Local and abrupt changes in mechanical forces play a fundamental role in control of tissue and organ development. While some investigators have varied material properties of tissue engineering scaffolds to influence cell behavior, no biomaterials have been developed that harness mechanical actuation mechanisms to induce new tissue formation. Here, we describe the development of mechanically-actuatable polymers that induce tissue differentiation by harnessing the physical induction mechanism that drives tooth organ formation in the embryo. The formation of many epithelial organs is triggered when sparsely distributed mesenchymal cells abruptly pack closely together and undergo a “mesenchymal condensation” response. For example, in tooth development, the associated physical compression and rounding of dental mesenchymal cells is sufficient to induce whole organ formation in vitro and in vivo.[1] Inspired by this developmental induction mechanism, we fabricated an artificial, shrink-wrap like polymer scaffold that can stimulate tooth tissue differentiation by abruptly inducing physical compaction of cells cultured within it when warmed to body temperature. A porous, GRGDS-modified, hydrogel scaffold was fabricated from poly(N-isopropylacrylamide) (PNIPAAm), which remains in an expanded form in the cold, and rapidly contracts volumetrically when placed at body temperature. When undifferentiated embryonic dental mesenchymal cells were seeded within this hydrogel sponge and polymer shrinkage was thermally induced by warming, the cells became physically compressed and exhibited a more compact, rounded morphology, as they do when they undergo mesenchymal condensation during tooth organ development in the embryo. This physical change in cell shape stimulated tooth differentiation, as measured by the induction of key odontogenic transcription factors in vitro and stimulation of mineralization in vivo. This polymer-based mechanical actuation mechanism represents a new bioinspired approach to induce organ-specific tissue differentiation that could be useful for stem cell biology, tissue engineering and regenerative medicine.
Current design strategies used to fabricate materials for tissue engineering and regenerative medicine focus on the chemistry, structural properties, and three-dimensional (3D) spatial organization of the components that comprise these scaffolds (e.g., polymers, ceramics, biomaterials).[2,3] While current tissue scaffold designs can support cell survival and maintenance of some differentiated cell functions, they do not exhibit the ability to induce major developmental lineage switches that can drive whole organ formation. Thus, we set out to develop materials that mimic the organ inductive properties of certain embryonic tissues. The formation of most organs in the embryo results from complex interactions between adjacent epithelial and mesenchymal tissues.[4–7]An initial instructive signal, provided by one of the tissue layers, is followed by reciprocal exchange of inductive signals, resulting in stepwise differentiation of both tissue components into an integrated organ structure. One of the simplest examples of organ formation is the development of the tooth. In the mouse, the embryonic day (E10) dental epithelium induces a ‘mesenchymal condensation’ response in which underlying mesenchymal cells are stimulated to migrate towards the base of the epithelium, resulting in formation of a tightly packed, dense cell aggregate in this region.[1,8,9] Recent studies have revealed that the physical compression of cells caused by this condensation response mechanically triggers the mesenchymal cells to express odontogenic (tooth forming) genes including Pax9, Msx1 and BMP4.[1,10,11] Moreover, once induced in this manner, the mesenchyme can support whole tooth formation when recombined with normal embryonic dental epithelium and implanted under the kidney capsule in a mouse.[1] Importantly, similar mesenchymal condensation processes are crucial for the formation of many other epithelial organs, including the salivary gland, pancreas, kidney, bone, and cartilage among others[8,12–16], and thus, harnessing this induction mechanism could have much broader value for the field of tissue engineering.
Inspired by this mechanical organ induction mechanism, we set out to explore whether it is possible to develop artificial polymer scaffolds that can abruptly shrink in 3D, and thereby physically compress mesenchym cells cultured within the lattice to induce their differentiation. We designed a temperature-responsive polymer scaffold composed of poly(N-isopropylacrylamide) (PNIPAAm), which upon heating to physiological temperatures contracts in volume in 3D. PNIPAAm has been previously shown to autonomously change volume through alteration of temperature within physiologically relevant ranges.[17–20] The thermosensitivity of PNIPAAm is governed by its lower critical solution temperature (LCST): at temperatures below the LCST, the polymer is swollen, whereas it contracts when the polymer is heated to higher temperatures.[21,22] PNIPAAm’s LCST also can be manipulated by adding other chemical moieties that change the hydrophilicity of the polymer, thereby allowing its thermoresponsiveness to be tailored for specific biological applications.[17, 19, 22] Other parameters such as volume change, porosity, and biocompatibility also can be controlled by altering cross-linking density or adding adhesion-promoting components.
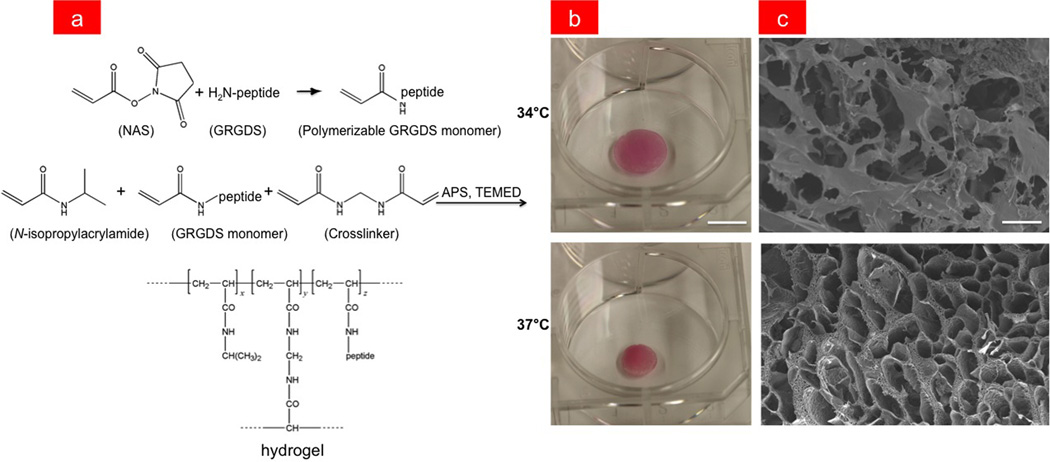
In the present study, we incorporated a GRGDS peptide into the cross-linked PNIPAAm polymer gel that mediates adhesion to cell surface integrin receptors in order to promote cell anchorage and survival.[23–25] The peptide was first modified with an acrylate moiety so that it could be polymerizable and then mixed into the hydrogel precursor solution containing 10% N-isopropylacrylamide (NIPAAm) monomer and 1% N,N’–methylenebisacrylamide (BIS) cross-linker by weight in water (Figure 1a). The hydrogel was polymerized by radical initiation using ammonium persulfate (APS) and tetramethylethylenediamine (TEMED), and then subsequently lyophilized (see Supporting Information for details). While PNIPAAm without peptide has an LCST of 32°C, the addition of even low concentrations of GRGDS significantly increased the LCST of the gel. For this reason, we experimentally determined the optimal concentration of peptide monomer necessary to yield hydrogels with an LCST of ~36°C, which is close to body temperature (Supplementary Figure S1). Swollen gels without cells were found to have an average pore size of 2398 + 211 µm and 1618 + 108 µm in their contracted state as determined from scanning electron micrographs of flash-frozen, lyophilized hydrogels, and these hydrogels contracted volumetrically by approximately 45% when heated from 34°C to 37°C in medium containing 10%fetal bovine serum (Figure 1b-c). The volumetric response was also reversible in that GRGDS-PNIPAAm gels that contracted at 37°C could be induced to swell back to their original size by lowering the temperature.
Figure 1.
(a) Chemical synthesis of GRGDS-PNIPAAm. First the GRGDS peptide is modified with an acrylate moiety by reaction with N-acryloxysuccinimide (NAS) and is then mixed with a NIPAAm and BIS precursor solution. The hydrogel is then polymerized through radical initiation using APS and TEMED. (b) GRGDS-PNIPAAm in swollen and contracted states at 34°C and 37°C, respectively (bar, 1 cm). The red color arises from the hydrogel being submerged in serum-containing medium. (c) SEMs of GRGDS-PNIPAAm gels in swollen (top) and contracted (bottom state showing reduction in pore size with gel contraction (bar, 100 µm).
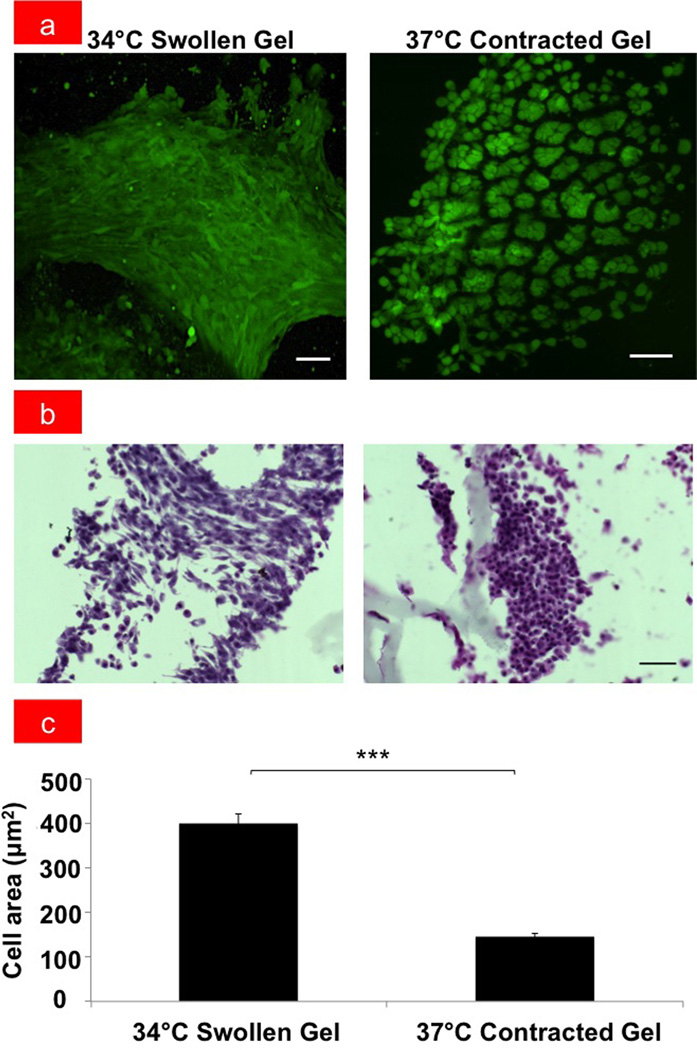
To determine whether cells cultured within the temperature-responsive scaffolds could be physically compressed by inducing gel shrinkage, dental mesenchymal cells originally isolated from E10 mouse embryos were implanted within the GRGDS-modified PNIPAAm gel, cultured overnight at 34°C, and then either maintained at the same temperature or shifted to 37°C for 48hours. Importantly, while cells maintained at the lower temperature exhibited polygonal cell morphology (Figure 2a) similar to that observed on conventional culture substrates, these cells formed tighly compacted, dense aggregates and exhibited a round morphology when the the hydrogel was induced to shrink by raising the temperature to 37°C (Fig. 2a-c). Computerized morphometric analysis confirmed that projected cell areas reduced by more than 60% when this compression response was mechanically triggered (Fig. 2c), which is similar to the rounding and reduction in cell size that was shown to be sufficient to induce these dental mesenchymal cells to undergo odontogenic differentiation in vitro and in vivo.[1] Moreover, time-lapse 2D and 3D imaging of the same gel revealed that cell rounding response occurred in parallel with gel contraction and that this occurred rapidly (< 15 min) after the temperature was raised (Supplementary Videos V1 and V2, respectively), thus, confirming that this a direct effect of mechanical compaction. Although the rate of the cell shape change varies depending on how quickly the heating chamber of the microscope equilibrated to the warmer temperature, the cell volume decreased at approximately 1.64x105 µm3/min in our 3D time-lapse study (Supplementary Figure S2). Importantly, most of the cells cultured at 37°C in the contracted GRGDS-gels remained viable for at least two weeks in culture as determined by cell viability, cytotoxicity, and growth assays, whereas cells on PNIPAAm gels without GRGDS rapidly lost their viability with virtually all cells dying within the first week (Supplementary Figures S3& S4).
Figure 2.
(a) Fluorescent micrographs of E10 dental mesenchymal cells in swollen GRGDS-PNIPAAm hydrogel at 34°C and in contracted GRGDS-PNIPAAm hydrogel at 37°C (bar, 50 µm). (b) Light micrographs showing hematoxylin and eosin (H&E) staining of mesenchymal cells that appear spread in the swollen GRGDS-PNIPAAm hydrogel, where as they are compact and rounded in the contracted GRGDS-PNIPAAm hydrogel at 37°C (bar, 50 µm). (c) Graph showing the quantification of the corresponding projected cell areas; ***p < 0.001.
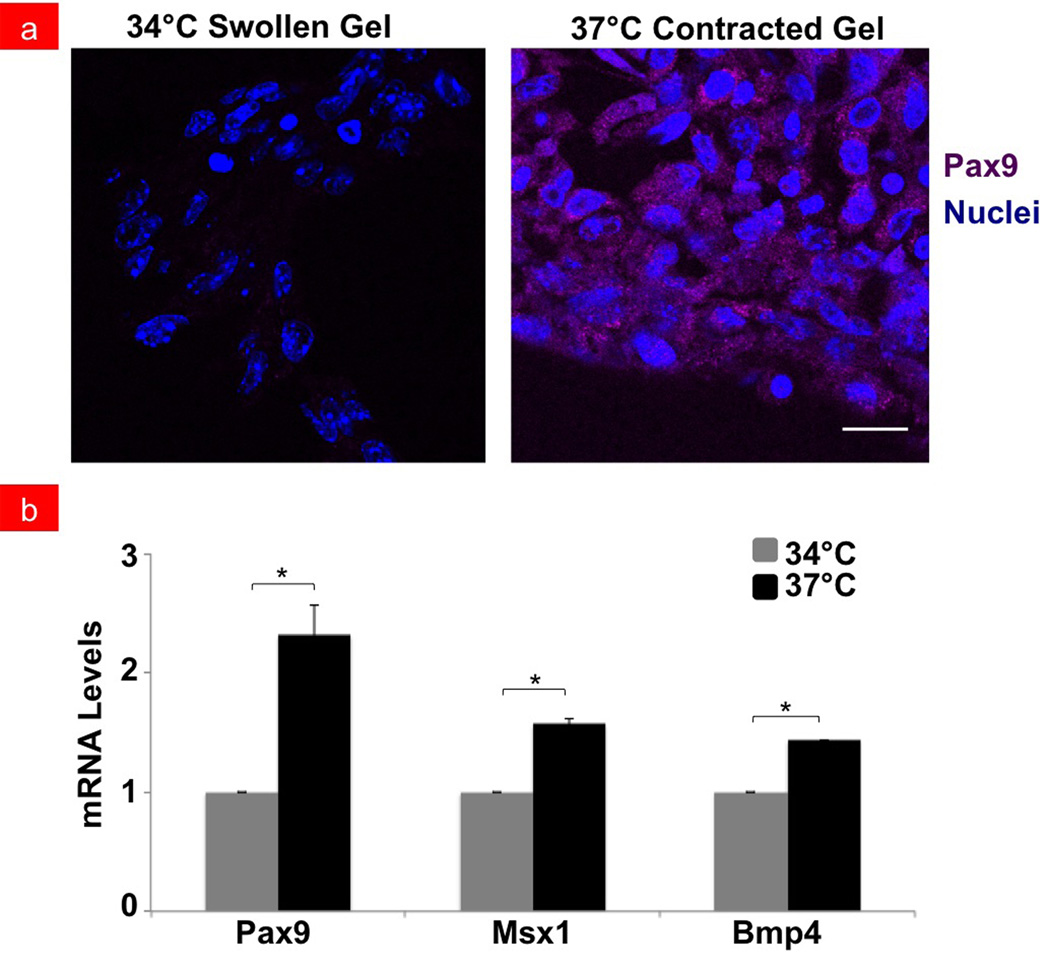
Next, we explored whether the cell shape change and compaction induced by the hydrogel compression influenced expression of three different genes that are critical drivers of tooth formation - Pax9, Msx1, and Bmp4.[1,26,27] Immunofluorescence staining for Pax9 protein confirmed that increased numbers of cells expressed this odontogenic transcription factor when cultured in contracted gels placed at 37°C that induced cell rounding compared to swollen gels maintained at the lower temperature where no compaction was observed (Figure 3a). Similar responses were observed when we used PCR to quantitate changes in mRNA levels for Pax9 as well as for two other genes – Msx1 and Bmp4 – that are also key drivers of tooth formation [1,26,27] (Figure 3b). Pax9 mRNA levels doubled in contracted gels relative to cells in gels maintained at 34°C, and Msx1 and Bmp4 increased by ~1.5-fold within 2 days after stimulation from hydrogel contraction (p < 0.05). Expression of these odontogenic markers was not affected by these temperature differences alone as confirmed by control experiments with cells cultured at 34°C and 37°C under standard culture conditions (Supplementary Figure S5).
Figure 3.
Fluorescent micrographs showing (a) Pax9 protein expression (purple) in mesenchymal cells with their nuclei stained with DAPI (blue) cultured in swollen versus contracted gels at 34°C or 37°C, respectively (bar, 20 µm). Pax9 expression is noted in contracted gels and absent in swollen gels. (b) Graph showing the quantification of mRNA expression levels of Pax9, Msx1 and Bmp4 odontogenic markers in compacted mesenchymal cells within contracted PNIPAAm gels. mRNA levels in cells cultured at 37°C (black bars) are shown relative to 34°C controls (grey bars); *p < 0.05.
The long-range goal of this effort is to design and fabricate biomimetic inductive scaffolds developmental induction for in vivo tissue engineering. To begin to explore the feasibility of this approach, GRGDS-PNIPAAm gels seeded with 1x106 dental mesenchymal cells were implanted under the kidney capsule of an adult mouse using a published method for in vivo analysis of tooth formation (Figure 4a & Supplementary Figure S6).[1,28] As these gels spontaneously contract when placed at body temperature, a cell pellet containing the same number of cells without a scaffold was implanted as a control. Additional in vivo controls included implantation of the GRGDS-PNIPAAm gel alone without DM cells and use of a GRGDS-PNIPAAm gel designed with an LCST above 37°C containing the same number of DM cells.
Figure 4.
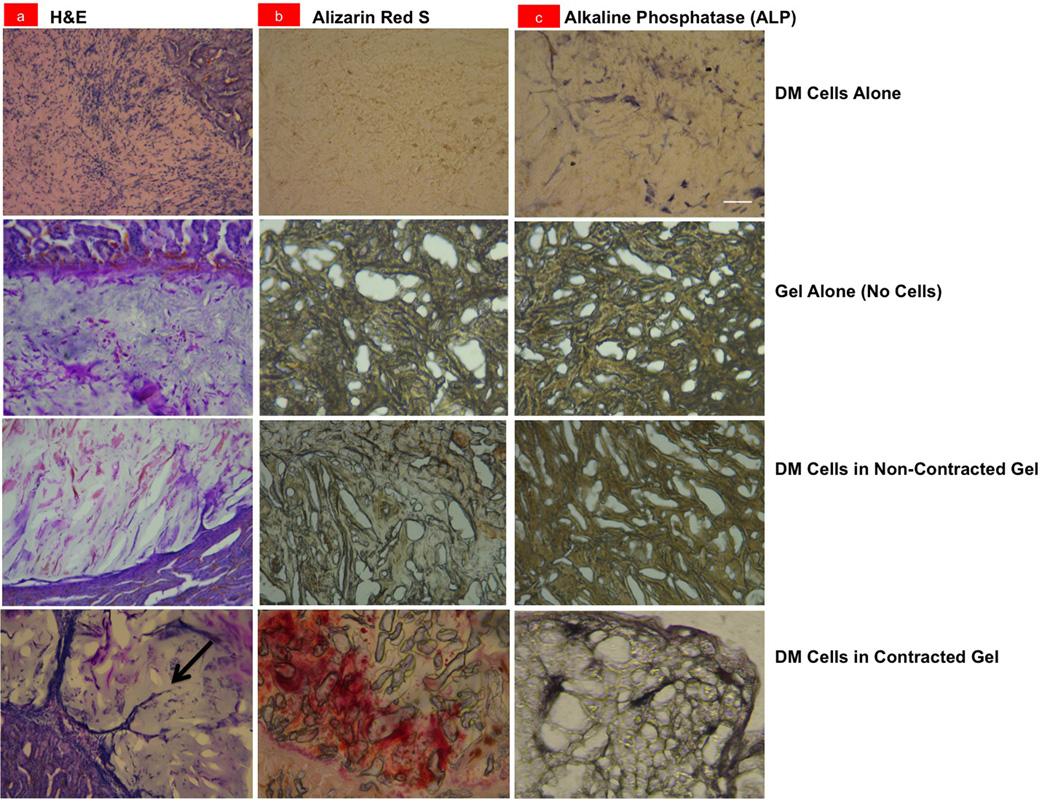
Light micrographs of histological sections of the control dental mesenchymal (DM) cell pellet alone (DM Cells Alone), GRGDS-PNIPAAm gel without cells (Gel Alone/No Cells), DM cells in a non-contracted gel with a LCST > 37°C (DM Cells in Non-Contracted Gel), and a contracted GRGDS-PNIPAAm gel containing DM cells (DM Cells in Contracted Gel) when implanted for 2 weeks under the kidney capsule of a mouse. Sections were stained with (a) Hematoxylin and eosin (H&E) or (b) Alizarin Red S, or analyzed for (c) Alkaline Phosphatase (ALP) activity; arrow indicates a new capillary sprout (bar, 100 µm).
Histological analysis of these implants after 2 weeks revealed that only the contracted gel containing cells implanted within the shrink wrap GRGDS-PNIPAAm polymer induced neovascularization (Figure 4a), and physical compaction of the DM cells could be detected in vivo (Supplementary Figure S7). Staining with Alizarin Red S and alkaline phosphatase (ALP) revealed that only the implants containing cells within contracted GRGDS-PNIPAAm gels were positive for deposition of calcium and mineralization, respectively (Figure 4b-c), which are indicative of later stages of tooth formation.[1,16,27,28] In contrast, neither mineralization nor vascularization was observed when the cell pellet or gel was implanted alone, or when the higher LCST gel (that did not contract at 37°C) with DM cells was implanted (Fig. 4b-c). Taken together, these results clearly demonstrate that mechanical compression of DM cells within the contracting gel was required fort he induction of the mineralization and vascularization we observed.
These findings confirm that a developmentally-inspired biomimetic scaffold that induces mesenchymal condensation mechanically can potentially be used to therapeutically stimulate cell and tissue differentiation in vitro as well as in vivo. In past studies, we showed that physical compression of cells during the mesenchymal condensation process is the key signal that triggers tooth formation, and that this is mediated by cell shape-dependent changes in the expression of two key odontogenic transcription factors (Pax9 and Msx1) and one important morphogen (Bmp4).[1] The results of the present study confirm that physical compaction of dental mesenchymal cells is indeed the key regulator of this tooth differentiation pathway. Responsive polymers have been previously used for controlled release of drugs and cells [19,29], and PNIPAAm has been employed to control cell adhesion and release tissues from substrates after they have formed.[30] But to our knowledge, this is the first study demonstrating the use of a responsive polymer, such as PNIPAAm, to induce tissue differentiation specifically by mechanically actuating a cell compaction response. It is also the first to promote tissue engineering by mimicking a developmental organ induction response.
We only focused on the effects of polymer shrinkage-induced compression of dental mesenchymal cells on tissue differentiation in the present study because inclusion of dental epithelial cells would have complicated our analysis. However, previous work has shown that induced dental mesenchymal cells must be recombined with dental epithelial cells in order to produce fully formed teeth in vivo. [1,28] Thus, tissue recombination studies should be explored in the future to fully define the value of this approach for organ engineering applications.
Many other organs require mesenchymal condensation for their induction and formation, including salivary gland, pancreas, kidney, bone, and cartilage, [12–16] and so these inductive polymer gels could have value for engineering of many tissues. Mechanically actuating polymer systems potentially could be used to suppress cancer growth as past studies have shown that tumor expansion can be accelerated or suppressed by altering tissue mechanics and cell distortion.[31,32] Thus, this shrink wrap polymer strategy will likely have broad applications for tissue engineering, regenerative medicine, and clinical therapy as well as value for basic research aimed at understanding and manipulating organ formation and regeneration.
Supplementary Material
Acknowledgements
This work was conducted with support by grants from the NIH Common Fund (RL1DE019023 to D.E.I.), the Wyss Institute for Biologically Inspired Engineering at Harvard University, and partially from the DOE BES (DE-SC0005247 to J.A.). We would like to thank E. Jiang and M. Kowalski for their technical assistance and T. Ferrante for assistance in imaging.
Footnotes
Experimental
Experimental materials and methods are provided in the Supporting Information.
Contributor Information
Basma Hashmi, Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, MA 02115 (USA); Harvard School of Engineering and Applied Sciences, Cambridge, MA 02138 (USA).
Lauren D. Zarzar, Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, MA 02115 (USA) Department of Chemistry and Chemical Biology, Harvard University, Cambridge, MA 02138 (USA).
Tadanori Mammoto, Vascular Biology Program, Boston Children’s Hospital and Harvard Medical School, Boston, MA 02115 (USA)
Akiko Mammoto, Vascular Biology Program, Boston Children’s Hospital and Harvard Medical School, Boston, MA 02115 (USA)
Amanda Jiang, Vascular Biology Program, Boston Children’s Hospital and Harvard Medical School, Boston, MA 02115 (USA).
Joanna Aizenberg, Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, MA 02115 (USA) Harvard School of Engineering and Applied Sciences, Cambridge, MA 02138 (USA); Department of Chemistry and Chemical Biology, Harvard University, Cambridge, MA 02138 (USA).
Donald E. Ingber, Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, MA 02115 (USA); Harvard School of Engineering and Applied Sciences, Cambridge, MA 02138 (USA); Vascular Biology Program, Boston Children’s Hospital and Harvard Medical School, Boston, MA 02115 (USA).
References
- 1.Mammoto T, Mammoto A, Torisawa YS, Tat T, Gibbs A, Derda R, Mannix R, de Bruijn M, Yung CW, Huh D, Ingber DE. Dev. Cell. 2011;21:758–769. doi: 10.1016/j.devcel.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Place ES, Evans ND, Stevens MM. Nat. Mater. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 3.Huebsch N, Mooney DJ. Nature. 2009;462:426–432. doi: 10.1038/nature08601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson AG. Science. 1966;152:25–34. doi: 10.1126/science.152.3718.25. [DOI] [PubMed] [Google Scholar]
- 5.Grobstein C. Natl. Cancer Inst. Monogr. 1967;26:279–299. [PubMed] [Google Scholar]
- 6.Bernfield M, Banerjee SD, Koda JE, Rapraeger AC. Ciba Found. Symp. 1984;108:179–196. doi: 10.1002/9780470720899.ch12. [DOI] [PubMed] [Google Scholar]
- 7.Gurdon JB. Development. 1987;99:285–306. doi: 10.1242/dev.99.3.285. [DOI] [PubMed] [Google Scholar]
- 8.Saxén L, Thesleff I. Ciba Found. Symp. 1992;165:183–192. doi: 10.1002/9780470514221.ch11. [DOI] [PubMed] [Google Scholar]
- 9.Jernvall J, Thesleff I. Mech. Dev. 2000;92:19–29. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- 10.Mina M, Kollar EJ. Arch. Oral Biol. 1987;32:123–127. doi: 10.1016/0003-9969(87)90055-0. [DOI] [PubMed] [Google Scholar]
- 11.Bei M, Kratochwil K, Maas RL. Development. 2000;127:4711–4718. doi: 10.1242/dev.127.21.4711. [DOI] [PubMed] [Google Scholar]
- 12.Grobstein C. Nature. 1953;172:869–870. doi: 10.1038/172869a0. [DOI] [PubMed] [Google Scholar]
- 13.Golosow N, Grobstein C. Dev. Biol. 1962;4:242–255. doi: 10.1016/0012-1606(62)90042-8. [DOI] [PubMed] [Google Scholar]
- 14.Smith MM, Hall BK. Biol. Rev. Camb. Philos. Soc. 1990;65:277–373. doi: 10.1111/j.1469-185x.1990.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 15.Hall BK, Miyake T. Anat. Embryol. 1992;186:107–124. doi: 10.1007/BF00174948. [DOI] [PubMed] [Google Scholar]
- 16.Thesleff I, Vaahtokari A, Partanen AM. Int. J. Dev. Biol. 1995;39:35–50. [PubMed] [Google Scholar]
- 17.Tekin H, Anaya M, Brigham M, Nauman C, Langer R, Khademhosseini A. Lab Chip. 2010;10:2411–2418. doi: 10.1039/c004732e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tekin H, Sanchez JG, Landeros C, Dubbin K, Langer R, Khademhosseini A. Adv. Mater. 2012;24:5543–5547. doi: 10.1002/adma.201201805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klouda L, Perkins KR, Watson BM, Hacker MC, Bryant SJ, Raphael RM, Kasper FK, Mikos AG. Acta Biomater. 2011;7:1460–1467. doi: 10.1016/j.actbio.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmaljohann D. Adv. Drug Deliv. Rev. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Schild HG. Prog. Polym. Sci. 1992;17:163–249. [Google Scholar]
- 22.Klouda L, Mikos AG. Eur. J. Pharm. Biopharm. 2008;68:34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hern DL, Hubbell JA. J. Biomed. Mater. Res. 1998;39:266–276. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Hersel U, Dahmen C, Kessler H. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 25.Yang XB, Roach HI, Clarke NMP, Howdle SM, Quirk R, Shakesheff KM, Oreffo ROC. Bone. 2001;29:523–531. doi: 10.1016/s8756-3282(01)00617-2. [DOI] [PubMed] [Google Scholar]
- 26.Thesleff I. Acta Odontol. Scand. 1998;56:321–325. doi: 10.1080/000163598428248. [DOI] [PubMed] [Google Scholar]
- 27.Thesleff I. J. Cell Sci. 2003;116:1647–1648. doi: 10.1242/jcs.00410. [DOI] [PubMed] [Google Scholar]
- 28.Ohazama A, Modino SA, Miletich I, Sharpe PT. J. Dent. Res. 2004;83:518–522. doi: 10.1177/154405910408300702. [DOI] [PubMed] [Google Scholar]
- 29.Zhao X, Kim J, Cezar CA, Huebsch N, Lee K, Bouhadir K, Mooney DJ. Proc. Natl. Acad. Sci. U.S.A. 2011;108:67–72. doi: 10.1073/pnas.1007862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi H, Matsuzaka N, Nakayama M, Kikuchi A, Yamato M, Okano T. Biomacromolecules. 2012;13:253–260. doi: 10.1021/bm201545u. [DOI] [PubMed] [Google Scholar]
- 31.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paszek MJ, Weaver VM. J. Mammary Gland Biol. Neoplasia. 2004;9:325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.