Abstract
Non-cognitive neuropsychiatric symptoms (NPS) of dementia include aggression, agitation, depression, anxiety, delusions, hallucinations, apathy, and disinhibition. These affect dementia patients nearly universally across dementia stages and etiologies. NPS are associated with poor patient and caregiver outcomes including excess morbidity and mortality, increased health care utilization, and earlier nursing home placement, as well as caregiver stress, depression and reduced employment. While there is no FDA-approved pharmacotherapy for NPS, psychotropic medications are frequently used to manage these symptoms. However, in the few cases of proven pharmacologic efficacy, benefit may be off set by significant risk of adverse effects. Non-pharmacologic treatments, typically considered first-line, also show evidence for efficacy as well as a limited potential for adverse effects. However, their uptake as preferred treatments remains inadequate in real-world clinical settings. Thus, the field currently finds itself between a “rock and a hard place” in terms of management of these difficult symptoms. It was in this context that the University of Michigan Program for Positive Aging, working in collaboration with the Johns Hopkins Alzheimer’s Disease Research Center and Center for Innovative Care in Aging sponsored and convened a multidisciplinary expert panel in Detroit Michigan in Fall 2011 with three objectives, to: 1) define key elements of care for NPS in dementia; 2) construct an approach describing the sequential and iterative steps of managing NPS in real-world clinical settings that can be used as a basis for integrating non-pharmacologic and pharmacologic approaches; 3) discuss how the approach generated could be implemented in research and clinical care.
Keywords: behavior, behavioral management, non-pharmacological management
INTRODUCTION
Although cognitive impairment is the clinical hallmark of dementia, non-cognitive neuropsychiatric symptoms (NPS) are exceedingly common and dominate the presentation [1]. NPS occur in all types of dementia in clusters or syndromes identified as depression, psychosis, agitation, aggression, apathy, sleep disturbances, and disinhibition [2]. NPS are universal, affecting 98% of individuals at some point in disease course [2]. Thirty percent of the cost of caring for community-dwelling patients with dementia is directly attributable to NPS management [3]. NPS appear to be a consequence of the confluence of multiple, but sometimes modifiable, interacting factors internal and external to persons with dementia, are closely linked to the underlying brain disease causing cognitive symptoms, and also result in part from heightened vulnerability to the environment as cognitive ability declines.
NPS, as opposed to core cognitive symptoms, tend to create the most difficulties for patients, caregivers and providers, and commonly lead to earlier nursing home placement [4, 5], excess morbidity, mortality, hospital stays [6], caregiver stress and depression, and reduced caregiver employment income [7, 8]. These symptoms pose threats to caregivers’ own health and quality of life [9]. Caregivers of individuals with NPS are more distressed and depressed than those not managing NPS [10]. Clinically significant NPS, if untreated, are associated with more rapid disease progression than in the absence of such symptoms [11]. Therefore, effective treatments may have the potential to modify disease course, lower costs and improve quality of life for patients and caregivers.
In real-world settings, few well-proven treatment options are currently available for NPS. Although there are no FDA-approved medications for NPS, it is common clinical practice to use psychotropic medications such as antipsychotics to control symptoms. However, antipsychotics show modest efficacy in improving NPS [1] and have significant risks for patients including side effects and mortality [12].
Non-pharmacologic management of NPS is increasingly recognized as a critical part of comprehensive, state-of-the-art dementia care [13, 14]. Non-pharmacologic approaches tend to conceptualize behaviors as stemming from unmet needs, environmental overload and interactions of patient, caregiver and environmental factors. The goals of non-pharmacologic treatment are prevention, symptom relief and reduction of caregiver distress [15]. Non-pharmacologic strategies are recommended by multiple medical organizations and expert groups (including the American Geriatrics Society, the American Psychiatric Society and American Association for Geriatric Psychiatry) as the preferred first-line treatment approach to NPS [16–19], except in emergency situations when NPS could lead to imminent danger or otherwise compromise safety. In the latter cases, the standard of care supports psychotropic use in the absence of data. Unfortunately, effective non-pharmacologic strategies for NPS have not been translated into real-world clinical management and standard care [20]. Despite major concerns about safety and efficacy, psychotropics remain the primary “go-to” treatment approach, often without systematic assessment of potential underlying causes [21].
There are several reasons for the predominant use of medications over non-pharmacologic strategies. Most specialties have some training in psychotropic use for NPS but few receive instruction in non-pharmacologic approaches and are aware of their effectiveness [21]. Even when aware of these techniques and their value, most providers lack basic training in assessing NPS and choosing or communicating strategies to patients and caregivers. Assessing NPS and using non-pharmacologic strategies can be time-prohibitive and not reimbursed. Approaches developed in research trials [22–25] typically circumvent provider time limitations by training non-medical personnel to deliver interventions, and thus, strategies remain outside standard clinical practices. Enhancing clinician decision-making for NPS is timely and critical in view of a national movement to improve dementia care [26], new AMA Pay for Performance Guidelines [27] that will require physicians to evaluate and treat NPS as part of on-going standard care, as well as the Centers for Medicare and Medicaid Services efforts to reduce unnecessary use of antipsychotics in nursing homes [28].
Critically needed is an evidence-informed standardized approach to managing NPS that integrates pharmacological and non-pharmacological treatments for real-world implementation. To address this issue, the University of Michigan Program for Positive Aging, in collaboration with the Johns Hopkins Alzheimer’s Disease Research Center and Center for Innovative Care in Aging, sponsored and convened an Expert Panel in Detroit Michigan in 2011. Panel members had clinical and/or research expertise in managing NPS in dementia and reducing distress in family caregivers. This paper summarizes the approach developed by the panel.
Key Care Elements for NPS
Treatments for NPS can be categorized as pharmacologic, medical, or non-pharmacologic (which the expert panel referred to as “behavioral and environmental modifications”). The non-pharmacologic strategies underlying the DICE approach are those with the strongest evidence base, including thorough assessments for underlying causes and family caregiver interventions [22–25, 29–31]. Members of the expert panel discussed different decision-making approaches that had common elements of identifying and treating NPS [18, 32–35], including the “4 D Approach” [13]. The latter served as the starting point from which the panel further elaborated and relabeled steps to enhance clarity and ease of use by clinicians and from which a mnemonic was developed, “DICE”(Describe, Investigate, Create and Evaluate).
The DICE Approach
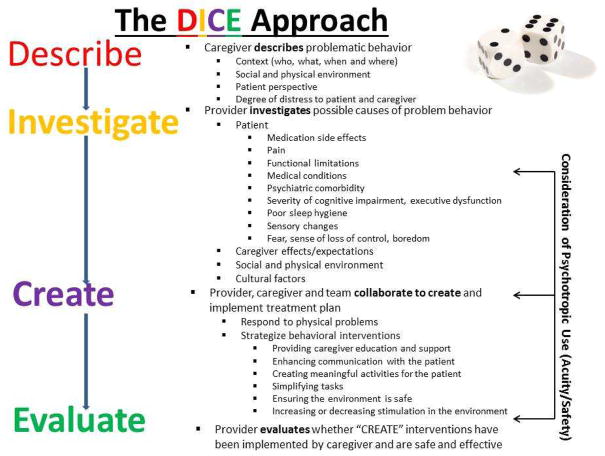
The DICE approach assumes that a problem NPS has been identified and brought to the provider’s attention (Figure 1). To flesh out each step of the approach, the panel used a case example of an 80 year-old woman with moderate dementia who strikes out at her daughter during bathing. This case reflects a typical clinical scenario often resulting in prescription of a psychotropic, and for which research supports behavioral and environmental modifications
Figure 1.
The DICE Approach
Step 1–Describe
The first step is to “DESCRIBE” the presenting behavior to derive an accurate characterization of the NPS and the context in which it occurs through discussion with caregiver and patient (if possible).
Strategies for eliciting details include asking caregivers to play back the NPS “as if in a movie”. A basic problem-solving approach identifies antecedents, describes the specifics of the NPS, and then details consequences in order to uncover the context in which NPS occur and potential underlying modifiable patterns or contributory factors. Caregivers can be encouraged to record NPS and the related patient, caregiver and environmental considerations (see Figure 2) in diaries or patient logs at home if possible. Except in severe dementia where communication may be problematic, the patient’s perspective should be elicited and clinicians should probe to determine what he/she can describe about the NPS. It is important to understand what aspect of the symptom is most distressing or problematic for the patient and caregiver and her/his treatment goal. Eliciting this information helps to evaluate the caregiver’s knowledge of dementia and NPS and leads to specific treatment strategies.
Figure 2.
Linkage of DICE Steps with Patient/Caregiver/Environmental Considerations
In the case, the caregiver uses the term “agitation” yet this could encompass a range of symptoms (anxiety, repetitive questions, aggression, wandering), each of which might have a different underlying cause and corresponding management strategy. The DESCRIBE step reveals that “agitation” referred to the patient becoming physically and verbally aggressive at bath time with the caregiver. The patient expresses that bathing “hurts” (i.e. she experienced pain when the caregiver put her in the bath). The caregiver indicates while she is not afraid for her own safety, she believes the patient is “doing this on purpose”. The caregiver’s goal is to have the patient bathe daily. There are no symptoms to suggest psychosis, and the patient does not have depressive symptoms.
Step 2-Investigate
Once the NPS is well-characterized, the next step is for the provider to examine, rule out and identify possible underlying and modifiable causes (Figure 2). Similar to the workup of delirium, the key to managing NPS is a thorough assessment of underlying causes. Undiagnosed medical conditions are important contributors. Individuals with dementia may suffer from pain and undiagnosed illnesses (such as urinary tract infection and anemia) disproportionately more than those without cognitive impairment [29, 36].
Patient considerations
This includes evaluating the current medication profile and presence of undetected medical conditions or pain. Compiling a list of patient medications, optimally by having the caregiver bring in bottles, including prescription and over-the-counter drugs and supplements, is an important first measure. Providers should assess the contributions of medication side effects, particularly those with anticholinergic properties, as well as considering possible drug interactions. Investigating medical conditions such as urinary tract and other infections, constipation, dehydration, and pain is critical. Obtaining blood work such as chemistries (including blood glucose and electrolytes), complete blood count with differential and a urinalysis may be helpful. Providers should also consider the impact of underlying prior psychiatric comorbidity (e.g., lifelong major depressive or anxiety disorder). Other important patient considerations include limitations in functional abilities, cognitive impairment severity, poor sleep hygiene, sensory changes and boredom. Psychological factors including feelings of inadequacy and helplessness and fear of “being a burden” to the family may play a role in the development and exacerbation of NPS.
Caregiver Considerations
These include understanding the historical and current quality of relationship between the patient and caregiver. Caregivers may lack an understanding of the link between dementia and NPS and believe the patient is “doing this to them on purpose”. Caregivers’ communication styles, expectations, over and/or underestimation of patient abilities, and their own stress and depression may inadvertently exacerbate behaviors. Finally, understanding the family cultural context is important. Beliefs will differentially impact caregiver and patient behaviors. In some families, nursing home placement may not be acceptable, and tremendous strain may be created in attempting to keep a person with severe limitations at home. In other families, discussing NPS may be difficult, and viewed as “airing dirty laundry” to “outsiders.”
Environmental Considerations
An environment that is over- or under-stimulating, presents way-finding challenges; lack of predictable routines and pleasurable activities can also impact NPS. Home safety is important: considered should be whether the patient can easily leave home; if the patient has access to dangerous objects (knives, guns), if the patient can navigate safely from one room to the next, if there are grab bars, other equipment and adaptations (e.g., use of labels, adequate task lighting) that compensate for patient functional difficulties.
In the case, the provider learns that the patient has a diagnosis of arthritis, but is not taking pain medications. When the caregiver moves the patient’s limbs quickly it causes pain which may contribute to her aggression to stop it. The caregiver’s communication is also overly complex for the patient’s dementia stage. The caregiver appears to lack an understanding of the link between dementia and behaviors (“she is doing this on purpose”)The caregiver’s tone with the patient when frustrated is harsh and confrontational (“I can’t have you acting like this. I have to give you a bath now!”). The caregiver’s goal to bathe the patient daily reflects the caregiver’s own values and preferences and intent to keep daily life as it was prior to dementia onset. The bathtub does not have a grab bar or bath mat which may be contributing to the patient’s fear of getting into and out of the tub.
Step 3 - CREATE
In this step, the provider, caregiver, person with dementia (if possible) and team collaborate to create and implement a treatment plan (Figure 2). The provider initially needs to respond to physical problems detected in the INVESTIGATE step (prescribing antibiotics for a UTI, giving fluids to a dehydrated patient, managing constipation). This may also include discontinuing medications with the potential to cause behavioral side effects if possible and evaluating whether other medication side effects may be contributing to NPS. Effective pain management also has an important role and can lead to reducing unnecessary psychotropic prescriptions [37].
For patients with an underlying psychiatric condition (predating the dementia such as schizophrenia or bipolar disorder), the psychotropic regimen for the disorder should be optimized with close monitoring and discontinuation of medications that are ineffective or not tolerated. Good sleep hygiene measures should be instituted. Sensory impairments (hearing, vision) should be addressed.
This step requires creativity with providers brainstorming approaches with the caregiver, patient (when possible), and other team members (visiting nurse, social work, occupational therapists). Brainstorming with the caregiver is important to address an active problem, to model problem-solving, and to obtain buy-in for recommendations. Behavioral and environmental strategies deployed at this stage can be categorized as generalized or targeted (Figure 3). Generalized strategies are non-behavior specific and involve enriching the environment and improving caregiver skills and well-being. Targeted strategies are directed at eliminating a specific NPS (e.g., aggression at bath time)[15]. Although there are multiple potentially effective strategies depending upon behavior, person, caregiver and environmental considerations, four key domains of generalized strategies represent “low-hanging fruit”: 1) providing caregiver education; 2) enhancing effective communication between caregiver and patient; 3) assisting the caregiver in creating meaningful activities for the patient; and 4) helping the caregiver to simplify tasks and establish structured routines for the patient. Additional problem-solving should occur concerning ensuring safety and simplifying/enhancing the environment. Also, caregivers provide important information regarding “what has worked and what has not”, patient interests, and a glimpse into life-long personality and care styles.
Figure 3.
Behavioral and Environmental Modification Strategies for Managing NPS
In the case, the provider starts pain medication, makes are ferral for physical therapy, and educates the caregiver about dementia and that behaviors are not intentional. The provider also suggests ways to improve communication (calmer tones, simpler single-step commands, light touch to reassure) with the patient and avoid negative interactions (harsh tone, complex multi-step commands, open ended questioning, screaming). Helping the caregiver establish a “new normal” routine that promotes patient safety and well being such as using sponge baths or having patient bathe less frequently, and when taking a bath, using a tub bench and grab bar for safety is promoted.
Step 4 - EVALUATE
The final step is for the provider to assess whether recommended strategies were attempted and effective. If the caregiver did not implement an intervention, it is important to understand why and brainstorm solutions. If an intervention was attempted, it is important to evaluate if the strategy was implemented effectively, if the NPS improved, and if the caregiver’s distress was reduced. The patient’s reaction to the intervention(s) is important to assess, as are any unintended side effects or consequences; it is possible that a behavioral intervention makes a behavior worse or has unintended consequences. If so, the provider must understand if the negative outcome is a consequence of the intervention or whether the intervention had not been implemented as intended. If psychotropic medications were judged to be needed, it is important to consider a trial of dose reduction or discontinuation to ensure that the medication continues to be necessary. Because NPS change and fluctuate over the course of dementia, ongoing monitoring of behaviors is essential and removal of interventions, especially medications, should at times be considered.
In the case, several interventions addressing pain (medication, physical therapy), caregiver education, communication, personal preferences and values, and the environment (bath safety measures) are suggested. The provider follows up with the caregiver regarding which were deployed. If she chose not to deploy some, why? Of those she did use, which were effective and judged helpful?
Psychotropic Medication Use
Given a mixed evidence-base for efficacy of most psychotropics used for NPS [1, 38], several in the group were hesitant to recommend first-line treatment with medications under any circumstances. Others noted that past trials have been plagued by lack of homogeneity of NPS phenotype (e.g. trials of antipsychotics for “agitation”) and that medications might show more efficacy with better attention to such. However, current research is limited. Given the limitations in the evidence-base, the panel consensus was that psychotropics should be used only after significant efforts are made to mitigate NPS using behavioral and environmental modifications and medical interventions if needed, with three exceptions. In each of these cases, use would follow a concern for significant and imminent risk): 1) Major depression with or without suicidal ideation; 2) Psychosis causing harm or with great potential of harm; and 3) Aggression causing risk to self or others. The panel also reinforced the need for close follow-up to monitor for adverse effects potentially caused by psychotropics, and that use should be time-limited, as behaviors and symptoms may resolve over time with or without drug treatment. If providers elect to use psychotropics, it is important to remember that there is no FDA approval for their use in the treatment of NPS and that the risk-benefit ratio of medication use must be carefully evaluated.
Psychotropics are not likely to impact the following: unfriendliness; poor self-care; memory problems; not paying attention or caring about what is going on; repetitive verbalizations/questioning; rejection or refusal of care; shadowing; and wandering.
For additional information on the evidence base for and appropriate use of psychotropic medications in dementia, we refer the reader to the papers of Sink et al, Ballard et al and Gauthier et al [1, 39, 40]. For additional information on the evidence base for non-pharmacologic interventions, we refer the reader to the papers of Gitlin et al, Brodaty and O’Neil et al [15, 31, 41].
Research and Clinical Considerations
Since its development, the DICE approach has been reviewed by the Centers for Medicare and Medicaid Services and will be included in their toolkit to promote nonpharmacologic approaches in dementia. Although the DICE approach is evidence-informed, it requires further research testing in clinical settings. Content for each step may need additional and better specification for use in specific settings or by particular providers. The approach also has utility in clinical trials of treatments for NPS, particularly in testing new pharmacologic agents. It can be used to better subtype NPS or focus on particular NPS at randomization coupled with a systematic treatment approach (e.g., behavioral or environmental modification using DICE method first, followed by psychotropic testing). The new Medicare Pay for Performance guidelines [27] might compensate providers for time spent in DICE or similar approaches. As use of DICE may result in fewer hospitalizations or readmissions, it may be of interest to Accountable Care Organizations. Application of this approach in social service agencies involved with dementia patients deserves special attention. Ultimately, developing technology applications of DICE (e.g., an “app”) may simplify its use, save time, standardize its application and facilitate evaluation of its effectiveness.
Conclusions
NPS are among the most significant challenges in dementia care, yet remain under- or mistreated. Psychotropic medications are currently the most commonly deployed management strategies in real-world settings. However, they often have sub-optimal risk-benefit profiles and may not impact some of the most frequently occurring symptoms most distressing to families and which may trigger hospitalizations or nursing home placement. Non-pharmacologic techniques have a substantial evidence-base but are currently under-utilized in standard care. Innovative approaches that include training and support of providers are needed to be able to serve the burgeoning older population with neuropsychiatric symptoms [42].
The DICE approach offers clinicians an evidence-informed structured approach that can be integrated into diverse practice settings. The approach is inherently patient-and caregiver-centered as patient and caregiver concerns are integral to each step of the process. DICE enables clinicians to consider conjointly the role of non-pharmacologic, medical and pharmacologic treatment; it offers a clinical reasoning approach through which clinicians can more efficiently and effectively choose optimal treatment plans. The DICE approach, endorsed by the Detroit Expert Panel, can enhance clinical practice and outcomes and advance research.
Acknowledgments
Funding: Dr. Kales and Gitlin were supported in part by R01NR014200-0. Dr. Lyketsos was supported in part by the Johns Hopkins Alzheimer’s Disease Research Center (P50AG005146). Resources were also contributed by the Program for Positive Aging and The Geriatrics Center, University of Michigan, Ann Arbor, MI.
Panel Members:
| Mary GuerrieroAustrom, PhD | Indiana University |
| Frederic C. Blow, PhD | VA Ann Arbor Healthcare System/University of Michigan |
| Kathleen C. Buckwalter, PhD | University of Iowa |
| Christopher M. Callahan, MD | Indiana University |
| Ryan M. Carnahan Pharm.D., M.S., B.C.P.P. | University of Iowa |
| Laura N. Gitlin, PhD | Johns Hopkins University |
| Helen C. Kales, MD | VA Ann Arbor Healthcare System/University of Michigan |
| Dimitris N. Kiosses, PhD | Weill Cornell Medical College |
| Mark E. Kunik, MD | VA Houston/Baylor College of Medicine^ |
| Constantine G. Lyketsos, MD | Johns Hopkins University |
| Linda O. Nichols, PhD | VA Memphis/University of Tennessee |
| Daniel Weintraub, MD | VA Philadelphia/University of Pennsylvania |
Unable to be present on day of panel, post-hoc participation
Footnotes
Author Contributions: All authors participated in study concept and design and preparation of the manuscript.
Sponsor’s Role: The sponsors had no role in the design or preparation of the paper.
Conflict of Interest: Dr. Kales: grant support through NIH, VA.
Dr. Gitlin: grant support through NIH, Alzheimer’s Association; Member on the Fall Advisory Committee for Phillips Lifeline; honoraria for various speaking engagements.
Dr. Lyketsos: grant support (research or CME) from the National Institute of Mental Health (NIMH), the National Institute on Aging (NIA), Associated Jewish Federation of Baltimore, Weinberg Foundation, Forest, GlaxoSmithKline, Eisai, Pfizer, AstraZeneca, Lilly, Ortho-McNeil, Bristol-Myers, Novartis, the National Football League (NFL), Elan, and Functional Neuromodulation Inc; consultant/advisor for AstraZeneca, GlaxoSmithKline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lund beck, Merz, Lilly, Pfizer, Genentech, Elan, NFL Players Association, NFL Benefits Office, Avanir, Zinfandel; and receipt of an honorarium or travel support from Pfizer, Forest, GlaxoSmithKline, and Health Monitor.
References
- 1.Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: A review of the evidence. JAMA. 2005;293:596–608. doi: 10.1001/jama.293.5.596. [DOI] [PubMed] [Google Scholar]
- 2.Lyketsos CG, Carrillo MC, Ryan JM, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011;7:532–539. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beeri MS, Werner P, Davidson M, et al. The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer’s disease patients. Int J Geriatr Psychiatry. 2002;17:403–408. doi: 10.1002/gps.490. [DOI] [PubMed] [Google Scholar]
- 4.Kales HC, Chen P, Blow FC, et al. Rates of clinical depression diagnosis, functional impairment, and nursing home placement in coexisting dementia and depression. Am J Geriatr Psychiatry. 2005;13:441–449. doi: 10.1176/appi.ajgp.13.6.441. [DOI] [PubMed] [Google Scholar]
- 5.Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287:2090–2097. doi: 10.1001/jama.287.16.2090. [DOI] [PubMed] [Google Scholar]
- 6.Wancata J, Windhaber J, Krautgartner M, et al. The consequences of non-cognitive symptoms of dementia in medical hospital departments. Int J Psychiatry Med. 2003;33:257–271. doi: 10.2190/ABXK-FMWG-98YP-D1CU. [DOI] [PubMed] [Google Scholar]
- 7.Borson S, Raskind MA. Clinical features and pharmacologic treatment of behavioral symptoms of Alzheimer’s disease. Neurology. 1997;48:S17–24. doi: 10.1212/wnl.48.5_suppl_6.17s. [DOI] [PubMed] [Google Scholar]
- 8.Clyburn LD, Stones MJ, Hadjistavropoulos T, et al. Predicting caregiver burden and depression in Alzheimer’s disease. J Gerontol B Psychol Sci Soc Sci. 2000;55:S2–13. doi: 10.1093/geronb/55.1.s2. [DOI] [PubMed] [Google Scholar]
- 9.Van Den Wijngaart MA, Vernooij-Dassen MJ, Felling AJ. The influence of stressors, appraisal and personal conditions on the burden of spousal caregivers of persons with dementia. Aging Ment Health. 2007;11:626–636. doi: 10.1080/13607860701368463. [DOI] [PubMed] [Google Scholar]
- 10.de Vugt ME, Stevens F, Aalten P, et al. Do caregiver management strategies influence patient behaviour in dementia? Int J Geriatr Psychiatry. 2004;19:85–92. doi: 10.1002/gps.1044. [DOI] [PubMed] [Google Scholar]
- 11.Rabins P, Schwartz S, Tschanz J, et al. Risk factors for severe dementia from a population-based sample of incident Alzheimer’s disease: The Cache County Dementia Progression Study. Alzheimer Dement. 2011;7:S356. [Google Scholar]
- 12.Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164:1568–1576. doi: 10.1176/appi.ajp.2007.06101710. quiz 623. [DOI] [PubMed] [Google Scholar]
- 13.Rabins PV, Lyketsos CG, Steele C. Practical Dementia Care. New York: Oxford University Press; 2006. [Google Scholar]
- 14.Physician Consortium for Performance Improvement. Dementia: Performance Measure Set American Medical Association; 2010. [Google Scholar]
- 15.Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308:2020–2029. doi: 10.1001/jama.2012.36918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Geriatrics Society; An Initiative of the ABIM Foundation, editor. Choosing Wisely. Philadelphia, PA: 2013. Five Things Physicians and Patients Should Question. [Google Scholar]
- 17.National Institute for Health and Clinical Excellence; National Institute for Health and Clinical Excellence: Social Care Institute for Excellence, editor. Dementia: Supporting people with dementia and their careers in health and social care. London: National Institute for Health and Clinical Excellence; 2012. [Google Scholar]
- 18.Ouslander J, Bartels S, Beck C, et al. Consensus statement on improving the quality of mental health care in US nursing homes: Management of depression and behavioral symptoms associated with dementia. J Am Geriatr Soc. 2003;51:1287–1298. doi: 10.1046/j.1532-5415.2003.51415.x. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association; An Initiative of the ABIM Foundation, editor. Choosing Wisely. 2013. Five Things Physicians and Patients Should Question. [Google Scholar]
- 20.Molinari V, Chiriboga D, Branch LG, et al. Provision of psychopharmacological services in nursing homes. J Gerontol B Psychol Sci Soc Sci. 2010;65:57–60. doi: 10.1093/geronb/gbp080. [DOI] [PubMed] [Google Scholar]
- 21.Cohen-Mansfield J, Juravel-Jaffe A, Cohen A, et al. Physicians’ practice and familiarity with treatment for agitation associated with dementia in Israeli nursing homes. Int Psychogeriatr. 2013;25:236–244. doi: 10.1017/S104161021200172X. [DOI] [PubMed] [Google Scholar]
- 22.Nichols LO, Martindale-Adams J, Burns R, et al. Translation of a dementia caregiver support program in a health care system--REACH VA. Arch Intern Med. 2011;171:353–359. doi: 10.1001/archinternmed.2010.548. [DOI] [PubMed] [Google Scholar]
- 23.Gitlin LN, Winter L, Dennis MP, et al. A bio behavioral home-based intervention and the well-being of patients with dementia and their caregivers. JAMA. 2010;304:983–991. doi: 10.1001/jama.2010.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gitlin LN, Winter L, Dennis MP, et al. Targeting and managing behavioral symptoms in individuals with dementia: A Randomized Trial of a nonpharmacological intervention. J Am Geriatr Soc. 2010;58:1465–1474. doi: 10.1111/j.1532-5415.2010.02971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gitlin LN. The cost-effectiveness of a nonpharmacologic intervention for individuals with dementia and family caregivers: the tailored activity program. Am J Geriatr Psychiatry. 2010;18:510–519. doi: 10.1097/JGP.0b013e3181c37d13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Medicare & Medicaid Services, Health and Human Services; Department of Health and Human Services, Centers for Medicare and Medicaid Services, editor. Providing the Annual Wellness Visit (AWV) 2012. [Google Scholar]
- 27.Odenheimer G, Borson S, Sander AE, et al. Quality improvement in neurology: Dementia Management Quality Measures. Neurology. 2013;81:1545–1549. doi: 10.1212/WNL.0b013e3182a956bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonner A. Center for Medicare and Medicaid Services, editor. Medicare Learning Network. 2013. Improving dementia care and reducing unnecessary use of antipsychotic medications in nursing homes. [Google Scholar]
- 29.Hodgson NA, Gitlin LN, Winter L, et al. Undiagnosed illness and neuropsychiatric behaviors in community residing older adults with dementia. Alzheimer Dis Assoc Disord. 2011;25:109–115. doi: 10.1097/WAD.0b013e3181f8520a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gitlin LN. Good news for dementia care: caregiver interventions reduce behavioral symptoms in people with dementia and family distress. Am J Psychiatry. 2012;169:894–897. doi: 10.1176/appi.ajp.2012.12060774. [DOI] [PubMed] [Google Scholar]
- 31.Brodaty H, Arasaratnam C. Meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry. 2012;169:946–953. doi: 10.1176/appi.ajp.2012.11101529. [DOI] [PubMed] [Google Scholar]
- 32.Teri L. STAR: A Dementia-Specific training program for staff in assisted living residences: Nancy Morrow-Howell, Editor. Gerontologist. 2005;45:686–693. doi: 10.1093/geront/45.5.686. [DOI] [PubMed] [Google Scholar]
- 33.Tariot PN. Treatment of agitation in dementia. J Clin Psychiatry. 1999;60(Suppl 8):11–20. [PubMed] [Google Scholar]
- 34.Flaherty JH. The evaluation and management of delirium among older persons. Med Clin N Am. 2011;95:555–577. doi: 10.1016/j.mcna.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Smith M, Schultz S, Seydel L, et al. Improving antipsychotic agent use in nursing homes: Development of an algorithm for treating problem behaviors in dementia. J Gerontol Nurs. 2013;39:24–35. doi: 10.3928/00989134-20130314-04. quiz 36–37. [DOI] [PubMed] [Google Scholar]
- 36.Hodgson N, Gitlin LN, Dennis MP, et al. Relationship of pain to behavioral and psychiatric symptoms in older community residing adults with dementia. Clin J Pain. doi: 10.1097/AJP.0000000000000018. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Husebo BS, Ballard C, Sandvik R, et al. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: Cluster randomised clinical trial. BMJ. 2011 Jul 15;343:d4065. doi: 10.1136/bmj.d4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyketsos CG, DelCampo L, Steinberg M, et al. Treating depression in Alzheimer disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction: The DIADS. Arch Gen Psychiatry. 2003;60:737–746. doi: 10.1001/archpsyc.60.7.737. [DOI] [PubMed] [Google Scholar]
- 39.Ballard CG, Gauthier S, Cummings JL, et al. Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol. 2009;5:245–255. doi: 10.1038/nrneurol.2009.39. [DOI] [PubMed] [Google Scholar]
- 40.Gauthier S, Cummings J, Ballard C, et al. Management of behavioral problems in Alzheimer’s disease. Int Psychogeriatr. 2010;22:346–372. doi: 10.1017/S1041610209991505. [DOI] [PubMed] [Google Scholar]
- 41.O’Neil M, Freeman M, Christensen V, et al. Non-pharmacological Interventions for behavioral symptoms of dementia: A systematic review of the evidence. VA-ESP Project. 2011:05-225. [PubMed] [Google Scholar]
- 42.Ickowicz E. The American Geriatrics Society and American Association for Geriatric Psychiatry recommendations for policies in support of quality mental health care in US nursing homes. J Am Geriatr Soc. 2003;51:1299–1304. doi: 10.1046/j.1532-5415.2003.51416.x. [DOI] [PubMed] [Google Scholar]