Abstract
Objective
To conduct a cancer education intervention with racially diverse communities in South Carolina.
Methods
The study was conducted at eight different sites in six counties in SC. The intervention included a 3-hour general cancer knowledge and 30-minute prostate cancer knowledge component. Pre- and post-intervention surveys were administered. Maximum scores were 31, 10 and 5 for the general cancer knowledge, prostate cancer knowledge and perceived self-efficacy in patient-physician interaction instruments, respectively. Analyses were completed using SPSS 16.0, SAS 9.1.3, and R v2.6.1.
Results
The study sample consisted of 164 predominantly African American participants. Most of the participants who reported age were 50+ years (62.5%). Among those who reported income, 46.1% had an annual household income < $40,000. The mean general cancer knowledge pre-test score was 26.2 (standard deviation (SD) 3.7) with a mean post-intervention increase of 2.15 points (p<0.01). The mean pre-test prostate cancer knowledge score was 7.3 (SD 2.0) with a post-intervention increase of 0.48 points (p<0.01). Perceived self-efficacy in patient-physician interaction scores had a ceiling effect.
Conclusions
General cancer knowledge and prostate cancer knowledge scores increased following the intervention.
Practice Implications
The intervention was successful in the short-term. It could be continued by community members.
Keywords: African American, Cancer Knowledge, Racially Diverse Communities
Introduction
South Carolina (SC) ranks among the top 20 states in the U.S. with the highest number of cancer deaths [1]. One of five SC residents will have cancer during their lives [1]. As shown in the following data, African Americans (AAs) in SC have significantly higher cancer death rates than European Americans (EAs) [2].
Prostate cancer is the second most common cause of cancer death among men in the U.S.[3] AA men have the highest incidence and mortality rates due to prostate cancer of any other racial or ethnic group in the U.S.[4–8] In SC, prostate cancer death rates are almost 2.5 times higher among AAs than among EAs.
Lack of Cancer Knowledge among AA Community Members
Previous studies show that members of AA communities may require additional knowledge about cancer screening, prevention, early detection, and treatment. Lack of knowledge likely contributes to cancer disparities [9–12]. For example, Sadler et al. [10] conducted a cancer knowledge assessment in San Diego beauty salons with AA women and reported low pre-intervention breast cancer knowledge levels and low adherence to recommended breast cancer screening guidelines. Only 30% of the women reported being confident about their level of knowledge of the disease [10].
Perceived self-efficacy in patient-physician interactions refers to patients’ self-confidence in their ability to obtain needed health information and to have physicians pay attention to their health concerns. In Bandura’s social cognitive theory (SCT), self-efficacy is defined as confidence in being able to exert personal control.[13, 14]
Low levels of knowledge are associated with low self-efficacy and low rates of participation in prostate, breast and cervical cancer screening.[13–17] For example, many AA men report that clinicians do not communicate effectively with them about prostate cancer screening.[10] Lack of knowledge precludes patients’ feelings of self-efficacy to actively engage in shared decision making about screening with their clinicians. Therefore, as cancer knowledge increases, participants’ confidence in their ability to effectively communicate with their clinicians about cancer would be expected to increase commensurately. [18, 19]
Methods
Study Sample
To maximize the impact of the intervention, our study included a convenience sample of participants from communities with large racial disparities in cancer mortality rates (Table 1) [20]. Although most of the community leaders who took responsibility for recruiting participants to the intervention were AA, we also included American Indians/Alaskan Natives due to their high cancer mortality rates.[21]
Table 1.
Age-Adjusted Cancer Mortality Rates for the Intervention Counties*
| 2006 Cancer Mortality Rate Intervention Counties in South Carolina | ||||||
|---|---|---|---|---|---|---|
| Race | Berkeley | Charleston | Georgetown | Greenville | Orangeburg | Richland |
| Caucasian | 197.83 | 176.89 | 179.93 | 171.77 | 180.38 | 190.19 |
| African American | 206.16 | 222.10 | 187.23 | 244.71 | 261.08 | 237.20 |
South Carolina Department of Environmental Control website (http://www.scandhec); accessed 7/10/09; age adjusted rates; 2000 US standard population[7]
We did not exclude participants on the basis of their race or ethnicity. Caucasians participated in the study as well. We conducted the intervention at eight sites in six different counties representing several different geographic regions of the state (Figure 1).
Figure 1.
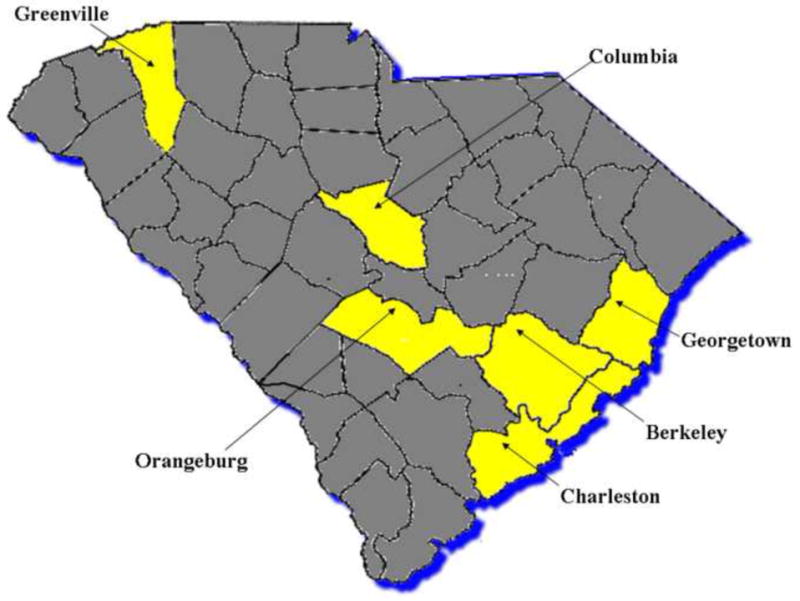
Six countied in SC where the intervention took place
Inclusion criteria thus included:
Residence in the communities near the location of the intervention site
Male or female gender
Any race or ethnicity
Ages 21 years or older
Institutional Review Board Approval
The Institutional Review Board (IRB) at the Medical University of South Carolina approved the study protocol. The pre-/post-intervention surveys that were completed by participants were linked by an identifier that was not connected to their name, date of birth, or any other personal identifier.
Intervention
The intervention consisted of a 3.5-hour evidence-based cancer education program in which a 3-hour component focused on general cancer knowledge and a 30-minute component focused on prostate cancer knowledge. The intervention was developed by the SC Cancer Alliance (SCCA) for general audiences with no expert knowledge about cancer. The SCCA is an 800-member statewide non-profit organization with membership from the lay community, public health associations and academia. A pre-/post-intervention survey was administered at each site.
The rationale for the dual focus of the intervention on general cancer and prostate cancer in particular is based on cancer mortality data from SC. For every major cancer, the state ranks among the highest in the nation in cancer mortality and there are large racial disparities within these cancer subtypes. Additionally, the state has the 3rd highest prostate cancer death rate in the nation.[22] For these reasons, we felt that while gaining increased knowledge about many different cancer subtypes was important, it was of particular importance to include a separate training module focusing on prostate cancer.
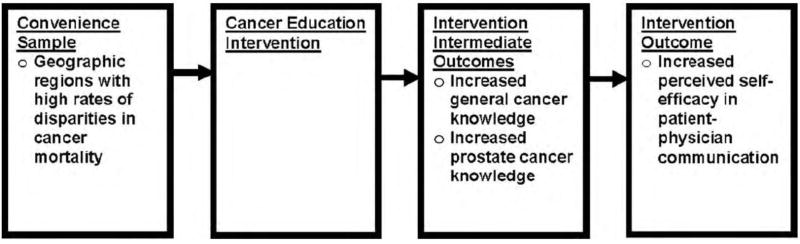
The study design focused on three different outcomes: perceived self-efficacy in patient-physician interaction, general knowledge of cancer and prostate cancer-specific knowledge. Figure 2 depicts our conceptual framework. We hypothesized that after the intervention, we would see increases in the following outcomes: general cancer knowledge, prostate cancer knowledge and perceived self-efficacy in patient-physician interaction.
Figure 2.
Conceptual framework of the hypothesized relationships.
While the intervention focused on three different outcomes, it was not delivered through three different modalities. The same modality was used in each session and with each group of participants. The structure and content of the intervention was identical across all study sites.
Measures
For general cancer knowledge, a 19-item instrument was developed by the investigative team. The instrument included one item on prostate cancer, three items on breast cancer, one item on cervical cancer, one item on the HPV vaccine, one item on colon cancer, three items on skin cancer, and nine other cancer-related items addressing diet, exercise, tobacco use, family history, and myths.
Prostate cancer knowledge was measured by the 10-item PROCASE instrument [23]. The PROCASE was developed in a sample of male patients aged 50+ years receiving primary care at four participating VA Medical Centers in the Midwest. In terms of its reliability, the Kuder-Richardson 20 (KR-20), which measures the average intercorrelation of items with dichotomous responses, was 0.68. [23]
Perceived self-efficacy in patient-physician communication about cancer was measured by a cancer adapted version of the 5-item Perceived Self-Efficacy in Patient-Physician Interactions (PEPPI) Scale developed by Maly et al. [18]. These investigators created the scale to quantify older patients’ self-efficacy in medical interactions with physicians. In their study, Maly et al. [18] reported that the Cronbach’s alpha was 0.83, indicating high reliability. Higher PEPPI scores are associated with higher levels of perceived self-efficacy in patient-physician interactions [18].
Six additional survey items were included. These items assessed sociodemographic characteristics, including Hispanic ethnicity, race, education level, marital status, household income, age and gender.
Statistical Methods
The survey data were double-entered and compared for verification of data entry. Analyses were completed using SPSS 16.0, SAS 9.1.3, and R v2.6.1. A knowledge score was created by scoring one point for each question an individual answered correctly. The mean knowledge scores, standard deviations, and 95% confidence intervals were calculated. Chi-square tests were used to compare demographics across the different sites, and paired t-tests were used to compare pre- and post-test scores.
Results
Table 2 shows the demographic characteristics of the participants (n=164, 94% response rate). The majority (78.6%) were AA, and most (76.4%) had at least a college education. Half (50.0%) were married or living as married. Among those who reported income, 46.1% had an annual household income < $40,000. The majority of participants (79.7%) were female. The demographic analysis across all sites shows a statistically significant difference in gender, race and education by site (Table 3).
Table 2.
Summary of Demographic of Participants at Pre-Test (N=164)
| Variable | N (%) |
|---|---|
| Age* | |
| Less than 50 years | 60 (37.5%) |
| 51–64 years | 63 (39.4%) |
| 65–75 years | 33 (20.6%) |
| More than 76 years | 4 (2.5%) |
| Hispanic* | |
| Yes | 3 (1.9%) |
| No | 157 (98.1%) |
| Race* | |
| African American or Black | 125 (78.6%) |
| American Indian or Alaskan Native | 15 (9.4%) |
| Asian | 0 (0.0%) |
| Caucasian or White | 19 (11.9%) |
| Pacific Islander | 0 (0.0%) |
| Education* | |
| Less than 8 years | 4 (2.5%) |
| 8–11 years | 7 (4.3%) |
| 12 years or completed high school | 17 (10.6%) |
| Post high school training other than college | 10 (6.2%) |
| Some college | 30 (18.6%) |
| College graduate | 43 (26.7%) |
| Postgraduate | 50 (31.1%) |
| Marital Status* | |
| Married or living as married | 80 (50.0%) |
| Widowed | 16 (10.0%) |
| Divorced | 21 (13.1%) |
| Separated | 3 (1.9%) |
| Never married | 40 (25.0%) |
| Household Income | |
| S0–S19,999 | 32 (20.8%) |
| S20,000–S39,999 | 39 (25.3%) |
| S40,000–S59,999 | 37 (24.0%) |
| S60,000–S79,999 | 21 (13.6%) |
| S80,000+ | 25 (16.2%) |
| Gender | |
| Male | 24 (20.3%) |
| Female | 94 (79.7%) |
Some participants were missing data on this variable
Table 3.
Cross-site comparison of socio-demographic characteristics of participants.
| Variable | p-Value |
|---|---|
| Age | 0.068 |
| Hispanic ethnicity | 0.514 |
| Gendera | <0.001 |
| Race | <0.001 |
| Education | <0.001 |
| Marital status | 0.663 |
| Household income | 0.313 |
Not all sites collected information regarding gender.
General Cancer and Prostate Cancer Knowledge
Table 4 shows the results for the general cancer knowledge and prostate cancer knowledge scores. Nineteen items assessed general cancer knowledge and several items had multiple correct responses, each of which was scored separately. Therefore, the maximum score for the cancer knowledge items was 31. The pre-survey general cancer knowledge score had a mean (SD) of 26.2 (3.7), equivalent to 85±12%. Across the eight study sites, the mean change in general cancer knowledge was an increase of 2.15 points (p<0.01).
Table 4.
General Cancer Knowledge Score by Site
| Site | Pre-test score | Point increase from pre- to post-test | p-value | |||
|---|---|---|---|---|---|---|
| General Cancer Knowledge | Prostate Cancer Knowledge | General Cancer Knowledge | Prostate Cancer Knowledge | General Cancer Knowledge | Prostate Cancer Knowledge | |
| Georgetown (n = 15) | 25.9 | 6.2 | 1.6 | 0.7 | 0.072 | 0.081 |
| Ridgeville (n = 24) | 24.7 | 6.2 | 3.6 | 1.2 | <0.001 | <0.001 |
| Charleston (n = 17) | 26.9 | 8.1 | 2.5 | 0.5 | 0.003 | 0.179 |
| Greenville (n = 19) | 27.0 | 7.9 | 0.8 | −0.3 | 0.316 | 0.479 |
| Orangeburg (n = 33) | 25.0 | 7.1 | 3.0 | 0.5 | <0.001 | 0.055 |
| Columbia (n = 20) | 27.5 | 8.3 | 1.9 | −0.2 | 0.014 | 0.679 |
| Orangeburg 2 (n = 15) | 27.8 | 8.2 | 0.1 | 0.1 | 0.880 | 0.873 |
| Johns Island (n = 12) | 26.3 | 6.5 | 2.3 | 1.3 | 0.020 | 0.008 |
Only those participants that had both pre- and post-test scores included in the average pre-test score and the point increase.
Statistically significant increases in prostate cancer knowledge from pre-test to post-test were seen in Ridgeville and Johns Island, the two sites with the most culturally homogeneous participants (predominantly Native American in Ridgeville and predominantly Sea Island residents on Johns Island). Across all eight sites, the mean change in prostate cancer knowledge score was an increase of 0.48 points (p<0.01).
Perceived Efficacy in Patient-Physician Interaction
For the PEPPI scale, five items were used to determine how self-efficacy was related to knowledge of cancer. On a scale from 1 to 5, with 1 being Not at all confident and 5 being Very Confident, participants rated their confidence in speaking with their physician about cancer [18]. For example, for the first question: “How confident are you in your ability to know what questions about cancer to ask a doctor?” The Ridgeville site, which had the lowest initial PEPPI score for each of the five questions, showed the most significant point increases from pre- to post- test. The other sites demonstrated smaller pre-/post-test increases in PEPPI scores for the five items. Because some of the scores were initially high (on the five-point scale), there was a ceiling effect since as there was little room for scores to improve.
Discussion and Conclusion
The purpose of this study was to examine the impact of a cancer knowledge intervention on general cancer knowledge, prostate cancer knowledge, and perceived self-efficacy in patient-physician interactions among predominantly AA communities in SC.
In our previous study focusing on recruitment of AA men to a cancer clinical trial, female spouses or partners were found to serve as gatekeepers in terms of access to the male study participants. We also learned that women transmitted health information to the men in their lives.[24, 25]
Therefore, in the present study, while we made efforts to include males by publicizing the cancer education sessions in each area with male-dominated organizations such as fraternities, Masonic orders and ministerial alliances, we felt confident that the women who participated in the sessions would share the information with their husbands, sons, nephews, etc.
A possible explanation for the initial high cancer knowledge level among study participants could be their relatively high educational level. According to the 2006 U.S. Census Bureau estimates, only 15.1% of the SC population over the age of twenty-five has completed their bachelor’s degree [26]. Thus, the participants in this study were more highly educated than the general population of SC.
Two large cultural groups were represented in the sample. The first group is the Wassamasaw Tribe of Varnertown/Ridgeville and the second group is the Sea Island community of Johns Island. Both groups are medically underserved and tend to be relatively isolated from health care settings [27]. The most statistically significant study findings were in these groups.
South Carolina’s cancer mortality rates, and racial/ethnic disparities in these rates, rank among the highest in the nation [28]. Cancer knowledge may play a large role in these disparities. Our results show that the cancer education intervention that we tested had a strongly positive and significant impact on the study outcomes.
Practice Implications
Conducting cancer education training with racially and ethnically diverse populations led to increased cancer knowledge and feelings of self-efficacy in patient-physician interactions. Continued efforts will be needed to assess whether the short-term gains are sustained over time and whether these gains lead to positive changes in cancer prevention activities.
Future Directions
Cancer knowledge scores and perceived self-efficacy increased following the intervention. Therefore, future interventions could incorporate more intensive (i.e., repeated sessions) cancer education programs as well as an assessment of the impact of the interventions on the communication dynamics between patients and their healthcare providers. Such interventions are needed to combat cancer disparities in SC.
Less educated populations may have lower knowledge of cancer risk factors [29]. Therefore, future educational interventions should target populations with educational levels similar to or lower than the general population to attempt to examine knowledge levels and perceived self-efficacy to empower these patients and to increase their perceived self-efficacy in talking with doctors about cancer. Nevertheless, it is important to point out the fact that cancer disparities in SC persist regardless of the education level of the population. Cancer disparities occur at every stage of the socioeconomic status spectrum in the state.
Acknowledgments
The authors would like to thank Dr. Heather Brandt and Ms. Marylou Stinson for their review of the manuscript.
Footnotes
Author Statement
I confirm all patient/personal identifiers have been removed or disguised so the patient/person(s) described are not identifiable and cannot be identified through the details of the story.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marvella Ford, Email: fordmar@musc.edu, Associate Director, Cancer Disparities Program, Medical University of South Carolina, Hollings Cancer Center, 86 Jonathan Lucas Street, Charleston, South Carolina, 29425, Office Phone: (843) 876-1116, Fax Number: (843) 792-4233
Amy E. Wahlquist, Email: herrin@musc.edu, Research Associate, Department of Medicine, Division of Biostatistics and Epidemiology, Medical University of South Carolina, Hollings Cancer, Center Office Phone: (843) 876-1054, Fax Number: (843) 876-1126
Celina Ridgeway, Email: Clinaridgeway@yahoo.com, Student, Voorhees College.
June Streets, Email: Junebug0322@yahoo.com, jstreets322@msn.com, Student, Georgetown University.
Katie A. Mitchum, Email: mitchu@musc.edu, Medical Student, Medical University of South Carolina
Reverend Remus Harper, Jr., Email: remusharperjr@aol.com, Pastor, Mt. Carmel AME Church Office Phone: (843) 797-3628
Ian Hamilton, Email: Ian.Hamilton@sccanceralliance.org, Prevention Coordinator, South Carolina Cancer Alliance Office Phone: (803) 356-7583.
Jim Etheredge, Email: etherjam@musc.edu, Program Coordinator, Cancer Disparities Program, Medical University of South Carolina, Hollings Cancer Center, Office Phone: (843) 792-8192, Fax Number: (843) 792-4233.
Melanie Sweat, Email: sweatma@musc.edu, Program Coordinator, Cancer Disparities Program, Medical University of South Carolina, Hollings Cancer Center, Office Phone: (843) 876-1569, Fax Number: (843) 792-4233.
Heidi Varner, Email: cfeliz2003@aol.com, Varner Town Indian Community.
Katora Campbell, Email: katora.campbell@palmettohealth.org, Facilitator, South Carolina Cancer Alliance, Office Phone: (803) 356-7583.
Elizabeth Garrett-Mayer, Email: garrettm@musc.edu, Associate Professor, Division of Biostatistics, Bioinformatics, and Epidemiology, Medical University of South Carolina, Office Phone: (843) 792-7764
References
- 1.CDC. SIP 11. Atlanta, Georgia: CDC; 2004. [Google Scholar]
- 2.Cullen KW, Bartholomew LK, Parcel GS, Koehly L. Measuring stage of change for fruit and vegetable consumption in 9- to 12-year-old girls. J Behav Med. 1998;21(3):241–54. doi: 10.1023/a:1018764932609. [DOI] [PubMed] [Google Scholar]
- 3.Sanderson M, Coker AL, Perez A, Du XL, Peltz G, Fadden MK. A multilevel analysis of socioeconomic status and prostate cancer risk. Ann Epidemiol. 2006;16(12):901–7. doi: 10.1016/j.annepidem.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghafoor A, Jemal A, Cokkinides V, et al. Cancer statistics for African Americans. CA Cancer J Clin. 2002;52(6):326–41. doi: 10.3322/canjclin.52.6.326. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54(1):8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 6.Pierce R, Chadiha LA, Vargas A, Mosley M. Prostate cancer and psychosocial concerns in African American men: literature synthesis and recommendations. Health Soc Work. 2003;28(4):302–11. doi: 10.1093/hsw/28.4.302. [DOI] [PubMed] [Google Scholar]
- 7.Powell IJ, Banerjee M, Novallo M, et al. Prostate cancer biochemical recurrence stage for stage is more frequent among African-American than white men with locally advanced but not organ-confined disease. Urology. 2000;55(2):246–51. doi: 10.1016/s0090-4295(99)00436-7. [DOI] [PubMed] [Google Scholar]
- 8.Hu SY, Liu T, Liu Z, et al. Identification of a novel germline missense mutation of the androgen receptor in African American men with familial prostate cancer. Asian J Androl. doi: 10.1038/aja.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yabroff KR, Washington KS, Leader A, Neilson E, Mandelblatt J. Is the promise of cancer-screening programs being compromised? Quality of follow-up care after abnormal screening results. Med Care Res Rev. 2003;60(3):294–331. doi: 10.1177/1077558703254698. [DOI] [PubMed] [Google Scholar]
- 10.Sadler GR, Ko CM, Cohn JA, White M, Weldon RN, Wu P. Breast cancer knowledge, attitudes, and screening behaviors among African American women: the Black cosmetologists promoting health program. BMC Public Health. 2007;7(147):57. doi: 10.1186/1471-2458-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green PM, Kelly BA. Colorectal cancer knowledge, perceptions, and behaviors in African Americans. Cancer Nurs. 2004;27(3):206–15. doi: 10.1097/00002820-200405000-00004. quiz 216–7. [DOI] [PubMed] [Google Scholar]
- 12.Eggleston KS, Coker AL, Das IP, Cordray ST, Luchok KJ. Understanding barriers for adherence to follow-up care for abnormal pap tests. J Womens Health (Larchmt) 2007;16(3):311–30. doi: 10.1089/jwh.2006.0161. [DOI] [PubMed] [Google Scholar]
- 13.Bandura A, Adams NE. Analysis of Self-Efficacy Theory of Behavior Change. Cognitive Therapy Research. 1977;1 [Google Scholar]
- 14.Fernandez ME, Diamond PM, Rakowski W, et al. Development and validation of a cervical cancer screening self-efficacy scale for low-income Mexican American women. Cancer Epidemiol Biomarkers Prev. 2009;18(3):866–75. doi: 10.1158/1055-9965.EPI-07-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogenmiller JR, Atwood JR, Lindsey AM, Johnson DR, Hertzog M, Scott JC., Jr Self-efficacy scale for Pap smear screening participation in sheltered women. Nurs Res. 2007;56(6):369–77. doi: 10.1097/01.NNR.0000299848.21935.8d. [DOI] [PubMed] [Google Scholar]
- 16.Kendrick L, Montgomery S, Ouattara D, Flaskerud JH. African american men and self-efficacy in preventing prostate cancer. Issues Ment Health Nurs. 2009;30(5):342–3. doi: 10.1080/01612840902754669. [DOI] [PubMed] [Google Scholar]
- 17.Woods VD, Montgomery SB, Herring RP. Recruiting Black/African American men for research on prostate cancer prevention. Cancer. 2004;100(5):1017–25. doi: 10.1002/cncr.20029. [DOI] [PubMed] [Google Scholar]
- 18.Maly RC, Frank JC, Marshall GN, DiMatteo MR, Reuben DB. Perceived efficacy in patient-physician interactions (PEPPI): validation of an instrument in older persons. J Am Geriatr Soc. 1998;46(7):889–94. doi: 10.1111/j.1532-5415.1998.tb02725.x. [DOI] [PubMed] [Google Scholar]
- 19.Maly RC, Leake B, Silliman RA. Breast cancer treatment in older women: impact of the patient-physician interaction. J Am Geriatr Soc. 2004;52(7):1138–45. doi: 10.1111/j.1532-5415.2004.52312.x. [DOI] [PubMed] [Google Scholar]
- 20.South Carolina Department of Enivornmental Control. Age-Adjusted Rates 2005 [Google Scholar]
- 21.SEER Stat Database. Incidence. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, April 2008, based on the November 2007 submission.
- 22.South Carolina Cancer Facts & Figures 2004–2005. Columbia: South Carolina Central Cancer Registry, Office of Public Health Statistics and Information Services, South Carolina Department of Health and Environmental Control; 2005. [Google Scholar]
- 23.Radosevich DM, Partin MR, Nugent S, et al. Measuring patient knowledge of the risks and benefits of prostate cancer screening. Patient Educ Couns. 2004;54(2):143–52. doi: 10.1016/S0738-3991(03)00207-6. [DOI] [PubMed] [Google Scholar]
- 24.Ford DW, Nietert PJ, Zapka J, Zoller JS, Silvestri GA. Barriers to hospice enrollment among lung cancer patients: a survey of family members and physicians. Palliat Support Care. 2008;6(4):357–62. doi: 10.1017/S1478951508000564. [DOI] [PubMed] [Google Scholar]
- 25.Ford ME, Havstad SL, Davis SD. A randomized trial of recruitment methods for older African American men in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Clin Trials. 2004;1(4):343–51. doi: 10.1191/1740774504cn029oa. [DOI] [PubMed] [Google Scholar]
- 26.Selected Social Characteristics in the United States. United States Census Bureau, 2006.
- 27.American Indians & Cancer. http://iccnetwork.org/cancerfacts/ICC-CFS2.pdf.
- 28.Group USCSW. United States Cancer Statistics: 1999–2004 Incidence and Mortality Web-Based Report. Atlanta: U.S. Department of Health and Human Services, Center for Disease Control and Prevention and National Cancer Institute; 2007. [Google Scholar]
- 29.Breslow RA, Sorkin JD, Frey CM, Kessler LG. Americans’ knowledge of cancer risk and survival. Prev Med. 1997;26(2):170–7. doi: 10.1006/pmed.1996.0136. [DOI] [PubMed] [Google Scholar]