considering that MCCs have a mean diameter of ∼15 μm and cover only 46% of the electrodes’ active areas, the effective biosensing area of the NCD array was proportionally reduced to 70 μm2. Following this, the scaled frequency to the active surface of the CFEs ((11.4 ± 1.8) × 10−3 Hz μm−2) was not significantly different from that of the 9-Ch NCD-UMEA when using either electrical ((11.7 ± 2.5) × 10−3 Hz μm−2) or chemical stimulation ((10.9 ± 1.8) × 10−3 Hz μm−2; P > 0.05, ANOVA followed by Bonferroni post hoc comparison). Similarly, for recordings from BCCs, the spike frequency was calculated over 240 s recordings from 10 cells. Since BCCs covered the entire detection surface of the device, the scaled frequency for the two devices was (11.2 ± 1.3) × 10−3 Hz μm−2 for CFE and (11.1 ± 3.9) × 10−3 Hz μm−2 for UMEA (P > 0.05 with two-tailed Student's t test).
Spatial mapping of exocytosis in bovine chromaffin cells
Given that the 9-Ch NCD-UMEA was able to reveal single vesicle release with high time resolution, we next studied the subcellular localization of exocytotic events in bovine chromaffin cells using the MATLAB routine described in the Methods. Figure 7 shows the spatial localization of quantal events over the nine electrodes in four different BCCs. To the left are shown, in different colours, the nine electrodes drawn in an array of a 44 × 44 square grid. The colour indicates the number of quantal exocytic events detected by the sensitive area of the electrode, giving a direct chromatic view of the microdomain regions of different exocytotic activity. To the right are shown the corresponding raster plots of 2 min recordings of each electrode, as well as the occurrence of simultaneous spikes. It is evident that the nine electrodes detected remarkably different amounts of quantal events. There were channels with intense secretory activity and channels with no activity regardless of the size of the electrode. Obviously the larger electrodes (3, 6 and 9) on average detected more events. In the example of Fig. 7A (corresponding to the recordings of Fig. 5), channels 4, 5, 7 and 8 are associated to membrane domains with very low or no activity, while the remaining channels have higher activity, suggesting membrane areas with clear heterogeneous granule contents release.
As described in Methods, events were considered synchronous, and thus associated to the same secretory events, when their temporal separation was less than 10 ms. Examples of spikes simultaneously recorded from three different electrodes (1, 2 and 3) are indicated by the rectangle in the raster plot of Fig. 7A and their corresponding traces in Fig. 5. Since different electrodes detected variable amount of secretory events, independently of their active area, we quantified this variability using the coefficient of variation (CV) related to the number of events detected by the nine channels: CV = (σ/μ)100, where μ is the mean of the number of events recorded individually by the nine electrodes (normalized to the surface area) and σ is the standard deviation of the mean. We found that CV was greater than 71% for all the cells analysed (n = 10). In the examples of Fig. 7 the four CVs ranged between 71% and 106%, indicating a high variability of secretory activity in different areas of the cell. Notice that in the case of extreme homogeneity (when each electrode reveals the same normalized number of events) the expected CV is zero.
We should also remark that although secretion regularly occurred in all cells tested (n = 10), 22 of the total 90 channels analysed remained silent (white bar in Fig. 8A), corresponding to 24% of channels unable to detect secretory activity (Fig. 8B). From the histogram of the percentage of release recorded from each channel (Fig. 8A) it is evident that there are surface areas of very different activity. The histogram exhibits two clear minima around 30 and 65% that suggest the existence of areas that we intuitively defined as no or low activity with percentage of secretion below 30% (white and light-blue bars in Fig. 8A), medium activity with secretion between 30 and 65% (green bars) and high activity with secretion above 65%. Excluding the channels with no activity (silent; 24%), we found that on average all the other microelectrodes recorded an almost equal percentage of low (22%), medium (26%) and high activity (28%) (Fig. 8B). We also noticed that, although covering only 28% of the total area, the channels exhibiting high activity (dark-red colours) covered a continuous narrow area that crossed the cell along the diameter (see right panels in Fig. 7). This cell-crossing area of high activity seems a peculiarity of BCCs. It was observed in 8 of the 10 cells tested and occurred at different angular orientations (see the examples in Fig. 7), as expected due to the casual positioning of the cell on the chip.
Figure 8.

Histograms of the percentage of release revealed by the 9-Ch NCD-UMEA and percentage of surface areas with different secretory activities
A, histogram of the percentage of release detected by each of the 9 ultra-microelectrodes collected from 10 BBCs for a total of 90 recording channels. The colours indicate different percentage of activity: no activity (white bar), low activity (light-blue), medium activity (green) and high activity (dark-red). B, percentage of the channel areas recording either no release (white), low (light-blue), medium (green) or high secretory activity (dark-red) (see text for details).
In conclusion, secretion in BCCs viewed through the 9-Ch NCD-UMEA appears remarkably heterogeneous and organized in large microdomains. Activity is absent in 24% of the cell surface (‘silent zones’) and coexists with well-defined continuous areas of intense activity covering nearly the same percentage of cell surface (28%). Areas of high and medium activity cover more or less half of the cell surface (54%), comparable to the remaining 46% exhibiting either low or no activity. Thus, the new picture of chromaffin cells that we derive from the 9-Ch NCD-UMEA is of a cell with large fractions of non-secreting areas under physiological conditions whose structural organization and functional role remains to be investigated.
Discussion
Here we propose an improved version of a 9-Ch NCD-UMEA to resolve neurotransmitter secretion within microdomains of single chromaffin cells. Specifically, the new microchip is: (i) suitable for multi-site recordings within single living cells, (ii) responsive to micromolar neurotransmitter concentrations, and (iii) versatile in both chronoamperometric and voltammetric mode. The multi-site array highlights the existence of large microdomains with high, medium and low secretory activity as well as areas of silent zones with null activity in BCCs. On average, each region of activity and silent zones cover nearly 1/4 of the total area. The high-activity area seems to extend transversally along the cell diameter, suggesting the existence of a highly polarized secretory area, evocative of the arrangement of the intact gland, where chromaffin cells are packed in columns facing the capillary (Carmichael, 1987; Garcia et al. 2006). This ‘secretory pole’ may be preserved in isolated cells and gives origin to the ‘high-activity zones’ that are randomly oriented due to the casual cell positioning on the chip.
Another interesting finding with the 9-Ch NCD-UMEA is that only half of the cell surface exhibits high and medium secretory activity (54%), while the remaining 46% is either silent or weakly responding to stimulation. The ‘silent zones’ are randomly distributed and occupy 24% of the total surface area. Areas lacking secretion have been already reported in BCCs using small tip diameter CFEs (2 μm) (Schroeder et al. 1994; Robinson et al. 1995), but a quantitative estimation of the covered areas was biased by the complexity of monitoring secretion by randomly moving two small-size CFEs under the microscope. This issue can be now more easily tackled by the 9-Ch NCD-UMEA, given its planar configuration and high-resolution sensitive areas. It is worth noticing, however, that the present distribution of ‘hot’ and ‘silent’ zones in isolated cells may be different in adrenal gland slices or in vivo conditions.
Planar boron-doped diamonds vs. other chips for electrochemical signalling
Despite their high time resolution and low background noise (Chow et al. 1992), CFEs cannot be used for simultaneous fluorescence imaging and patch-clamping, and their use is limited to the anodic range. Thus, other materials and geometries have been proposed to fabricate electrochemical biosensors with improved performances. Lindau's lab was the first to adopt a planar array of Pt-microelectrodes to investigate vesicle localization and fusion pore formation (Dias et al. 2002; Hafez et al. 2005; Berberian et al. 2009) and more recently, arrays of indium tin oxide (ITO), nitrogen-doped diamond-like carbon and thin gold films on glass substrates, with improved transparency (Kisler et al. 2012). All arrays were capable of detecting amperometric signals with high resolution except ITO, which showed significantly lower sensitivity to catecholamines. An improved spatial resolution was recently achieved using microelectrode tips containing 8–15 carbon-ring fibres to detect amperometric signals from individual neurons or cell networks (Zhang et al. 2008; Lin et al. 2012). In good agreement with our findings, Lin et al. (2012) found that secretion in phaeochromocytoma (PC12) cells is extremely heterogeneous when recorded with an eight carbon-ring microelectrode array of 20 μm total diameter. They found evidence for microareas of high activity as well as ‘cold spots’ of low or no activity, which remained silent for more than 3 min. In PC12 cells 1 out of 8 channels was silent, while we found on average 2.2 channels out of 9 not responding in BCCs. Despite these differences, it is interesting to underscore how the increased spatial resolution with arrays of 8–9 sensitive microareas per cell helps to uncover microdomains of ‘no secretion’ (silent zones) which are overlooked in devices with one or a few microelectrodes per cell surface. ‘Inactive release zones’ were first reported by Schroeder et al. (1994) using small-diameter CFEs moved on top of the BCC surface.
The 9-Ch NCD-UMEA for resolving amperometric spikes in cell microdomains
As previously reported, the main advantages of using boron-doped NCD-UMEAs to record amperometric signals are: the electrochemical stability, the large hydrolysis window, robustness, the transparency from the far infrared to UV, and the possibility of using CVD, photolithography and dry-etching techniques to construct microarrays of defined geometries (Gao et al. 2010; Colombo et al. 2011). With respect to our previous NCD prototypes (Carabelli et al. 2010; Pasquarelli et al. 2011) here we provide an advanced array geometry to spatially identify microdomains of neurosecretion in single chromaffin cells. Amperometric signals collected from MCCs and BCCs with the 9-Ch NCD-UMEAs exhibited no significant difference from those recorded by CFEs, which represent the ‘gold standard’ for this approach. Mean amperometric charges are in good agreement with those measured by others using CFEs (Finnegan et al. 1996; Haller et al. 1998), thus confirming that the 9-Ch chip is sensitive enough for detecting the release of quantal fusion events from chromaffin cells.
Considering the high-density geometry of the array and that chromaffin cells were positioned and held on the detector surface by a patch pipette, a tight contact of the cell to the electrode was ensured. This reduces the rate-limiting diffusional dispersion of catecholamines (Jankowski et al. 1993) and optimizes their amperometric detection. In this regard, the estimated spike half-width is comparable with that measured by CFEs (14 ms with 9-Ch NCD-UMEA vs. 8–18 ms with CFEs) (Haller et al. 1998) and with the mean value estimated by Pt-microelectrodes (13.8 ms) (Berberian et al. 2009). Distribution of the t1/2 square root values (Schroeder et al. 1992) measured by the 9-Ch NCD-UMEA confirms that spike duration is also comparable with that of CFEs, thus confirming the excellent time-resolution of the diamond chip.
Our findings also suggest that quantal events recorded from the apex (with CFEs) or from the bottom of a chromaffin cell (with the 9-Ch NCD-UMEA) are comparable. This is in good agreement with recordings from cell bottom using planar arrays of different materials and geometry, and confirms the convenience of using planar microchips for detecting secretion (Chen et al. 1994; Hafez et al. 2005; Gao et al. 2010; Picollo et al. 2013). However, these observations are at variance with previous findings on BCCs (Amatore et al. 2007), reporting different secretion efficiency between the apex and the bottom of the cell using ITO devices. An explanation for this discrepancy could be due to the different cell plating conditions, besides the materials and the electrode geometry.
Spatial mapping of exocytosis with the 9-Ch NCD-UMEA
Our data clearly show that secretory granules can be independently detected by all the electrodes of the 9-Ch NCD-UMEA. Some electrodes detected high and medium secretory activity, while others detected low or no activity. The two areas covered more or less half of the cell, but most interestingly, 2.2 channels out of 9 remained silent, uncovering a remarkably heterogeneous spatial mapping of exocytosis in BCCs. The physiological meaning of the highly localized areas for secretion and large areas of silent activity remains to be clarified. A possibility is that secretion is highly polarized in the columnar organization of chromaffin cells in the intact gland. Chromaffin cells are innervated at one site and face blood capillaries at another site, which is presumably the ‘secretory pole’, since most of the vesicles are found there (Carmichael, 1987). If this geometry is preserved in isolated cells, it will give origin to the random diametrically distributed crossing-area of high activity illustrated in Fig. 7. There could obviously be other explanations to the existence of high-activity areas, but it is clear that the 9-Ch NCD-UMEA opens new perspectives through which secretion can be studied at higher spatial resolution and poses new questions on how the different cell microdomains are organized in terms of Ca2+ channel densities, SNARE protein availability and vesicle pool distribution. An intriguing possibility is that the ‘silent areas’ may undergo remodelling of ion channels, membrane receptors and membrane proteins controlling secretion (Guerineau et al. 2012) and become active during chronic stress or sustained neurogenic stimulation of the adrenal gland. New versions of the chip with an increased number of ultra-microelectrodes and improved transparency would certainly enhance the interest regarding this new nanotech approach allowing simultaneous measurements of secretion and fluorescence imaging and other parameters that diamond chips can measure in potentiometric mode (action potentials, pH) (Bitziou et al. 2008).
A final concern on the potential use of 9-Ch NCD-UMEA is related to the present dimensions of the electrodes which are 3–10 times larger than the 2 μm diameter CFEs (Schroeder et al. 1994). These dimensions do not allow any conclusions to be drawn on how secretion is organized (a) in CNS synapses, where docked vesicles are tightly coupled to presynaptic Ca2+ channels in nanodomains (Schneggenburger et al. 2012), or (b) in chromaffin cells, where Ca2+ channels and secretory granules are either co-localized in square micrometre hot-spots (Monck et al. 1994; Robinson et al. 1995; Wu et al. 2009) or uniformly distributed over membrane areas where Ca2+ channels are loosely coupled to chromaffin granules (Klingauf & Neher, 1997; Carabelli et al. 1998). Our findings suggest that, independently of the coupling of chromaffin granules to Ca2+ channels, there exists a macroscopic heterogeneity associated with the existence of large domains of cell surface where secretion is either absent or low for about half of the total area.
Acknowledgments
None declared.
Glossary
- [A]
adrenaline concentration
- BCC
bovine chromaffin cell
- CFE
carbon fibre electrode
- Ch
channel
- CVD
chemical vapour deposition
- ITO
indium tin oxide
- MCC
mouse chromaffin cell
- NCD
nanocrystalline diamond
- RIE
reactive ion etching
- UMEA
ultra-microelectrode array
Additional information
Competing interests
None declared.
Author contributions
S.G., C.F. and V.C. contributed to data collection and analysis of amperometric and voltammetric experiments, performed in the laboratories of the Department of Drug Science at the University of Turin, Italy. A.P. and El.Co. contributed to the design and production of the 9-Ch NCD-UMEA and to the hardware and software development, at the Department of Electron Devices and Circuits, University of Ulm. S.G. and M.T. developed the MatLab routine for the localization of the amperometric events. S.G., Em.Ca. and V.C. contributed to the conception and design of experiments and the drafting of the article as well as revising it critically for important intellectual content. All authors have approved the final version of the manuscript.
Funding
This work was supported by Regione Piemonte, P.O.R.-F.E.S.R. 2008/2014 project ‘Be-Free’ and P.O.R.-F.E.S.R. 2007/2013 project ‘MicroDiBi’; by the Italian Ministry for University and Research (MIUR), ‘FIRB-Futuro in Ricerca 2010’ project D11J11000450001 and PRIN 2010/2011 project 2010JFYFY2.
Author's present address
E. Colombo: Neuroscience and Brain Technologies, Italian Institute of Technology, 16163 Genoa, Italy.
References
- Amatore C, Arbault S, Lemaître F, Verchier Y. Comparison of apex and bottom secretion efficiency at chromaffin cells as measured by amperometry. Biophys Chem. 2007;127:165–171. doi: 10.1016/j.bpc.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Ayers S, Berberian K, Gillis KD, Lindau M, Minch BA. Post-CMOS fabrication of working electrodes for on-chip recordings of transmitter release. IEEE Trans Biomed Circuits Syst. 2010;4:86–92. doi: 10.1109/TBCAS.2009.2033706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barizuddin S, Liu X, Mathai JC, Hossain M, Gillis KD, Gangopadhyay S. Automated targeting of cells to electrochemical electrodes using a surface chemistry approach for the measurement of quantal exocytosis. ACS Chem Neurosci. 2010;1:590–597. doi: 10.1021/cn1000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberian K, Kisler K, Fang Q, Lindau M. Improved surface-patterned platinum microelectrodes for the study of exocytotic events. Anal Chem. 2009;81:8734–8740. doi: 10.1021/ac900674g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitziou E, O'Hare D, Patel BA. Simultaneous detection of pH changes and histamine release from oxyntic glands in isolated stomach. Anal Chem. 2008;80:8733–8740. doi: 10.1021/ac801413b. [DOI] [PubMed] [Google Scholar]
- Carabelli V, Carra I, Carbone E. Localized secretion of ATP and opioids revealed through single Ca2+ channel modulation in bovine chromaffin cells. Neuron. 1998;20:1255–1268. doi: 10.1016/s0896-6273(00)80505-x. [DOI] [PubMed] [Google Scholar]
- Carabelli V, Gosso S, Marcantoni A, Xu Y, Colombo E, Gao Z, Vittone E, Kohn E, Pasquarelli A, Carbone E. Nanocrystalline diamond microelectrode arrays fabricated on sapphire technology for high-time resolution of quantal catecholamine secretion from chromaffin cells. Biosens Bioelectron. 2010;26:92–98. doi: 10.1016/j.bios.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Carabelli V, Marcantoni A, Comunanza V, de Luca A, Diaz J, Borges R, Carbone E. Chronic hypoxia up-regulates α1H T-type channels and low-threshold catecholamine secretion in rat chromaffin cells. J Physiol. 2007;584:149–165. doi: 10.1113/jphysiol.2007.132274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SW. Morphology and Innervation of the Adrenal Medulla. Vol. 1. Boca Raton, Florida: CRC Press; 1987. [Google Scholar]
- Chen TK, Luo G, Ewing AG. Amperometric monitoring of stimulated catecholamine release from rat pheochromocytoma (PC12) cells at the zeptomole level. Anal Chem. 1994;66:3031–3035. doi: 10.1021/ac00091a007. [DOI] [PubMed] [Google Scholar]
- Chow RH, von Ruden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Cohen AE, Kunz RR. Large-area interdigitated array microelectrodes for electrochemical sensing. Sens Actuators B Chem. 2000;62:23–29. [Google Scholar]
- Colombo E, Men Y, Scharpf J, Pietzka C, Dipalo M, Herfurth P, Gao Z, Schneider M, Carabelli V, Carbone E, Kohn E, Pasquarelli A. Fabrication of a NCD microelectrode array for amperometric detection with micrometer spatial resolution. Diamond Rel Mat. 2011;20:793–797. [Google Scholar]
- Dias AF, Dernick G, Valero V, Yong MG, James CD, Craighead HG, Lindau M. An electrochemical detector array to study cell biology on the nanoscale. Nanotechnology. 2002;13:285–289. [Google Scholar]
- Dittami GM, Rabbitt RD. Electrically evoking and electrochemically resolving quantal release on a microchip. Lab Chip. 2010;10:30–35. doi: 10.1039/b911763f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan JM, Borges R, Wightman RM. Comparison of cytosolic Ca2+ and exocytosis responses from single rat and bovine chromaffin cells. Neuroscience. 1996;71:833–843. doi: 10.1016/0306-4522(95)00459-9. [DOI] [PubMed] [Google Scholar]
- Gao Z, Carabelli V, Carbone E, Colombo E, Demaria F, Dipalo M, Gosso S, Manfredotti C, Pasquarelli A, Rossi S, Xu Y, Vittone E, Kohn E. Transparent diamond microelectrodes for biochemical application. Diamond Rel Mat. 2010;19:1021–1026. [Google Scholar]
- Gao Z, Carabelli V, Carbone E, Colombo E, Dipalo M, Manfredotti C, Pasquarelli A, Feneberg M, Thonke K, Vittone E, Kohn E. Transparent microelectrode array in diamond technology. J Micro-Nano Mechatron. 2011;6:33–37. [Google Scholar]
- Garcia AG, Garcia-De-Diego AM, Gandia L, Borges R, Garcia-Sancho J. Calcium signaling and exocytosis in adrenal chromaffin cells. Physiol Rev. 2006;86:1093–1131. doi: 10.1152/physrev.00039.2005. [DOI] [PubMed] [Google Scholar]
- Gerhardt G, Adams RN. Determination of diffusion coefficients by flow injection analysis. Anal Chem. 1982;54:2618–2620. [Google Scholar]
- Ghosh J, Liu X, Gillis KD. Electroporation followed by electrochemical measurement of quantal transmitter release from single cells using a patterned microelectrode. Lab Chip. 2013;13:2083–2090. doi: 10.1039/c3lc41324a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosso S, Gavello D, Giachello CN, Franchino C, Carbone E, Carabelli V. The effect of CdSe-ZnS quantum dots on calcium currents and catecholamine secretion in mouse chromaffin cells. Biomaterials. 2011;32:9040–9050. doi: 10.1016/j.biomaterials.2011.08.031. [DOI] [PubMed] [Google Scholar]
- Graham ME, Fisher RJ, Burgoyne RD. Measurement of exocytosis by amperometry in adrenal chromaffin cells: effects of clostridial neurotoxins and activation of protein kinase C on fusion pore kinetics. Biochimie. 2000;82:469–479. doi: 10.1016/s0300-9084(00)00196-6. [DOI] [PubMed] [Google Scholar]
- Guerineau NC, Desarmenien MG, Carabelli V, Carbone E. Functional chromaffin cell plasticity in response to stress: focus on nicotinic, gap junction, and voltage-gated Ca2+ channels. J Mol Neurosci. 2012;48:368–386. doi: 10.1007/s12031-012-9707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafez I, Kisler K, Berberian K, Dernick G, Valero V, Yong MG, Craighead HG, Lindau M. Electrochemical imaging of fusion pore openings by electrochemical detector arrays. Proc Natl Acad Sci U S A. 2005;102:13879–13884. doi: 10.1073/pnas.0504098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller M, Heinemann C, Chow RH, Heidelberger R, Neher E. Comparison of secretory responses as measured by membrane capacitance and by amperometry. Biophys J. 1998;74:2100–2113. doi: 10.1016/S0006-3495(98)77917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski JA, Schroeder TJ, Ciolkowski EL, Wightman RM. Temporal characteristics of quantal secretion of catecholamines from adrenal medullary cells. J Biol Chem. 1993;268:14694–14700. [PubMed] [Google Scholar]
- Kim BN, Herbst AD, Kim SJ, Minch BA, Lindau M. Parallel recording of neurotransmitters release from chromaffin cells using a 10×10 CMOS IC potentiostat array with on-chip working electrodes. Biosens Bioelectron. 2012;41:736–744. doi: 10.1016/j.bios.2012.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisler K, Kim BN, Liu X, Berberian K, Fang Q, Mathai CJ, Gangopadhyay S, Gillis KD, Lindau M. Transparent electrode materials for simultaneous amperometric detection of exocytosis and fluorescence microscopy. J Biomater Nanobiotechnol. 2012;3:243–253. doi: 10.4236/jbnb.2012.322030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingauf J, Neher E. Modeling buffered Ca2+ diffusion near the membrane: Implications for secretion in neuroendocrine cells. Biophys J. 1997;72:674–690. doi: 10.1016/s0006-3495(97)78704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Trouillon R, Svensson MI, Keighron JD, Cans AS, Ewing AG. Carbon-ring microelectrode arrays for electrochemical imaging of single cell exocytosis: fabrication and characterization. Anal Chem. 2012;84:2949–2954. doi: 10.1021/ac3000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado JD, Morales A, Gomez JF, Borges R. cAmp modulates exocytotic kinetics and increases quantal size in chromaffin cells. Mol Pharmacol. 2001;60:514–520. [PubMed] [Google Scholar]
- Marcantoni A, Carabelli V, Vandael DH, Comunanza V, Carbone E. PDE type-4 inhibition increases L-type Ca2+ currents, action potential firing, and quantal size of exocytosis in mouse chromaffin cells. Pflugers Arch. 2009;457:1093–1110. doi: 10.1007/s00424-008-0584-4. [DOI] [PubMed] [Google Scholar]
- Monck JR, Robinson IM, Escobar AL, Vergara JL, Fernandez JM. Pulsed laser imaging of rapid Ca2+ gradients in excitable cells. Biophys J. 1994;67:505–514. doi: 10.1016/S0006-3495(94)80554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquarelli A, Carabelli V, Xu Y, Colombo E, Gao Z, Scharpf J, Carbone E, Kohn E. Diamond microelectrode arrays for the detection of secretory cell activity. Int J Environ Anal Chem. 2011;91:150–160. [Google Scholar]
- Picollo F, Gosso S, Vittone E, Pasquarelli A, Carbone E, Olivero P, Carabelli V. A new diamond biosensor with integrated graphitic microchannels for detecting quantal exocytic events from chromaffin cells. Adv Mater. 2013;25:4696–4700. doi: 10.1002/adma.201300710. [DOI] [PubMed] [Google Scholar]
- Robinson IM, Finnegan JM, Monck JR, Wightman RM, Fernandez JM. Colocalization of calcium entry and exocytotic release sites in adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1995;92:2474–2478. doi: 10.1073/pnas.92.7.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson DG, Anderson LB. Filar electrodes – steady-state currents and spectroelectrochemistry at twin interdigitated electrodes. Anal Chem. 1985;57:2388–2393. [Google Scholar]
- Schneggenburger R, Han Y, Kochubey O. Ca2+ channels and transmitter release at the active zone. Cell Calcium. 2012;52:199–207. doi: 10.1016/j.ceca.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Schroeder TJ, Jankowski JA, Kawagoe KT, Wightman RM, Lefrou C, Amatore C. Analysis of diffusional broadening of vesicular packets of catecholamines released from biological cells during exocytosis. Anal Chem. 1992;64:3077–3083. doi: 10.1021/ac00048a003. [DOI] [PubMed] [Google Scholar]
- Schroeder TJ, Jankowski JA, Senyshyn J, Holz RW, Wightman RM. Zones of exocytotic release on bovine adrenal medullary cells in culture. J Biol Chem. 1994;269:17215–17220. [PubMed] [Google Scholar]
- Shea TV, Bard AJ. Digital-simulation of homogeneous chemical-reactions coupled to heterogeneous electron-transfer and applications at platinum mica platinum ultramicroband electrodes. Anal Chem. 1987;59:2101–2111. [Google Scholar]
- Staal RG, Mosharov EV, Sulzer D. Dopamine neurons release transmitter via a flickering fusion pore. Nat Neurosci. 2004;7:341–346. doi: 10.1038/nn1205. [DOI] [PubMed] [Google Scholar]
- Strutwolf J, Williams DE. Electrochemical sensor design using coplanar and elevated interdigitated array electrodes. A computational study. Electroanalysis. 2005;17:169–177. [Google Scholar]
- Wightman RM. Microvoltammetric electrodes. Anal Chem. 1981;53:1125A–1134A. doi: 10.1021/ac00232a791. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, Jr, Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci U S A. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Schroeder TJ, Finnegan JM, Ciolkowski EL, Pihel K. Time course of release of catecholamines from individual vesicles during exocytosis at adrenal medullary cells. Biophys J. 1995;68:383–390. doi: 10.1016/S0006-3495(95)80199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Llobet A, Lagnado L. Loose coupling between calcium channels and sites of exocytosis in chromaffin cells. J Physiol. 2009;587:5377–5391. doi: 10.1113/jphysiol.2009.176065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Q, Wightman RM. Simultaneous detection of catecholamine exocytosis and Ca2+ release from single bovine chromaffin cells using a dual microsensor. Anal Chem. 1998;70:1677–1681. doi: 10.1021/ac970746o. [DOI] [PubMed] [Google Scholar]
- Zhang B, Adams KL, Luber SJ, Eves DJ, Heien ML, Ewing AG. Spatially and temporally resolved single-cell exocytosis utilizing individually addressable carbon microelectrode arrays. Anal Chem. 2008;80:1394–1400. doi: 10.1021/ac702409s. [DOI] [PMC free article] [PubMed] [Google Scholar]


