Abstract
Dendritic cell leukemia (DCL) or hematodermic tumor is an uncommon subtype of acute leukemia. In contrast to adult cases, children tend to have a less aggressive course. The diagnosis of DCL should be considered when its characteristic morphologic features are present and leukemic cells co-express CD4 and CD56. Cases of DCL among pediatric patients have been reported to respond to therapeutic regimens for acute lymphoblastic leukemia, but details regarding the specifics of therapy are lacking.
Keywords: dendritic cell, leukemia, CD4, CD56, hematodermic tumors
Introduction
Dendritic cells are antigen-presenting cells, originating from CD34+ progenitors in bone marrow. Dendritic cell leukemia (DCL), also known as hematodermic tumors, is uncommon with only 104 putative cases described, mostly in adults. Adult cases are generally associated with an unfavorable prognosis [1–3]. Childhood cases typically present with nonspecific signs, such as fever, pallor, lymphadenopathy, and hepatosplenomegaly [1, 4–7]. In one study of 1363 children with leukemia, only three (0.22%) had DCL [7]. Because of its rarity, the prognosis and optimal treatment for children with DCL have yet to be definitively established. We report four pediatric cases of plasmacytoid DCL treated at St. Jude Children’s Research Hospital (SJCRH) in the past decade (Table 1) and review them in the context of the existing literature.
TABLE 1.
Pediatric DCL – SJCRH - Clinical presentation, immunophenoytpe, cytogenetics, and treatment response
| Patient Age (years)/Sex | Initial Cutaneous Involvement | Immunophenotype | Cytogenetics | Response to Total XV, Time from Diagnosis | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| 7/F | − | CR, 5.8 years | |||
| Bone marrow | dim CD45, CD22, CD79a, TdT, HLA-DR, CD4, CD36, dim CD33, dim CD15, CD56, CD68, CD123 (IL3R alpha), BDCA-4, weakly positive for CD68 | MPO, CD19, CD10, CD34, other myeloid or monocytic antigens | Pseudodiploid (46,XX) t(2;9), add(3p), add(12p), del(13q), and del(14q) | ||
| 7/M | + | CR, 4.2 years | |||
| Bone marrow* | NA | NA | NA | ||
| Soft tissue biopsy | CD4, CD56, CD123, TdT, CD68, CD43, LCA | ALK-1, CD30, CD3, CD5, CD20, CD79a, CD10, CD117, MastCT320, CD34, MPO, TIA-1, granzyme B, EBER | Normal diploid | ||
| 11/M | + | CR** | |||
| Bone marrow* | NA | NA | NA | ||
| Soft tissue biopsy | CD34, dim CD45, dim TdT, CD56, CD4, CD123, HLA-DR, dim CD2, BCL2 | CD 20, CD10, cyclin D, CD99, myogenin, and multiple myeloid and lymphoid markers, lysozyme, EBER, Ig kappa and lamda light chains | Hypodiploid (45, XY), −9, del(12)(p11.2), −13, del(17)(p11.2), +mar[20] | ||
| 6/F | + | CR, 11.1 years | |||
| Bone marrow | CD56, CD4, CD7, CD8, TdT, weak cDC3, weak CD10, CD123 | CD2, CD3, CD5, CD19; MPO, CD20, lysozyme, CD1a, CD79a | 46, XX, del (6)(q21q25), der(7)t(7;9)(p13;q13), −9, add(11)(q23), , +mar | ||
| Skin punch biopsy | TdT, weak CD3, CD10 | CD20, MPO, lysozyme, CD1a, CD79a | |||
BDCA-4, blood dendritic cell antigen 4; CR, complete remission; DCL, dendritic cell leukemia; F, female; M, male; MPO, myeloperoxidase; NA, not applicable; SJCRH, St. Jude Children’s Research Hospital;
bone marrow was negative for tumor;
response after induction and surgical resection only; patient lost to follow-up
Cases
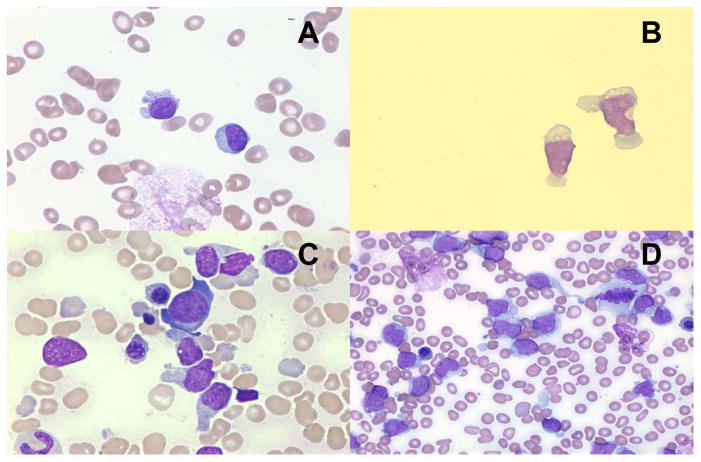
A 7-year-old female presented with pallor, fatigue, and anorexia. She had marked hepatosplenomegaly; scattered petechiae; multiple firm cervical, axillary, and inguinal lymph nodes. Laboratory results included white blood cell (WBC) count 11.6×109/L with 48% blasts (Fig. 1A), hemoglobin concentration 4 g/dL, platelet concentration 31×109/L, and serum lactate dehydrogenase concentration 590 U/L. The cerebrospinal fluid (CSF) contained 6 WBC and 0 red blood cells per microliter with 71% blasts (Fig. 1B). The cells’ phenotype indicated plasmacytoid DC differentiation (CD4+, CD56+), further confirmed by bilateral bone marrow biopsies. The leukemic-cell DNA index was 1.0.
Fig. 1.
A. Morphology of peripheral blood and B. cerebrospinal fluid shows blasts with scant, pale, basophilic, vacuolated cytoplasm. Bone marrow morphology reveals C. prominent cytoplasmic projections and D. peripheral vacuolization resembling a string of pearls.
The second patient, a 7-year-old male, had a 4-month history of enlarging subcutaneous right thigh mass. Complete blood count, chemistries, liver function tests and coagulation panel results were normal. Positron emission tomography (PET) showed an oblong focus of increased uptake along the right mid-lateral thigh. No malignant cells were seen in the CSF. The subcutaneous mass, measuring 4cm × 5cm, was excised and showed immunophenotypic features of plasmacytoid DCL. Ki-67 staining was positive in 70–75% of tumor cells. In situ hybridization detected no Epstein-Barr virus–encoded RNA. Flow cytometry revealed no malignant cells in the peripheral blood. Bilateral bone marrow aspirates and biopsies revealed normocellular marrow with no tumor.
The third patient, an 11-year-old male, presented with 1.5 year history of right calf mass. Magnetic resonance imaging delineated a well-defined subcutaneous mass along the right calf separate from the leg musculature, measuring 5.6cm × 3.4cm × 6.4cm. A needle biopsy of the lesion disclosed a plasmacytoid DC neoplasm. PET showed no evidence of metastatic disease; blood and bone marrow showed no malignancy. The lesion was surgically resected after one week of induction chemotherapy.
Lastly, a 6-year-old female presented with one-year history of venous discoloration on her right cheek. CT confirmed a 3.5cm × 3cm soft tissue mass just below the orbit. Scattered bruises were on her extremities and periorbital area. A 3.5 cm firm nodule was palpable beneath her right nipple. Her spleen extended 3 cm below the costal margin. Bone scan showed increased activity in the right facial region. Biopsy results of the right cheek lesion revealed a plasmacytoid DC neoplasm expressing CD4 and CD56. Her presenting laboratory results included WBC count 1.9×109/L with 20% blasts, hemoglobin 7.8 g/dL, platelet concentration 128×109/L, and an elevated serum uric acid concentration 6.4 mg/dL. CSF contained no blasts. Bone marrow aspirates revealed small blast cells with scant to moderate amounts of weakly basophilic cytoplasm and indistinct nucleoli. MLL was not rearranged.
Discussion
These 4 cases represent 0.4% of the 930 patients with acute lympoblastic leukemia (ALL) (n=750) and non-Hodgkin lymphoma (n=180) treated in a 10-year period at SJCRH. One patient enrolled on the standard/high-risk arm of our institutional ALL protocol, Total XV [8]; the other three patients were treated according to the same protocol. Induction chemotherapy included prednisone, vincristine, daunorubicin, asparaginase, cyclophosphamide, mercaptopurine, cytarabine, and triple intrathecal therapy (methotrexate, hydrocortisone, and cytarabine). Consolidation comprised 4 courses of high-dose methotrexate and oral mercaptopurine. Continuation therapy consisted of rotating drug pairs, intrathecal therapy and two courses of re-induction. Patients 1, 2 and 4 completed treatment and remain in continuous complete remission (CCR) 5.8 years, 4.2 months, and 11.1 years from diagnosis, respectively. The third patient was in remission after induction chemotherapy and surgical resection, but subsequently lost to follow-up after transferring care.
In plasmacytoid DCL, bone marrow morphology often reveals extensive replacement by blast cells, ranging from “lymphoid-like” to a more characteristic appearance with irregular nuclei, ample basophilic cytoplasm, prominent cytoplasmic projections and/or peripheral vacuolization resembling a string of pearls (Fig. 1C and D). Cytochemical stains for myeloperoxidase and alpha naphthyl acetate esterase are negative [1].
Specific markers of dendritic cell lineage include CD4, CD56, HLA-DR, blood DC antigen (BDCA)-2, BDCA-4, CD45A, and CD123 [9]. Most specific markers for myeloid (CD13, CD33, MPO), T-cell (CD3 and CD5), or B-cell (CD19 and CD20) lineages are absent, as is CD34 [1]. Thus, the diagnosis of DCL should be entertained when characteristic morphologic features are seen with Wright-Giemsa-staining and in leukemias with any morphologic features showing co-expression of CD4 and CD56, while negative for CD34 and cytoplasmic CD3.
Like those reported previously, our patients tumors’ were positive for CD4 and CD56. Individual cases, however, are bound to vary. None of the reported cases of DCL outside this series expressed the B-cell antigens CD22 and CD79a, as in our first patient. In addition, only rare cases published to date express TdT, which was detected in all four of our patients; therefore, the presence of B cell markers and TdT should not exclude the diagnosis of DCL. Likewise, expression of myeloid marker CD33 is compatible with a diagnosis of plasmacytoid DCL [8].
Cutaneous involvement may be more common among pediatric patients than previously reported in reviews of adult DCL [1, 2, 7, 10, 11]. It is important to distinguish the occurrence of cutaneous involvement in this entity from that of myeloid leukemia cutis (LC). Clinical morphology is not useful for diagnosis of leukemia type in LC given that individual lesions are not pathognomic for the different forms of leukemia [12–14]. More recently, Cronin and colleagues indicated that a panel including CD4, CD56, CD 123 and Tcl-1 can be used to distinguish DCL from LC [15]. Immunophenotyping on bone marrow for all 4 patients included CD4, CD56 and CD123. Jegalian et al. also noted focal positivity of S-100 in three of four patients with DCL within their series, which may provide another differentiating stain to be applied to future cases [5].
Prior publications indicate varying therapeutic approaches, with almost half of patients receiving an ALL-based regimen [1–11, 16–20]. The successful results achieved in our four patients support ALL-based therapy for plasmacytoid DCL, a malignancy subtype now included in the WHO classification of myeloid disease [11]. Based on our experience, we recommend intensive ALL chemotherapy for pediatric patients with DCL. It remains unclear; however, whether low-risk ALL therapies would be as effective as more intensified treatment regimens. Increasing awareness of DCL and further understanding of its molecular basis should lead to more accurate diagnosis, better defined prognostic markers for risk stratification and improved therapies.
Acknowledgments
This research was supported in part by a grant from the National Institutes of Health, USA (CA21765), and by the American Lebanese Syrian Associated Charities (ALSAC). We would also like to thank the Scientific Editing Department (Vani Shanker) at SJCRH for review of the manuscript.
Footnotes
Disclosures
The authors have no disclosures or competing financial interests.
References
- 1.Feuillard J, Jacob MC, Valensi F, et al. Clinical and biologic features of CD4+CD56+ malignancies. Blood. 2002;99:1556–1563. doi: 10.1182/blood.v99.5.1556. [DOI] [PubMed] [Google Scholar]
- 2.Petrella T, Bagot M, Willemze R, et al. Blastic NK-cell lymphomas (agranular CD4+ CD56+ hematodermic neoplasms): a review. Am J Clin Pathol. 2005;123:662–675. [PubMed] [Google Scholar]
- 3.Reimer P, Rudiger T, Kraemer D, et al. What is CD4+CD56+ malignancy and how should it be treated? Bone Marrow Transplant. 2003;32:637–646. doi: 10.1038/sj.bmt.1704215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anargyrou K, Paterakis G, Boutsis D, et al. An unusual case of CD4+ CD7+ CD56+ acute leukemia with overlapping features of type 2 dendritic cell (DC2) and myeloid/NK cell precursor acute leukemia. Eur J Haematol. 2003;71:294–298. doi: 10.1034/j.1600-0609.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 5.Jegalian AG, Buxbaum NP, Facchetti F, et al. Blastic plasmacytoid dendritic cell neoplasm in children: diagnostic features and clinical implications. Haematologica. 2010;95:1873–1879. doi: 10.3324/haematol.2010.026179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reineks EZ, Osei ES, Rosenberg A, et al. CD22 expression on blastic plasmacytoid dendritic cell neoplasms and reactivity of anti-CD22 antibodies to peripheral blood dendritic cells. Cytometry Part B: Clinical Cytometry. 2009;76:237–248. doi: 10.1002/cyto.b.20469. [DOI] [PubMed] [Google Scholar]
- 7.Rossi JG, Felice MS, Bernasconi AR, et al. Acute leukemia of dendritic cell lineage in childhood: incidence, biological characteristics and outcome. Leuk Lymphoma. 2006;47:715–725. doi: 10.1080/10428190500353216. [DOI] [PubMed] [Google Scholar]
- 8.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilichowska ME, Fleming MD, Pinkus JL, et al. CD4+/CD56+ hematodermic neoplasm (“blastic natural killer cell lymphoma”): neoplastic cells express the immature dendritic cell marker BDCA-2 and produce interferon. Am J Clin Pathol. 2007;128:445–453. doi: 10.1309/W9Q5AGYDE5LANN39. [DOI] [PubMed] [Google Scholar]
- 10.Hama A, Kudo K, Itzel BV, et al. Plasmacytoid dendritic cell leukemia in children. J Pediatr Hematol Oncol. 2009;31:339–343. doi: 10.1097/MPH.0b013e31819b7215. [DOI] [PubMed] [Google Scholar]
- 11.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. The International Agency for Research on Cancer (IARC) Press; Lyon: 2008. [Google Scholar]
- 12.Wagner G, Fenchel K, Back W, et al. Leukemia cutis – epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27–36. doi: 10.1111/j.1610-0387.2011.07842.x. [DOI] [PubMed] [Google Scholar]
- 13.Ratnam KV, Khor CJ, Su WP. Leukemia cutis. Dermatol Clin. 1994;12:419–431. [PubMed] [Google Scholar]
- 14.Kaddu S, Zenahlik P, Beham-Schmid C, et al. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966–78. doi: 10.1016/s0190-9622(99)70086-1. [DOI] [PubMed] [Google Scholar]
- 15.Cronin DM, George TI, Reichard KK, et al. Immunophenotypic analysis of myeloperoxidase-negative leukemia cutis and blastic plasmacytoid dendritic cell neoplasm. Am J Clin Pathol. 2012;137:367–376. doi: 10.1309/AJCP9IS9KFSVWKGH. [DOI] [PubMed] [Google Scholar]
- 16.Eguaras AV, Lo RW, Veloso JD, et al. CD4+/CD56+ hematodermic neoplasm: blastic NK cell lymphoma in a 6-year-old child: report of a case and review of literature. J Pediatr Hematol Oncol. 2007;29:766–769. doi: 10.1097/MPH.0b013e318159a4e6. [DOI] [PubMed] [Google Scholar]
- 17.Ruggiero A, Maurizi P, Larocca LM, et al. Childhood CD4+/CD56+ hematodermic neoplasm: case report and review of the literature. Haematologica. 2006;91:48. [PubMed] [Google Scholar]
- 18.Falcao RP, Garcia AB, Marques MG, et al. Blastic CD4 NK cell leukemia/lymphoma: a distinct clinical entity. Leuk Res. 2002;26:803–807. doi: 10.1016/s0145-2126(02)00014-0. [DOI] [PubMed] [Google Scholar]
- 19.Petrella T, Comeau MR, Maynadie M, et al. ‘Agranular CD4+ CD56+ hematodermic neoplasm’ (blastic NK-cell lymphoma) originates from a population of CD56+ precursor cells related to plasmacytoid monocytes. Am J Surg Pathol. 2002;26:852–862. doi: 10.1097/00000478-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Dijkman R, van Doorn R, Szuhai K, et al. Gene-expression profiling and array-based CGH classify CD4+ CD56+ hematodermic neoplasm and cutaneous myelomonocytic leukemia as distinct disease entities. Blood. 2007;109:1720–1727. doi: 10.1182/blood-2006-04-018143. [DOI] [PubMed] [Google Scholar]