Abstract
Previous preclinical studies have emphasized that drugs of abuse, through actions within and between mesocorticolimbic (MCL) regions, usurp learning and memory processes normally involved in the pursuit of natural rewards. To distinguish MCL circuit pathobiological neuroadaptations that accompany addiction from general learning processes associated with natural reward, we trained two groups of rats to self-administer either cocaine (IV) or sucrose (orally) followed by an identically enforced 30 day abstinence period. These procedures are known to induce behavioral changes and neuroadaptations. A third group of sedentary animals served as a negative control group for general handling effects. We examined low-frequency spontaneous fluctuations in the functional magnetic resonance imaging (fMRI) signal, known as resting-state functional connectivity (rsFC), as a measure of intrinsic neurobiological interactions between brain regions. Decreased rsFC was seen in the cocaine-SA compared with both sucrose-SA and housing control groups between prelimbic (PrL) cortex and entopeduncular nucleus and between nucleus accumbens core (AcbC) and dorsomedial prefrontal cortex (dmPFC). Moreover, individual differences in cocaine SA escalation predicted connectivity strength only in the Acb-dmPFC circuit. These data provide evidence of fronto-striatal plasticity across the addiction trajectory, which are consistent with Acb-PFC hypoactivity seen in abstinent human drug addicts, indicating potential circuit level biomarkers that may inform therapeutic interventions. They further suggest that available data from cross-sectional human studies may reflect the consequence of rather a predispositional predecessor to their dependence.
Key words: : CBV, fMRI, functional connectivity, mesocorticolimbic system, propofol, rsMRI, spontaneous fluctuation
Introduction
Relapse to drug use, even after prolonged withdrawal, remains a major problem in addiction treatment (Hunt et al., 1971; O'Brien, 1997). Decades of research have consistently identified the roles of the mesocorticolimbic (MCL) system in reward, motivation, learning, and memory. Whereas neuroplasticity changes within this system has been linked to compulsive drug seeking and taking behavior (Goldstein and Volkow, 2002; Robinson and Berridge, 2008; Wise and Rompre, 1989), such motivationally linked neural circuitry is also activated in response to natural rewards such as palatable food, although drugs of abuse are known to induce dopamine (DA) release that is far greater in magnitude and longer in duration than food delivery (Di Chiara and Imperato, 1988). Recent evidence suggests that in some circumstances, intermittent access to sugar can lead to behavioral and neurochemical changes that resemble the effects of a substance of abuse (Avena et al., 2008). However, since DA release is greater following abused drugs than natural rewards, one might postulate that after chronic exposure, drug-associated long-term alterations in neural function and circuit remodeling will not only at least partially overlap across reinforcers, the more profound drug-induced DA signaling may also induce maladaptation that lead to addiction. Nevertheless, differentiating neuroadaptations caused by natural and drug rewards remain to be explored.
The SA reinstatement model has been developed to investigate the behavioral, environmental, and neural mechanisms underlying drug relapse. In these studies, animals are typically trained to lever-press for drug infusions paired with discrete environmental cues, followed by abstinence or extinction training for a certain period of time (Epstein et al., 2006). Accumulating data suggest that this model captures at least part of the neurobiological aspects of human relapse, including persistent drug seeking, increased motivation to obtain drug, and relapse to drug use when subsequently exposed to stress, drug, or drug-related cues (Ahmed and Cador, 2006; Vanderschuren and Everitt, 2004). Although there have been extensive studies elucidating the molecular, cellular, and electrophysiological mechanisms of neuroadaptations in this model (Epstein et al., 2006; Shaham et al., 2003), systems-level investigation of brain circuitry changes resulting from drug exposure and withdrawal remains scarce.
Recent advances in understanding the functional connectivity of the brain present novel opportunities to pursue this question. Resting-state functional connectivity (rsFC) is the term given to task-independent low-frequency synchronized activity across distant brain regions. It has been observed not only in humans (Fox et al., 2005; Greicius et al., 2003), but also in nonhuman primates (Vincent et al., 2007) and rodents (Lu et al., 2007; Lu et al., 2012a; Majeed et al., 2009; Pawela et al., 2008; Zhang et al., 2010; Zhao et al., 2008). Connectivity patterns revealed by this technique show remarkable correspondence with networks explicitly activated during cognitive tasks (Smith et al., 2009). Furthermore, the strength of rsFC circuits can predict subsequent task activation (Zou et al., 2012) and behavioral performance (Kelly et al., 2008; Liang et al., 2013; Seeley et al., 2007; Zou et al., 2012). Specific changes in functional connectivity strength have been reported in drug-dependent populations, including cocaine (Gu et al., 2010; Tomasi et al., 2010), nicotine (Hong et al., 2009), and heroin (Ma et al., 2010) [Sutherland et al. (2012) for a recent review]. However, cross-sectional human imaging studies are inherently limited in their ability to distinguish whether differences in functional connectivity are caused by drug exposure per se or they reflect a pre-existing drug-dependent liability.
In the present study, we employed rsFC to investigate systems-level neuroplasticity in a reinstatement addiction model consisting of long access (LgA) cocaine self-administration (SA) followed by 30 days of forced abstinence. Given that a priming dose of cocaine will precipitate reinstatement of drug-seeking behavior (Shaham et al., 2003), we acquired resting-state functional magnetic resonance imaging (fMRI) data before and after an acute cocaine injection. Following the demonstration that specific MCL circuits have reduced connectivity strength in human cocaine addicts (Gu et al., 2010), we hypothesized: (1) cocaine SA specific reductions in rsFC between MCL system nodes, (2) the strength of such alterations will reflect cocaine SA intake history, and (3) an acute cocaine injection will enhance specific rsFC circuits known to mediate behavioral reinstatement. To control for nonspecific contextual and learning effects and motor behavior from those specifically caused by cocaine SA and abstinence, we also scanned a separate group of animals that underwent similar SA training procedures using oral sucrose as a reinforcer. We further hypothesized that difference in functional connectivity between cocaine and sucrose groups likely reflects alterations specific to cocaine SA and abstinence. Additionally, we also included a training- and drug-naïve group to establish a functional connectivity baseline that is unbiased by the behavioral training history.
Materials and Methods
Subjects
Thirty-eight male Long-Evans rats (Charles River Laboratories, Wilmington, MA) started the study at ∼12 weeks of age. All procedures were reviewed and approved by the Animal Care and Use Committee of both the University of Pennsylvania and the National Institute on Drug Abuse (NIDA-IRP).
Experimental design
The experiment involved two phases, a SA phase and an imaging phase, with the former conducted at the University of Pennsylvania and the latter at NIDA. Rats were divided into three groups: cocaine SA, sucrose SA, and sedentary controls. Rats destined for cocaine SA were surgically implanted with a jugular catheter, allowed 6 days for recovery and then trained to SA cocaine (see SA procedures). The sucrose group received identical SA training, but was reinforced with oral sucrose. After 14 days of 1 h SA sessions, both SA groups were subjected to 20 days of long-access (LgA) SA sessions and weekend discrimination test sessions followed by 1 month (30–34 days) of abstinence. The housing control group underwent neither surgery nor subsequent behavioral training. Approximately half way through the month-long abstinence period, animals were transported to the NIDA-IRP where the caging, bedding, food, and light cycle were identical to that used at the University of Pennsylvania. Imaging procedures were conducted approximately 2 weeks after the animals arrived at NIDA.
SA procedures
Surgery and postoperative maintenance
Animals from cocaine and sucrose groups were deeply anesthetized with ketamine (30 mg/kg, IP) and xylazine (5 mg/kg, IP). A catheter was implanted in the external jugular vein and passed subcutaneously to the top of the back where it exited into a connector. After surgery, animals were flushed daily with 0.2 mL of an ampicillin solution (0.1 g/mL) containing heparin (300 IU/mL) to maintain patency. Rats were given free access to water, but were restricted to 15–20 g of food each day to maintain their body weight at ∼370 g.
Apparatus
Training and testing sessions were conducted in Plexiglas operant chambers (34×23×29 cm; l×w×h) placed inside sound attenuating cubicles. Each chamber was equipped with a response lever, a cue light, which was located above the lever, a syringe pump for sucrose or cocaine delivery, and a speaker at the top of the chamber.
Training phase
Animals in the cocaine-SA group (n=13) were first trained to SA cocaine (0.75 mg/kg/infusion) in daily 1-h sessions under a fixed-ratio-1 schedule of reinforcement. A contextual odor cue consisting of a cotton swab soaked in either a lemon or a vanilla scent (Pure Lemon Extract and Pure Vanilla Extract; McCormick) was placed underneath the chamber floor. Scents were counterbalanced between animals. Each cocaine infusion (FR=1) was paired with a 7-sec illumination of a cue light and an auditory tone. An 8-sec time-out period followed each reinforced lever press, during which responding was recorded, but not reinforced.
Animals in the sucrose-SA group (n=13) were exposed to the same pretraining and odor exposure and training, except for the following: (1) each reinforced lever-press was followed by delivery of a 32% sucrose solution into a drinking well (0.2 mL over 10 sec) and (2) the number of sucrose infusions was matched to the daily number of cocaine infusions earned by the cocaine group. SA training continued for 14 days for both groups. Data from the odor discrimination and cue responses are presented in a companion paper.
Long-access SA sessions
After the initial training phase, cocaine- and sucrose-SA sessions were extended from 1 to 6-h per day (referred to as long-access, LgA sessions); LgA training continued for an additional 20 days.
Imaging procedures
MRI preparation procedures were similar to those previously reported (Lu et al., 2007). Briefly, on scan days, rats were anesthetized with 2% isoflurane in 1:1 mixture of oxygen and air. Catheters were placed in a femoral artery for blood sampling and blood pressure monitoring and a femoral vein for drug administration. Animals were intubated using a customized T-shaped trachea tube to maintain artificial ventilation and to bypass exhaled air into a gas analyzer for continuous monitoring of end-tidal CO2 and O2. Core body temperature was maintained at 37.5°C±0.5°C with a water-circulating pad. After surgery, anesthesia was switched to continuous IV infusion of propofol (35 mg/kg/h). We also conducted task-based fMRI experiments in the same cohorts of animals with contextual cues (odor) and cocaine challenge (Lu et al., 2012b, 2013). Given the profound pharmacological effects of anesthetics on neurovascular coupling and brain function, we had performed pilot studies with a number of commonly used anesthetics to find the “best” agent. Results suggested that animals anesthetized with propofol gave reasonable fMRI signals in all three types of experiments. We thus chose propofol for this study. A neuromuscular blocking agent (pancuronium bromide, 1.5 g/kg/h, IV) was administered to further minimize motion artifacts. This preparation allowed us to monitor and maintain critical autonomic parameters within a constant and normal physiological range. Superparamagnetic contrast agent Ferumoxtran-10 (Advanced Magnetics, Cambridge, MA) was administered (15 mg/kg, IV) to achieve cerebral blood volume contrast, which greatly enhances the sensitivity and functional specificity of the fMRI signal (Vanduffel et al., 2001).
Data acquisition
fMRI experiments were performed on a Bruker Biospin 9.4T scanner (Bruker Medizintechnik, Karlsruhe, Germany). A volume coil was used for RF excitation, and a circular surface coil for signal reception. The anterior commissure (−0.36 mm from bregma, Paxinos and Watson, 2007) was used as a fiducial landmark to localize slices consistently across animals. Following initial localization scans, a Rapid Acquisition with Relaxation Enhancement sequence was used to acquire high-resolution anatomical images in the coronal plane, which was used for subsequent image registration. Scan parameters were: 23 slices, slice thickness of 1 mm, TR=2650 ms, effective TE=40 ms, field of view (FOV)=30 mm, matrix size=192×192, data were zero-padded to 256×256 for image reconstruction. Resting-state BOLD fMRI data were acquired using a single-shot, gradient-echo EPI sequence with the following parameters: 11 slices, thickness=1 mm, TR=1s, TE=11 ms, FOV=30 mm, matrix size=64×64, in-plane resolution=0.469 mm2. A total of 520 volumes were collected during each session. Two resting-state fMRI scans were performed: one in the absence of any acute drug manipulation that modeled the addiction trait, and a second after a saline injection (0.3 mL, IV) and two cocaine injections (0.75 mg/kg/injection, IV), intended to model the drug-induced reinstatement condition. Data collection failed for one housing control rat during the second resting scan.
Data analysis
Resting-state fMRI data from individual animals were registered to a common space (Lu et al., 2007, 2010) and subjected to the following preprocessed pipeline using the Analysis of Functional NeuroImaging (AFNI) software package (Cox, 1996): slice timing correction, linear and quadratic trend removal, band-pass (0.005–0.1 Hz) filtering, and spatial smoothing (0.6 mm FWHM). The average signal time course from ventricle and white matter (WM) regions of interests (ROIs), thought to reflect global physiological noise in the BOLD signal, was removed from gray matter signal through linear regression. Even though there were no group differences in CO2 or BP levels, since both parameters could potentially influence the hemodynamic signal, we also performed the rsFC analysis with BP and end-tidal CO2 as confounding variables. The spatial patterns using these additional regressors were consistent with only using CSF and WM. Since these data were not available for all rats, only the CSF and WM regressed data are reported in this study.
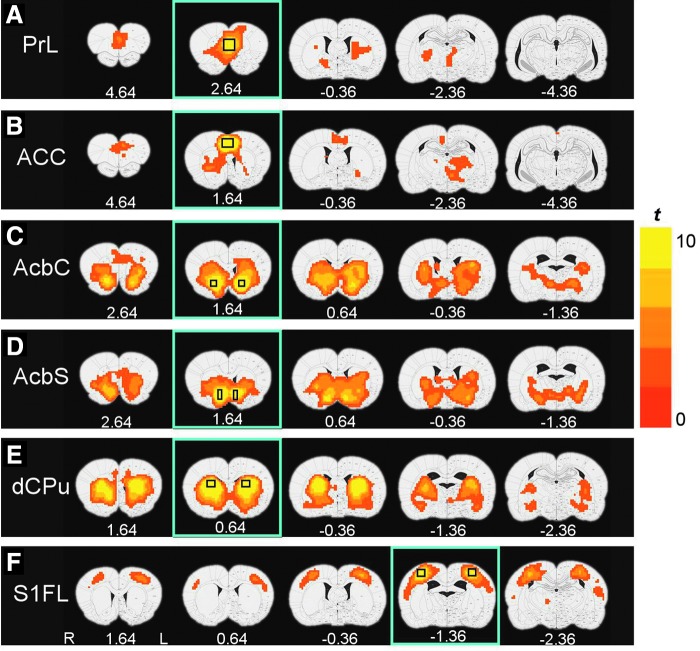
Five seed regions from the MCL system, serving as ROIs for the functional connectivity analysis, were anatomically defined (Paxinos and Watson, 2007) unilaterally across the midline in the prelimbic (PrL) (16 voxels) and anterior cingulate cortex (CG1/2, ACC) (12 voxels), and bilaterally in the nucleus accumbens core (AcbC, 8 voxels) and shell (AcbS, 12 voxels), and dorsal caudate-putamen (CPu, 18 voxels) (Fig. 1). Each seed ROI was manually drawn on the coronal slice with the largest representation of the region to minimize potential errors in registration and partial volume effects. A bilateral primary somatosensory cortex (S1FL) ROI (18 voxels) served to (1) validate our processing strategy and (2) as a control region for effect specificity. A cross-correlation coefficient (cc) map for each seed ROI was obtained by correlating the average time course from the seed with the time course of every voxel in the brain. The cc maps were then transformed by Fisher's z-transformation (z=½×ln[(1+cc)/(1−cc)]) to approach a normal distribution.
FIG. 1.
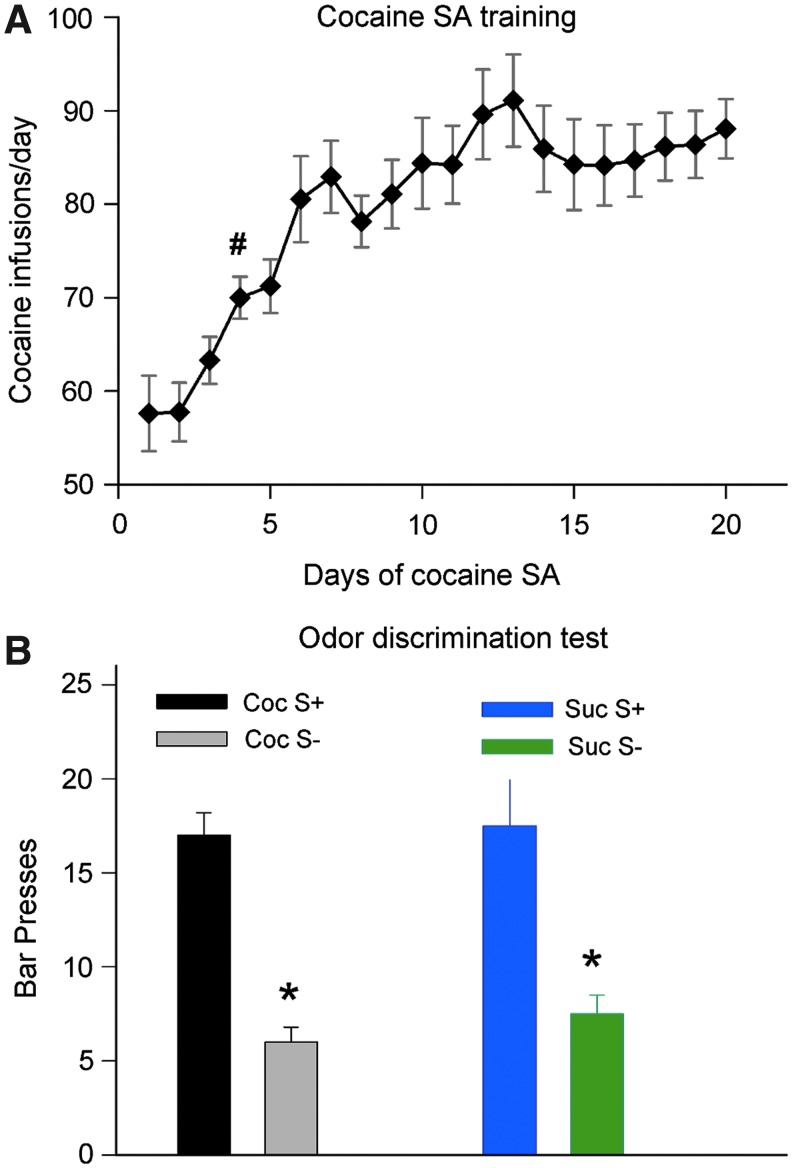
The amount of cocaine self-administered by rats escalated over time (A). Significant increase in cocaine infusions occurred on day 4 (#p<0.05, one-way ANOVA with post hoc Dunnett's test). (B) Both cocaine and sucrose SA rats showed significantly greater number of bar presses when presented with odors previously paired with the delivery of the reinforcers (S+) than no odor pairing (S−), indicating successful odor discrimination (*p<0.001).
One sample t-tests against the null hypothesis were conducted on each of the three groups (data from the two resting-state MRI scans, pre- and post-cocaine injections were averaged together). A threshold of p<0.005 uncorrected (t>3.49 for the control group, d.f.=11; t>3.42 for sucrose and cocaine groups, d.f.=12) together with a cluster size of 13 voxels was required to reach p<0.005, corrected for multiple comparisons, based on Monte Carlo simulations (Cox, 1996) was used to generate functional connectivity maps for each group. Linear mixed-effect (LME) modeling was constructed for 3 (GROUPS)×2 (DRUG condition; pre/post cocaine injection) analyses using 3dLME from the AFNI software package, a front-end to the LME routine from the R statistics package (R Development Core Team, 2010; www.R-project.org). To improve the sensitivity of detection while still controlling for the false positive rate, a threshold of uncorrected p<0.01 (F>5.26 for GROUP, F>7.44 for DRUG, and F>5.29 for interaction effects), combined with a cluster threshold of seven voxels, was used to obtain corrected p<0.05, with the further restriction that significant clusters must also overlap with regions shown to be significant in at least one group connectivity map (the group connectivity map applied in this study was corrected at a threshold of p<0.01). The variable cluster size thresholds reflect different number of comparisons across the five seed connectivity maps. For regions that showed significant GROUP main effects, whole brain, voxel-wise post hoc analyses were conducted to test for significance between the cocaine and sucrose, cocaine and control, and sucrose and control groups. A threshold of uncorrected p<0.01 combined with a cluster threshold of 7 voxels was used to obtain p<0.05 corrected post hoc results, which were considered significant only if they overlapped with the regions showing significant GROUP effects.
Exploratory connectivity-behavioral analyses were conducted between rsFC circuits that showed a main effect of either GROUP or DRUG against cocaine escalation index (CEI) defined as [cocaine intake during the last SA session minus intake on the first SA session]. For regions that showed significant cocaine GROUP effects, a Pearson correlation between the average rsFC of the two resting state scans and CEI was calculated for the cocaine-SA rats. For regions showing a significant DRUG effect, Pearson correlation was calculated separately for the two resting-state scans.
To test the robustness of our results and appropriateness of our analysis pipeline, we performed a reverse direction rsFC analysis, such that target regions from our seed ROIs that showed significant GROUP and DRUG main effects were redefined as new seeds and identical rsFC calculations and LME modeling were conducted as above. We expected that the new seed regions would show essentially similar GROUP or DRUG effect to the a priori anatomical seed regions as rsFC analysis measures the coherence between two regions, which should result in relatively consistent responses whether area A or B is taken as the seed. Furthermore, because rsFC cannot ascertain directionality (and thus none are implied in this report), in this article we use the term target only to distinguish the resultant coherent regions from the chosen seed location of the analysis.
Results
Behavioral characterization
As expected from previous studies, the amount of cocaine self-administered by rats escalated over the course of the SA sessions. Results are shown in Figure 1A. One-way repeated measures ANOVA with post hoc Dunnett's test revealed that starting on the fourth day, there was a significant increase in cocaine infusions (p<0.05), reaching 90±4 on the 20th day. Rats in the sucrose-SA group were individually matched with those in the cocaine-SA group for number of reinforcers/sessions; thus both groups had identical escalation patterns. Furthermore, both cocaine- and sucrose-SA rats learned to discriminate the odors pared with cocaine or sucrose administration, as shown in Figure 1B.
Functional connectivity in the sedentary control group
The rsFC maps derived from the six seed ROIs in the sedentary control group (Fig. 2) are generally consistent with known neuroanatomical connections as defined using traditional tract tracing methods (Groenewegen et al., 1999; McGeorge and Faull, 1989; Voorn et al., 2004). For example, the PrL (Fig. 2A) and ACC (Fig. 2B) seeds revealed significant overlapping rsFC maps with regions, including CPu and thalamus (Th) as well as with each other; the PrL functional connectivity pattern also extended to infralimbic cortex (IL) and ventral and medial orbital cortices. The AcbC (Fig. 2C) and AcbS (Fig. 2D) exhibited functional connectivity with PrL, most basal ganglia nuclei (CPu, Acb, globus pallidus [GP], and ventral pallidum [VP]), and extended amygdala, including basal nucleus and bed nucleus of the stria terminals (BNST). In addition, significant connectivity was observed between AcbC and ACC and between AcbS and IL. The dorsal CPu (Fig. 2E) was functionally connected bilaterally with dorsal and ventral CPu and with GP, ACC, amygdala, hypothalamus, and structures comprising the extended amygdala (VP, BNST). Finally, BOLD fluctuations in the control seed S1FL (Fig. 2F) were significantly correlated with bilateral S1 and S2 cortex, as expected from known neuroanatomy and previous rat imaging studies [e.g., Lu et al. (2007)].
FIG. 2.
rsFC maps derived from the six seed ROIs in the housing control group of rats (p<0.01, corrected for multiple comparison). The seed ROI locations are shown in rectangles in each row. (A) Prelimbic cortex (PrL); (B) anterior cingulated (ACC); (C) nucleus accumbens, core (AcbC); (D) nucleus accumbens, shell (AcbS); (E) dorsal caudate-putamen (dCPu); and (F), primary somatosensory cortex (S1FL). The number beneath each panel indicates coronal slice location relative to bregma. The right side of each brain section corresponds to the left hemisphere.
Effects of behavioral training on functional connectivity
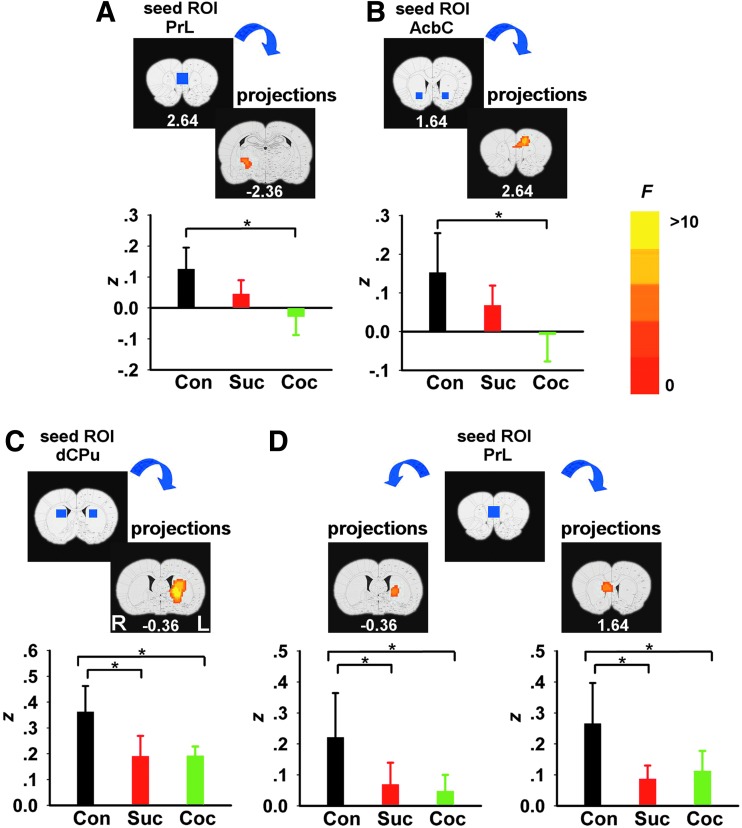
We next examined the effects of chronic operant behavioral training on functional connectivity. ANOVA revealed significant GROUP main effects between five seed–target pairs. However, post hoc analyses revealed that the cocaine-SA group uniquely showed reduced circuit strength only in two of these circuits: between PrL and right entopeduncular (EP) nucleus (Fig. 3A) and between AcbC and dorsomedial prefrontal cortex (dmPFC, including PrL and ACC/CG1) (Fig. 3B) when compared with the sedentary group, which did not differ from the sucrose-SA group. In contrast, rsFC decreased in both the cocaine- and sucrose-SA groups compared to the sedentary group between the right dorsal CPu and left CPu (Fig. 3C) and the PrL and bilateral CPu (Fig. 3D).
FIG. 3.
rsFC GROUP main effects differences between cocaine, sucrose, and housing control group. Brain areas showing a significant GROUP effect when seed ROIs (blue rectangles) were located at PrL (A, D), AcbC (B), and dCPu (C). Abbreviations are the same as in Figure 1. Bar plots compare mean Fisher's-z score between the seed region and target voxels depicted on the slices above the graph, averaged across the two resting-state scans in each group (mean±SD). “*” indicates significance at p<0.05. Decrease in rsFC strength between the two groups based on whole brain voxel-wise post hoc analyses.
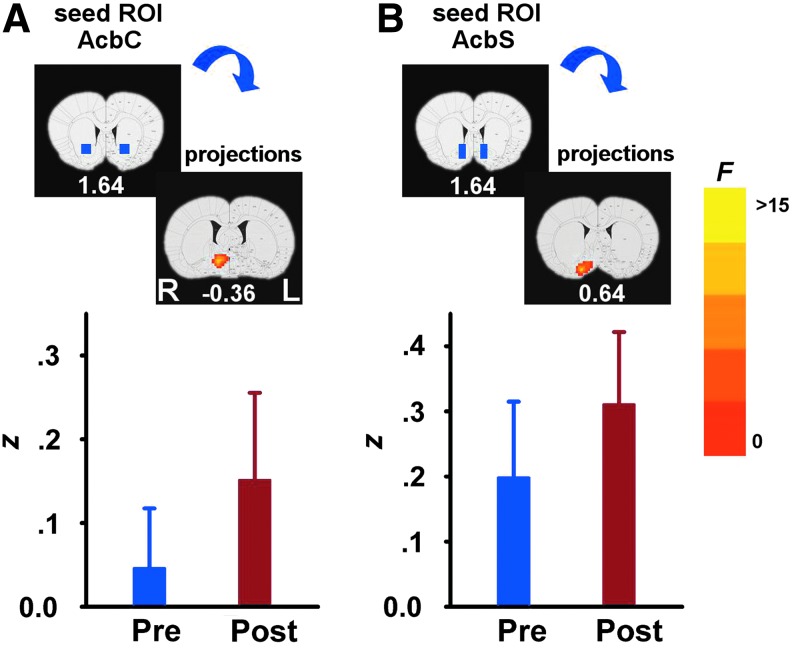
When examining the effects of acute cocaine administration on circuit strength, significant DRUG main effects were seen only between two pairs of seed–target areas: (1) AcbC and right hypothalamic preoptic area (POA) (Fig. 4A) and (2) AcbS and right VP-substantia innominata (SI) (Fig. 4B). Whole-brain post hoc analyses revealed that acute cocaine challenge increased rsFC in both regional pairs. There were no significant GROUP×DRUG interactions from any of the six seed ROIs. Importantly, there was no difference in either CO2 or BP during scan acquisition across the three groups following cocaine challenge; however, there was a significant DRUG main effect after the cocaine injection manifested as a decrease in BP (Table 1).
FIG. 4.
rsFC DRUG main effects pre- and postcocaine administration. Regions showing a significant increase in rsFC after cocaine administration when seed ROIs (blue rectangles) were located in the AcbC (A) and AcbS (B). Abbreviations are the same as in Figure. 1. Graph compares mean Fisher's-z score between the seed region and all the voxels in the region above the graph averaged across the three groups (mean±SD). Pre corresponds to the first resting-state scan before acute cocaine challenge, and post corresponds to the second resting-state scan after acute cocaine challenge.
Table 1.
Blood Pressure and End-Tidal CO2 Measurements Before and After Acute Cocaine Challenge
| Groups | BP (mmHg) (mean±SD) | CO2 (%) (mean±SD) |
|---|---|---|
| Precocaine | ||
| Control rats | 124.73±35.23 | 2.54±0.21 |
| Sucrose-SA rats | 130.02±32.24 | 2.47±0.12 |
| Cocaine-SA rats | 130.15±32.18 | 2.45±0.24 |
| Postcocaine | ||
| Control rats | 112.56±35.08 | 2.57±0.24 |
| Sucrose-SA rats | 114.96±30.95 | 2.41±0.21 |
| Cocaine-SA rats | 120.02±33.41 | 2.44±0.21 |
BP and end-tidal CO2 measurements were recorded during the two resting-state scans. Data from 1 cocaine-SA rat and 1 sucrose-SA rat were not available. Following 3dLME analysis in AFNI, there was no significant Group×Drug interaction, Group or Drug effect for neither BP nor end-tidal CO2 (p>0.05). There was a significant Drug effect of BP (p<0.05), with decreased BP in the post cocaine resting-state scan.
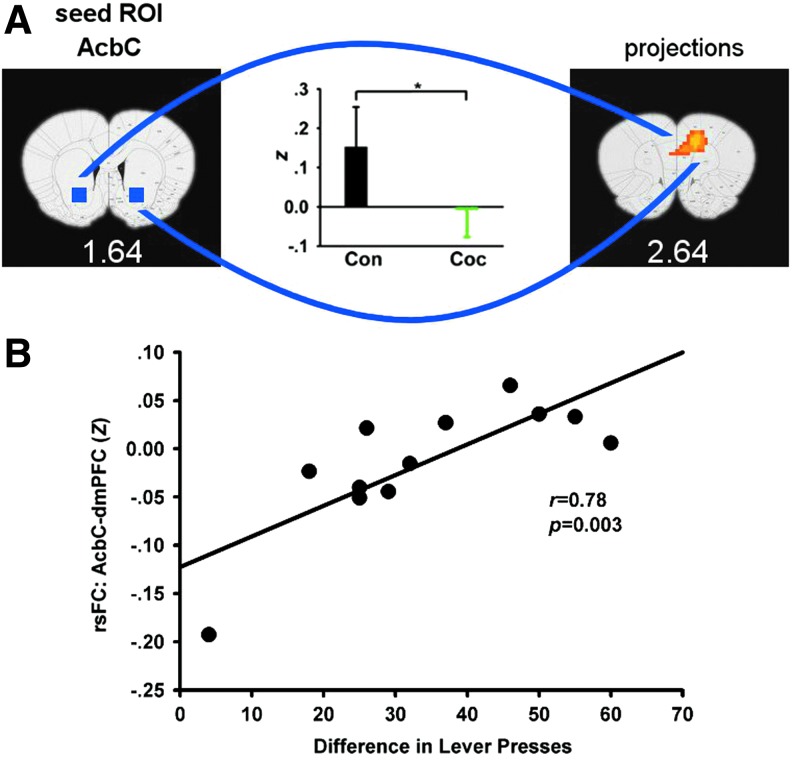
We next explored the relationship between the above rsFC alterations and individual animal cocaine SA history based upon CEI. We found that rsFC strength between AcbC and dmPFC, areas also showing a GROUP main effect (depicted in Fig. 3B), positively correlated with CEI (Fig. 5).
FIG. 5.
Positive correlation between rsFC strength and the escalation in cocaine SA. In this study, escalation is defined as the difference in total number of lever presses during the first and the last SA session. (A) Location of the AcbC seed ROI and the significant connectivity strength to dmPFC (PrL and ACC/Cg1). Reproduced from Figure 3B for easy comparison. The insert bar graph plots Fisher's-z score between the AcbC and target dmPFC from the control and cocaine SA groups (mean±SD). (B) Scatter plot of individual animals in the cocaine SA group versus rsFC data averaged over the two resting-state scans for individual rats. (*p<0.05).
As an important quality control for our analysis pipeline and to test the robustness of the above results, the target regions that showed significant GROUP (Fig. 3) and DRUG (Fig. 4) main effects were next taken as new seeds in an identical LME model analysis. As expected, the new rsFC circuits generally recapitulated the results from the original seeds, such that reduced connectivity was seen within CPu and between PrL and CPu in both SA groups compared to the housing control and increased connectivity seen between AcbC and POA and AcbS and VP/SI after an acute cocaine challenge (data not shown in this study).
Finally, and as expected, rsFC from the control S1FL seed ROI revealed no significant GROUP or DRUG main effects or GROUP×DRUG interactions, supporting the selectivity and specificity of the above MCL alterations. Table 2 details all significant connectivity results.
Table 2.
Main Effects in Brain Regions
| Seed | Projections | Number of voxels | Peak F value | Location relative to bregma |
|---|---|---|---|---|
| GROUP main effects | ||||
| dCPu | L- CPu/GP. Fig. 2C) | 75 | 13.09 | (−2.36)–1.64 |
| PrL | L- CPu/GP. Fig. 2D) | 9 | 8.32 | −2.36 |
| R- CPu/S. Fig. 2D) | 10 | 9.79 | (−0.36)–0.64 | |
| R- EP/Th. Fig. 2A) | 15 | 8.43 | 0.64–1.64 | |
| AcbC | PrL/ACC. Fig. 2B) | 23 | 11.63 | 1.64–4.64 |
| DRUG main effects | ||||
| AcbC | R- POA/BNST. Fig. 3A) | 10 | 17.51 | (−1.36)–(−0.36) |
| AcbS | R- VP/AcbS/SI/S/POA. Fig. 3B) | 15 | 20.14 | (−0.36)–1.64 |
Data from a 3. GROUP)×2. DRUG) ANOVA model. Voxel size: 0.469×0.469×1 mm3.
CPu, caudate-putamen; dCPu, dorsal CPu; GP, globus pallidus; PrL, prelimbic cortex; S, septum; EP, entopeduncular nucleus; Th, thalamus; AcbC, nucleus accumbens, core; ACC, anterior cingulate cortex; cingulate cortex, area 1; POA, hypothalamic preoptic area; BNST, bed nucleus of the stria terminalis; AcbS, nucleus accumbens, shell; VP, ventral pallidum; SI, substantia innominata; R/L, right/left.
Discussion
As the MCL system has long been implicated in processing motivated behaviors (Goldstein and Volkow, 2002; Kalivas and O'Brien, 2008), we employed a modification of a preclinical drug addiction and drug re-exposure priming model to test the hypothesis that specific regional alterations within key MCL circuits demonstrate persistent neuroadaptations manifested as differences in circuit coherence strength using rsFC analyses. We interrogated the system at a 30-day withdrawal time point consistent with that shown capable of reinstating cocaine-seeking behavior (Shaham et al., 2003).
Only two circuits, those between PrL and EP nucleus and AcbC and dmPFC, uniquely distinguished the cocaine-SA group, such that circuit strength was reduced compared with a housing group; circuit results from a sucrose-SA group was of intermediate strength and did not differ from either of the other two groups. Furthermore, the strength of the AcbC-dmPFC connection predicted the acceleration of cocaine intake during the SA period. Three additional circuits distinguished both SA groups from the housing control group, with decreased connectivity between the left and right CPu and between PrL and CPu. In contrast, acute cocaine challenge enhanced AcbC to POA and AcbS to VP/SI circuits.
Previous human imaging studies have revealed reduced gray matter density (Matochik et al., 2003) and metabolic activity in the PFC (Goldstein and Volkow, 2002) in drug-dependent individuals. Impaired frontal lobe and striatal functional responses are seen in cocaine-dependent individuals performing a number of cognitive tasks [e.g., Goldstein et al. (2009); Kaufman et al. (2003)]. Relevant to the current study, we have reported reduced resting circuit strength between dorsal anterior cingulate and ventral striatum in human cigarette smokers that was negatively correlated with nicotine addiction severity (Hong et al., 2009). Human studies have also consistently reported reduced striatal D2 receptor binding, reduced DA release, and reduced frontal metabolism in cocaine dependence (Volkow et al., 1993, 1997). Consistent with these findings, our cocaine-SA manipulation induced specific rsFC alterations limited to dmPFC-AcbC and PrL-EP circuits, suggesting unique functional circuit consequence to drug-induced plasticity. Taken together with other preclinical studies reporting altered cortical–striatal structural and functional consequences following chronic stimulant administration [e.g., Robinson and Kolb (2004)], these data may help explain the persistent cognitive and behavioral dysregulation seen in drug-dependent individuals (Kolb et al., 2003). Our neuroimaging data may thus provide a link for translating preclinical models directly to human clinical research.
We further found that the connectivity strength of the dmPFC-AcbC circuit was positively correlated with an animal's cocaine SA escalation pattern. Extracellular recordings in behaving animals have shown that basal firing rates of Acb neurons decrease across daily cocaine SA sessions (Peoples et al., 1999, 2007), which is associated with escalation in cocaine SA. Additionally, greater drug exposure leads to a greater compensatory glutamatergic adaptation during abstinence (Wolf, 2010). Altogether, these data demonstrate that repeated cocaine SA is associated with decreases in excitatory synaptic and neuronal activity in the Acb. It is possible that these drug exposure-induced neuroadaptations centered within the Acb contributed to the intake-dependent alterations in frontal–accumbens circuit connectivity in the present study.
The second circuit showing cocaine specific decreased rsFC was between PrL and EP. The EP in rodents is homologous to the internal segment of the primate GP, which sends direct excitatory projections (Shabel et al., 2012) to the lateral habenula (LHb). The habenula is uniquely positioned, anatomically and functionally, to modulate circuits involved in negative reward learning. Despite its emerging role in mediating reward signaling (Matsumoto and Hikosaka, 2007), with a few notable exceptions (Brown et al., 1992; Fowler et al., 2011; Zhang et al., 2005), the habenula has been little explored in drug addiction. Interestingly, the LHb and its primary output pathway, the fasciculus retroflexus, were identified as uniquely susceptible to cocaine- and nicotine-induced toxicity (Ciani et al., 2005; Ellison, 2002).
In contrast to the decreased MCL circuit connectivity during abstinence, enhanced circuit strength was seen following acute cocaine administration in a more restricted network between Acb and VP/SI/POA. These brain regions all send direct projections to the LHb, and together with the reduced PrL cortex circuitry to the EP discussed above, support a role for the habenula in mediating both the tonic and phasic cocaine effects in this preclinical addiction model. For example, as the LHb is responsible for communicating negative reward signals to midbrain DA neurons (Matsumoto and Hikosaka, 2007), decreased connectivity in circuitry targeting the habenula (PrL-EP) during prolonged abstinence might contribute to the negative/aversive state experienced during withdrawal, whereas enhanced connection strength after cocaine re-exposure may play a role in cocaine priming induced reinstatement as shown in a similar preclinical model (Shaham et al., 2003), perhaps by producing or modulating a positive affective state. Altogether, our findings provide some of the first functional evidence of drug-induced neuroplastic changes leading to altered LHb-related circuit activity and speak to its potential significance in drug addiction development.
We found decreased connectivity strength within a PrL to CPu circuit in both cocaine- and sucrose-SA groups. Since this circuit has been implicated in acquired habit behaviors (Hyman et al., 2006), these data might reflect common learning experience across the two operant groups. Well-learned instrumental skills result in neuroadaptations within loops associated with habit-driven behaviors (Belin and Everitt, 2008). Indeed, activation of CPU neurons decreased in overtrained rats (Carelli et al., 1997). A decrease in CPu to CPu rsFC following LgA SA further suggests that local circuit plasticity in information processing underlying well-learned instrumental skills (Reep et al., 2003) may reflect the transition from initial goal-directed behavior to habitual behavior, mechanisms not only specific for drugs of abuse but also observed with food reward (Barnes et al., 2005; Everitt et al., 2008; Jog et al., 1999).
The spatial resolution of fMRI is far from adequate to identify whether similar or different striatal cells contributed to the differential Acb to dmPFC circuit strength seen in the cocaine SA group. Recently, Cameron and Carelli (2012) identified populations of accumbens cells that responded to both sucrose and cocaine, but also distinct subpopulations that were uniquely tuned to each reinforcer. They further reported a shift in cell firing preference after a 30 day enforced abstinence period similar to that used in this study. Specifically, after withdrawal, there was a decrease in sucrose-sensitive cells concomitant with an increase in the percentage of cells responsive to cocaine. Their data suggested a functional segregation of circuits within the Acb that selectively compute distinct rewards, a conclusion also made by Koya and colleagues (2009) who demonstrated that a small subset of neurons in the Acb was responsible for altered drug–environmental context-specific behaviors.
rsFC as a potential biomarker of addiction is an emerging concept [Sutherland et al. (2012) for review]. Several recent studies have reported decreased MCL rsFC in active nicotine (Hong et al., 2009) and cocaine (Gu et al., 2010; Tomasi et al., 2010) users. Gu and colleagues (2010) also showed a VTA to striatal circuit that negatively predicted years of cocaine use, whereas Hong and colleagues (2009) reported a dorsal anterior cingulate to ventral striatal circuit that negatively predicted nicotine addiction severity. Although these human circuits do not perfectly overlap the reduction in MCL circuit strength reported in this study, species differences, ROI selection, anatomical specificity, anesthesia, and differences between the SA-forced abstinence model and the more uncontrolled and variable drug use pattern in human participants likely account for these small disparities. Nevertheless, these closely replicated data supports the use of preclinical imaging models in future addiction therapeutic development.
Compared with results from task-based fMRI
As stated above, we also performed fMRI studies in the same cohorts of animals using acute cocaine challenges (Lu et al., 2012b). Compared to the strong positive fMRI responses seen in the sedentary control group, both the cocaine- and sucrose-SA groups demonstrated very similar initial negative fMRI responses followed by attenuated positive responses. The most salient finding that distinguished the cocaine-SA from the sucrose-SA group was the fMRI response in the medial PFC (IL, PrL, Cg1/Cg2), which significantly correlated with the total amount of reinforcer intake during the training sessions for the cocaine-SA, but not for the sucrose-SA group.
Before the rsMRI scans, animals were also presented with odor cues previously associated with or without (S+/S−) reinforcer (cocaine/sucrose) availability while undergoing functional MRI scans (Liu et al., 2013). Results demonstrated a learning effect distinguishing S+ from S− in the insula and nucleus accumbens, with the insula response reflecting the individual history of cocaine SA intake. A main effect of group, distinguishing cocaine from sucrose, was seen in the mPFC and dorsolateral striatum. Critically, only the dorsomedial striatum demonstrated a double dissociation between the two SA groups and learning (S+ vs. S−).
Although rsMRI and task-based fMRI (acute cocaine challenge and contextual odor cues) probe dramatically different aspects of brain processes, data from the same cohort of animals point to neuroadaptations in mPFC, dorsomedial striatum, consistent with recent evidences emphasizing their roles in cocaine-seeking behavior (Belin and Everitt, 2008; Chen et al., 2013).
Caveats and technical considerations
An important caveat is required about all studies that employ rsFC. Although it is assumed that functional connectivity reflects mostly monosynaptic pathways, functional correlations are also possible through polysynaptic connections, suggesting that rsFC may reflect both direct and indirect synaptic influence (Honey et al., 2009). For example, both control groups showed connectivity between PrL and EP, whereas significant coherence was absent in the cocaine group, likely as a result of local circuit alterations. Although no direct anatomical connections are known between these areas, since direct PrL-CPu and CPu-EP anatomical projections are known to exist (Fink-Jensen and Mikkelsen, 1989; Vertes, 2004), the PrL-EP functional connectivity may have occurred through a CPu intermediary node. Present findings supporting this are strong PrL-CPu coherence in the housing group and decreased PrL-CPu and CPu-CPu connectivity in both SA groups, suggesting striatal mediation of the PrL-EP circuit. Indeed, the back projection analysis not only replicated our main results, but also provides evidence of complex brain circuitry consistent with convergent/divergent patterns of anatomical connections.
Several potential limitations of this study should be noted. The hypothesis-driven seed-based approach to interrogate specific MCL system plasticity should not be considered an exhaustive exploration of all circuit alterations, as other seed regions along with such data-driven methods as independent component analysis may reveal additional neuroplastic circuitry. Furthermore, as mentioned above, rsFC cannot distinguish directionality of a circuit; and while in vitro tract tracing can do so, it yields a pure anatomical measure. Virtually all brain circuits are known to be reciprocal, and virtually all integrate information from multiple relay regions (Llinas, 1988). Thus, anatomical directionality knowledge would not necessarily presage functional processing directionality. rsFC maps from certain seed areas, for example, the AcbS and AcbC, are very similar. This was likely caused by the intrinsic spillover effect of the hemodynamic response signal and the relatively coarse imaging resolution. High-resolution rsMRI is necessary to truly distinguish rsMRI networks in closely situated areas.
Another potential limitation is that imaging took place only at a single time point. Although vastly more difficult to perform, and with its own challenges and limitations, it would be useful to obtain rsFC data at baseline, at one or more time points during LgA SA and at several time points along the withdrawal period to determine the trajectory of the observed rsFC changes. Such within-subjects design is likely to reveal the time course of the putative neuroplasticity observed in this between-subjects design and may have demonstrated other more subtle circuit changes. Furthermore, additional behavioral anchors would then be available to better understand individual differences in the relationship between SA behavior and circuit strength alteration.
Finally, this study employed propofol as the anesthetics. We used relatively low doses, and a recent study from our lab suggested that this dose could maintain neurovascular coupling, and we were able to detect fMRI response to acute cocaine challenge in the same group of animals (Lu et al., 2012b). However, it is conceivable that the use of anesthesia may have modified the connectivity patterns. Nevertheless, spontaneous BOLD fluctuations, which are thought to have its neuronal origin (Liu et al., 2011), demonstrate generally consistent patterns in the anesthetized monkey (Vincent et al., 2007) and rat (Lu et al., 2007); and the connectivity patterns in sleeping humans (Horovitz et al., 2008) are similar to that seen in the awake human. The rsFC maps derived from our housing control group are in good agreement with known MCL anatomical connections (Alexander et al., 1986; Heidbreder and Groenewegen, 2003; Hoover and Vertes 2007; Jones et al., 2005; Voorn et al., 2004), are consistent with previous rsFC studies (Lu et al., 2007, 2011; Pawela et al., 2010), support our analysis pipeline examining group differences, and speak to the neurobiological relevance of the findings. Nevertheless, we recently found a default mode network in rats anesthetized with low dose of isoflurane in combination with low dose of dexmedetomidine (Lu et al., 2012a), which was not seen in animals anesthetized with propofol, suggesting that propofol may have somewhat compromised rsMRI signal, at least with the dose applied in this study.
To summarize, we demonstrated reduced rsFC within PrL-EP and AcbC-dmPFC circuits in abstinent rats following extended access cocaine SA; connectivity strength of the latter was positively correlated with drug exposure history. Furthermore, cocaine re-exposure increased rsFC between Acb and brain regions projecting to the LHb, a region emerging as critical in aversive learning. Altogether, these data describing specific drug-induced brain network abnormalities extend our understanding of MCL system plasticity. Finally, these data also suggest that differences seen in cross-sectional studies between human drug addicts and nonusing controls may be the result of, rather than a predisposition to drug dependence, and by identifying potential circuit level biomarkers of addiction, support the application of this translational model to explore therapeutic interventions to address recidivism.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH) and by an NIH Director's Bench-to-Bedside grant to L. L. Peoples and E. A. Stein. The project was also partially supported by the Institute for Translational Medicine and Therapeutics of the University of Pennsylvania and Grant UL155024134 from the National Center for Research Resources.
Author Disclosure Statement
No competing financial interests exist.
References
- Ahmed SH, Cador M. 2006. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology 31:563–571 [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. 1986. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 9:357–381 [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. 2008. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev 32:20–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. 2005. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature 437:1158–1161 [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. 2008. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron 57:432–441 [DOI] [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. 1992. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci 12:4112–4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron CM, Carelli RM. 2012. Cocaine abstinence alters nucleus accumbens firing dynamics during goal-directed behaviors for cocaine and sucrose. Eur J Neurosci 35:940–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Wolske M, West MO. 1997. Loss of lever press-related firing of rat striatal forelimb neurons after repeated sessions in a lever pressing task. J Neurosci 17:1804–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. 2013. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496:359. [DOI] [PubMed] [Google Scholar]
- Ciani E, Severi S, Bartesaghi R, Contestabile A. 2005. Neurochemical correlates of nicotine neurotoxicity on rat habenulo-interpeduncular cholinergic neurons. Neurotoxicology 26:467–474 [DOI] [PubMed] [Google Scholar]
- Cox RW. 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res Int J 29:162–173 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. 1988. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85:5274–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison G. 2002. Neural degeneration following chronic stimulant abuse reveals a weak link in brain, fasciculus retroflexus, implying the loss of forebrain control circuitry. Eur Neuropsychopharmacol 12:287–297 [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. 2006. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 189:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. 2008. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci 363:3125–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink-Jensen A, Mikkelsen JD. 1989. The striato-entopeduncular pathway in the rat. A retrograde transport study with wheatgerm-agglutinin-horseradish peroxidase. Brain Res 476:194–198 [DOI] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. 2011. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Carrillo JH, Maloney T, Woicik PA, Wang R, Telang F, Volkow ND. 2009. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc Natl Acad Sci U S A 106:9453–9458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. 2002. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159:1642–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Galis-de Graaf Y, Smeets WJ. 1999. Integration and segregation of limbic cortico-striatal loops at the thalamic level: an experimental tracing study in rats. J Chem Neuroanat 16:167–185 [DOI] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. 2010. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. NeuroImage 53:593–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. 2003. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev 27:555–579 [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. 2009. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A 106:2035–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA. 2009. Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry 66:431–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. 2007. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212:149–179 [DOI] [PubMed] [Google Scholar]
- Horovitz SG, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ, Duyn JH. 2008. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum Brain Mapp 29:671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt WA, Barnett LW, Branch LG. 1971. Relapse rates in addiction programs. J Clin Psychol 27:455–456 [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. 2006. Neural mechanisms of addiction: the role of reward-related learning and memory. Ann Rev Neurosci 29:565–598 [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. 1999. Building neural representations of habits. Science 286:1745–1749 [DOI] [PubMed] [Google Scholar]
- Jones BF, Groenewegen HJ, Witter MP. 2005. Intrinsic connections of the cingulate cortex in the rat suggest the existence of multiple functionally segregated networks. Neuroscience 133:193–207 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. 2008. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 33:166–180 [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. 2003. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci 23:7839–7843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. 2008. Competition between functional brain networks mediates behavioral variability. NeuroImage 39:527–537 [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Li Y, Samaha AN, Robinson TE. 2003. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc Natl Acad Sci U S A 100:10523–10528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, Mitchell TB, Farquhar D, Ghosh SC, Mattson BJ, Hope BT. 2009. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci 12:1069–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zou Q, He Y, Yang Y. 2013. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci U S A 110:1929–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HS, Chefer S, Lu H, Guillem K, Rea W, Kurup P, Yang Y, Peoples L, Stein EA. 2013. Dorsolateral caudate nucleus differentiates cocaine from natural reward-associated contextual cues. Proc Natl Acad Sci U S A 110:4093–4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu XH, Zhang Y, Chen W. 2011. Neural origin of spontaneous hemodynamic fluctuations in rats under burst-suppression anesthesia condition. Cereb Cortex 21:374–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR. 1988. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. New York, NY: 242:1654–1664 [DOI] [PubMed] [Google Scholar]
- Lu H, Chefer S, Kurup PK, Guillem K, Vaupel DB, Ross TJ, Moore A, Yang Y, Peoples LL, Stein EA. 2012b. fMRI response in the medial prefrontal cortex predicts cocaine but not sucrose self-administration history. Neuroimage 62:1857–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Scholl CA, Zuo Y, Demny S, Rea W, Stein EA, Yang Y. 2010. Registering and analyzing rat fMRI data in the stereotaxic framework by exploiting intrinsic anatomical features. Magn Reson Imaging 28:146–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zou Q, Gu H, Raichle ME, Stein EA, Yang Y. 2012a. Rat brains also have a default mode network. Proc Natl Acad Sci U S A 109:3979–3984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA, Rea W, Yang Y, Stein EA. 2007. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci U S A 104:18265–18269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR. 2010. Addiction related alteration in resting-state brain connectivity. NeuroImage 49:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed W, Magnuson M, Keilholz SD. 2009. Spatiotemporal dynamics of low frequency fluctuations in BOLD fMRI of the rat. J Magn Reson Imaging 30:384–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. 2003. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. NeuroImage 19:1095–1102 [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. 2007. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447:1111–1115 [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. 1989. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience 29:503–537 [DOI] [PubMed] [Google Scholar]
- O'Brien CP. 1997. Progress in the science of addiction. Am J Psychiatry 154:1195–1197 [DOI] [PubMed] [Google Scholar]
- Pawela CP, Biswal BB, Cho YR, Kao DS, Li R, Jones SR, Schulte ML, Matloub HS, Hudetz AG, Hyde JS. 2008. Resting-state functional connectivity of the rat brain. Magn Reson Med 59:1021–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawela CP, Biswal BB, Hudetz AG, Li R, Jones SR, Cho YR, Matloub HS, Hyde JS. 2010. Interhemispheric neuroplasticity following limb deafferentation detected by resting-state functional connectivity magnetic resonance imaging (fcMRI) and functional magnetic resonance imaging (fMRI). NeuroImage 49:2467–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 2007. The Rat Brain in Stereotaxic Coordinates, 6th Edition. San Diego: Academic Press [Google Scholar]
- Peoples LL, Kravitz AV, Lynch KG, Cavanaugh DJ. 2007. Accumbal neurons that are activated during cocaine self-administration are spared from inhibitory effects of repeated cocaine self-administration. Neuropsychopharmacology 32:1141–1158 [DOI] [PubMed] [Google Scholar]
- Peoples LL, Uzwiak AJ, Gee F, West MO. 1999. Tonic firing of rat nucleus accumbens neurons: changes during the first 2 weeks of daily cocaine self-administration sessions. Brain Res 822:231–236 [DOI] [PubMed] [Google Scholar]
- Reep RL, Cheatwood JL, Corwin JV. 2003. The associative striatum: organization of cortical projections to the dorsocentral striatum in rats. J Comp Neurol 467:271–292 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. 2008. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci 363:3137–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. 2004. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47Suppl 1:33–46 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ, Proulx CD, Trias A, Murphy RT, Malinow R. 2012. Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron 74:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. 2003. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 168:3–20 [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. 2009. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A 106:13040–13045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. 2012. Resting state functional connectivity in addiction: lessons learned and a road ahead. NeuroImage 62:2281–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, Woicik PA, Telang F, Goldstein RZ. 2010. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One 5:e10815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. 2004. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305:1017–1019 [DOI] [PubMed] [Google Scholar]
- Vanduffel W, Fize D, Mandeville JB, Nelissen K, Van Hecke P, Rosen BR, Tootell RB, Orban GA. 2001. Visual motion processing investigated using contrast agent-enhanced fMRI in awake behaving monkeys. Neuron 32:565–577 [DOI] [PubMed] [Google Scholar]
- Vertes RP. 2004. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58 [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. 2007. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447:83–86 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. 1993. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14:169–177 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. 1997. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 386:830–833 [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. 2004. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci 27:468–474 [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. 1989. Brain dopamine and reward. Annu Rev Psychol 40:191–225 [DOI] [PubMed] [Google Scholar]
- Wolf ME. 2010. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci 33:391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Zhou W, Liu H, Zhu H, Tang S, Lai M, Yang G. 2005. Increased c-Fos expression in the medial part of the lateral habenula during cue-evoked heroin-seeking in rats. Neurosci Lett 386:133–137 [DOI] [PubMed] [Google Scholar]
- Zhang N, Rane P, Huang W, Liang Z, Kennedy D, Frazier JA, King J. 2010. Mapping resting-state brain networks in conscious animals. J Neurosci Methods 189:186–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Zhao T, Zhou L, Wu Q, Hu X. 2008. BOLD study of stimulation-induced neural activity and resting-state connectivity in medetomidine-sedated rat. NeuroImage 39:248–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q, Ross TJ, Gu H, Geng X, Zuo XN, Hong LE, Gao JH, Stein EA, Zang YF, Yang Y. 2013. Intrinsic resting-state activity predicts working memory brain activation and behavioral performance. Hum Brain Mapp 34:3204–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]