Summary
Sodium–hydrogen exchanger isoform 1 (NHE1) plays a role in survival and migration/invasion of several cancers and emerges as a new therapeutic target. In this study, we investigated roles of NHE1 in glioma cells in counteracting chemo reagent temozolomide.
Abstract
Sodium–hydrogen exchanger isoform 1 (NHE1) plays a role in survival and migration/invasion of several cancers and is an emerging new therapeutic target. However, the role of NHE1 in glioblastoma and the interaction of NHE1 expression and function in glioblastoma cells with cytotoxic temozolomide (TMZ) therapy remain unknown. In this study, we detected high levels of NHE1 protein only in primary human glioma cells (GC), glioma xenografts and glioblastoma, but not in human neural stem cells or astrocytes. GC exhibited an alkaline resting pHi (7.46±0.04) maintained by robust NHE1-mediated H+ extrusion. GC treatment with TMZ for 2–24h triggered a transient decrease in pHi, which recovered by 48h and correlated with concurrent upregulation of NHE1 protein expression. NHE1 protein was colocalized with ezrin at lamellipodia and probably involved in GC migration. The TMZ-treated GC exhibited increased migration and invasion, which was attenuated by addition of NHE1 inhibitor HOE-642. Most importantly, NHE1 inhibition prevented prosurvival extracellular signal-regulated kinase activation and accelerated TMZ-induced apoptosis. Taken together, our study provides the first evidence that GC upregulate NHE1 protein to maintain alkaline pHi. Combining TMZ therapy with NHE1 inhibition suppresses GC migration and invasion, and also augments TMZ-induced apoptosis. These findings strongly suggest that NHE1 is an important cytoprotective mechanism in GC and presents a new therapeutic strategy of combining NHE1 inhibition and TMZ chemotherapy.
Introduction
Glioblastoma multiforme (GBM) is the most malignant World Health Organization Grade IV glioma, associated with median survival of <2 years (1,2). Current standard therapies for GBM include maximal safe surgical resection, radiation and temozolomide (TMZ) chemotherapy and only modestly improve survival (2-year survival rate of 27%) (3). Moreover, several recent clinical trials that target vascular endothelial growth factor-mediated angiogenesis and epidermal growth factor receptor mutation-dependent GBM proliferation failed to improve overall survival (http://www.rtog.org/clinicaltrials/protocoltable/studydetails.aspx?study=). It is hypothesized that much of the morbidity and high recurrence rate of GBM is due to migration and invasion of cells into adjacent brain. Therefore, there is an urgent need to develop more effective GBM therapeutic strategies.
Intracellular alkalinization and microenvironmental acidification play crucial roles in cell migration, invasion and apoptosis in solid tumors (4). Recent studies reveal that tumor cells have alkaline intracellular pH (pHi; 7.12–7.65 versus 6.99–7.20 in normal tissues) and acidic interstitial extracellular pH (pHo; 6.2–6.9, compared with 7.3–7.4) in breast cancer, fibrosarcoma and hepatoma (5). Several malignant glioma cell lines including human U87, U251 and U118 and murine C6 maintain an alkaline pHi between 7.2 and 7.4 determined in a bicarbonate-free environment (6). It has been suggested that this reversed pH gradient across cancer cell membrane may be an optimal microenvironment that promotes tumor progression (7). Exposure to low pHo promotes malignancy via induction of a cancer stem cell phenotype and increase of tumor cellular heterogeneity (8).
Sodium–hydrogen exchanger isoform 1 (NHE1) protein is overexpressed in many tumor types and maintains intracellular pH (pHi) of tumor cells by extruding H+ (9). Constitutive NHE1 activity in cancer cells also leads to acidification of the extracellular tumor microenvironment (10). In addition to maintaining pH homeostasis, NHE1 also regulates cell migration via direct interactions with ezrin, radixin and moesin (ERM) cytoskeletal proteins and promotes cell mobility of either solid tumor or fibroblast (11). However, it is unknown whether NHE1 protein plays a role in glioma migration. In particular, the interaction of NHE1 activity with TMZ chemotherapy in GBM also remains unknown.
In this study, we discovered that primary glioma cells (GC) selectively expressed a high basal level of NHE1 protein and maintained an alkaline pHi. NHE1 colocalized with ezrin at lamellipodia and is probably involved in GC migration and invasion. TMZ treatment is associated with elevated NHE1 protein expression and GC migration. Increased NHE1 expression enhanced GC resistance to TMZ-mediated apoptosis, in part via activation of extracellular signal-regulated kinase (ERK) signaling pathways. Taken together, these new findings demonstrate that GC sensitivity to TMZ chemotherapy is enhanced by blocking NHE1 activity, suggesting a novel combination therapeutic strategy.
Materials and methods
Cell cultures
All studies involving human tissues were performed with approval from the University of Wisconsin–Madison and University of Pittsburgh Institutional Review Board with informed consent obtained from patients.
U87 cell line was purchased from American Type Culture Collection. Primary glioma cell lines (GC#22 and GC#99) were established as described before (12). Both cell lines were validated for tumor initiation before experiments were conducted (13). Passages 20–40 were used in this study. Human cortex fetal neural stem cells (NSCs) and human astrocytes (a kind gift from Dr Clive Svendsen) were used as controls and maintained as described previously (14).
RNA interference knockdown of NHE1
Knockdown of NHE1 protein expression was induced by small interfering RNA (siRNA) with a final concentration of 15nM. The scrambled siRNA (Silencer® Negative Control No. 1 siRNA, Cat. No. AM4635) and siRNAs targeting human NHE1 (ID: s13023) were purchased from Invitrogen. The sequences of siRNAs are sense 5′-AGCUCAACCGGUUUAAUAAtt-3′; antisense 5′-UUAUUAAACCGGUUGAGCUtg-3. The cells were incubated at 37°C and subjected to experiments 48h after transfection as described before (15).
pHi measurement
pHi measurement in GC#22 was performed with a 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester dye as described previously (16). Images were collected under the Nikon TiE 300 inverted epifluorescence microscope (a ×40 oil immersion objective lens). The cells were excited every 20 s at 440 and 490nm, and the emission fluorescence at 535nm was recorded (16). The ratio of the background-corrected fluorescence emissions (F 490/F 440) was calibrated using the high-K+/nigericin technique (17). For NHE1 inhibitor HOE-642 treatment, cells were exposed to 1 μM HOE-642 (Aventis Pharma, Frankfurt, Germany) at 2min after the pHi baseline recording. For TMZ treatment, GC was incubated with 100 µM TMZ for 2–48h prior to the pHi recording. HOE-642-sensitive pHi was calculated at 8min of the drug application by subtracting the pHi under HOE-642 treatment from baseline pHi in both control and TMZ-treated conditions.
Cell motility measurement with time-lapse imaging
GCs were placed in a stage top incubator at 37°C with 5% CO2 + 95% air (TIZ model, Tokai Hit; Shizuoka-ken, Japan). Cell motility was monitored with a ×20 objective lens using a time-lapse video microscope system (the Nikon TiE 300 inverted epifluorescence microscope) and MetaMorph software (Molecular Devices; Sunnyvale, CA) under different treatment conditions. Time-lapse differential interference contrast images were acquired in 5 min intervals for 5h and images were analyzed. Cell tracking was performed using the ImageJ software (National Institute of Health) and Manual Tracking plugin. Total distance traveled was determined by tracking the movement of the cell gravity center, and its coordinates were used to calculate the distances.
Transwell chemotaxis
Dissociated GCs (6×104 cells) in 100 μl serum-free Dulbecco’s modified Eagle’s medium with different treatment regimens [control medium (CON), 1 µM HOE-642, 100 µM TMZ or 100 µM TMZ plus 1 µM HOE-642] were seeded on top of transwell membrane cell culture inserts (8.0 μm pore size, Becton Dickinson) for 5h. The migrated cells on the bottom surface were subjected to 4′,6-diamidino-2-phenylindole staining (2 µg/ml in phosphate-buffered saline). Images of five random fields were captured under the ×40 objective lens. Migrated cells in all five fields were averaged to give a mean cell count for each experiment as described (15).
Gelatinase zymography
Gelatinase zymography was performed in 10% Novex precast sodium dodecyl sulfate (SDS)–polyacrylamide gel in the presence of 0.1% gelatin under non-reducing conditions. Culture media (20 µl) were mixed with sample buffer and loaded for SDS–polyacrylamide gel electrophoresis with Tris–glycine–SDS buffer, as suggested by the manufacturer (Novex). Following electrophoresis, the gels were washed and incubated at 37°C overnight in the substrate buffer and stained with 0.5% Coomassie Blue R250 in 50% methanol and 10% glacial acetic acid for 30min. The intensity of gelatinase-digested gelatin bands were analyzed.
Annexin V assay for apoptosis
Annexin V-FITC Apoptosis Kits (Invitrogen) were used to determine apoptosis under different treatments conditions. Cells were incubated with 5 µl fluorescein isothiocyanate Annexin V in darkness at room temperature for 15min. After incubation, 50 μl binding buffer was added to each sample. Annexin V positive cells were analyzed using the Nikon TiE300 microscope as described before (18).
Immunoblotting assay
Samples (30 µg total proteins) were denatured and electrophoretically separated on 10% SDS gels. Blots were blocked and incubated with a primary antibody at 4°C overnight with primary anti-NHE1 monoclonal antibody (1:500), primary anti-caspase-3 polyclonal antibody (1:1000). After rinsing, the blots were incubated with horseradish peroxidase-conjugated secondary immunoglobin G (1:2000) for 1h at room temperature. Bound antibody was visualized with an enhanced chemiluminescence assay (Amersham, Piscataway, NJ).
Immunoprecipitation was conducted to examine interactions between NHE1 and ezrin proteins using the Pierce® Classic IP Kit (ThermoScientific, Rockford, IL). Cell lysates (0.2mg protein) were incubated with 2mg of mouse anti-ezrin antibody at 4°C overnight. Immunocomplexes were mixed with 20 μl protein A/G beads (50% slurry) in a Pierce spin column and incubated for 2h. The immunocomplexes were washed and analyzed as described before (16).
Immunofluorescence and immunohistochemistry
GC#22 cells were blocked with 5% normal goat serum with 0.3% triton X-100 for 1h at 37oC, followed by overnight incubation of mouse anti-NHE1 antibody (1:100) and rabbit anti-ezrin antibody (1:100) at 4oC. Cells were incubated with the fluorescence-labeled secondary antibodies (1:200) and fluorescence images were captured with a Leica DMIRE2 confocal microscope (×40) and Leica confocal software (Leica Microsystems, Mannheim, Germany), as described before (15).
After blocking for endogenous peroxidase and biotin (15), tissue sections (5 µm) were incubated with the rabbit anti-NHE1 antibody (1:50) overnight at 4°C. Secondary antibodies were applied for 2h at room temperature. The Elite Vector Stain ABC System was used for the subsequent immunodetection with diaminobenzidene as described previously (15).
GBM tissue microarray
A clinically annotated tissue microarray from 205 GBM patients diagnosed between 1999 and 2009 was created from the University of Wisconsin Pathology archives as reported (13). Overall survival data were available for 138 patients. Rabbit anti-NHE1 antibody (1:50) was used to label the tissue microarray. Each punch was subjectively scored as described before (15).
Statistical analysis
The results are expressed as the mean ± SEM. Comparisons between groups were made by Student’s t-test or one-way analysis of variance using the Bonferroni post hoc test in the case of multiple comparisons (SigmaStat, Systat Software, Point Richmond, CA). P < 0.05 was considered statistically significant. n values represent the number of independent cultures or tissue samples.
Results
Glioma cells express high levels of NHE1 protein
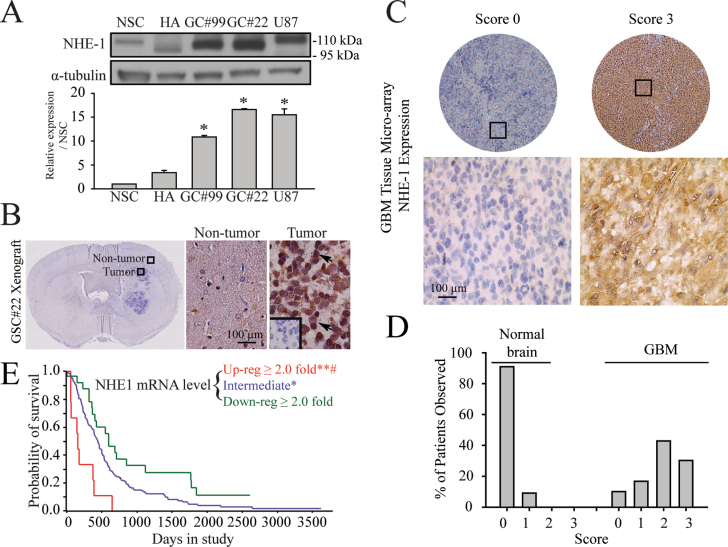
Expression of NHE1 protein was first characterized in human neural stem cell (NSC), human astrocytes (HA), primary human glioma cell lines (GC#99 and GC#22), and the standard U87 GBM cell line. NSC and HA showed relatively low NHE1 expression (Figure 1A). In contrast, all three human glioma cell lines exhibited significantly upregulated expression of NHE1 protein, with NHE1 immunoreactive bands ranged from 95–110kDa. Human NHE1 protein consists of 815 amino acids with a calculated molecular weight of 85kDa. However, because of the N-linked and O-linked glycosylation in its extracellular loop 1, the actual molecular weight of NHE1 can be varied from 85 to ~110kDa in different cell types (19–21). Compared with NHE1 expression level in NSC, NHE1 protein was 9.8±0.3 fold higher in GC#99, 15.6±0.2 fold higher in GC#22 and 14.5±1.2 fold in U87.
Fig. 1.
Abundant expression of NHE1 proteins in GC. (A) Upper panel: representative immunoblotting showing the expression of NHE1 protein in human NSCs, HAs, primary glioma cell lines (GC#99 and GC#22) or GBM cell line U87. Lower panel: summary data of immunoblotting. Expression of each protein was first normalized by α-tubulin and relative expression level in each cell type was then normalized to NSC. Data are mean ± SEM. n = 3. *P < 0.05 versus NSC. (B) Representative immunohistochemistry staining of NHE1 in xenograft brain tissues of structured clinical interview for DSM mouse derived from glioma stem cell (GSC#22). Arrow: representative cells positively stained for NHE1 protein. Inset: negative control with primary antibody omitted. (C) Representative images of NHE1 immunohistochemistry with different intensity in a tissue microarray of GBM. Rabbit anti-NHE1 was used to label the tissue microarray. Each punch was subjectively scored for negative (score 0), mild (score 1), moderate (score 2) and strong (score 3) of NHE1 expression by light microscopic visualization of intensity of cytoplasmic diaminobenzidene. Nuclear or fibrillary labeling was not scored as positive. In cases of multiple punches/cores for one patient tumor sample, the score given represented the most frequent expression level. Upper panel: examples of score 0 and score 3 NHE1 labeling from the GBM tissue microarray. Black boxes indicate magnified sections in subsequent panels. Lower panel: high magnification photomicrograph of the region in the black box. (D) Summary data of different NHE1 expression scores in normal or GBM tissues as described in Materials and methods. Data are mean ± SEM. n = 3. *P < 0.05 versus normal brain. (E) Kaplan–Meier survival probability curve for patients from Repository for Molecular Brain Neoplasia Data with differential NHE1 expression. The red, blue and green lines indicate the survival of patients with GBMs of upregulated, intermediate and downregulated NHE1 expression, respectively. *P < 0.05 and **P < 0.001 versus downregulated GBMs. # P < 0.01 versus intermediate GBMs.
NHE1 protein expression was also detected in GBM xenografts derived from human GSC#22. Shown in Figure 1B, most cells within the GBM xenograft exhibited positive immunostaining for NHE1 protein. NHE1 protein was localized in the cytoplasm and processes of GC. GBM tissue array samples revealed a similar NHE1 expression pattern (Figure 1C). All normal brain samples exhibited no or low NHE1 signals (Figure 1C). In contrast, some GBM specimens displayed intense NHE1 immunostaining (score 3, arrow, Figure 1C). Over 70% of GBM on the tissue microarray showed moderate to strong NHE1 expression (Figure 1D). We further surveyed whether NHE1 gene expression correlates with clinical outcomes in GBM patients with the Repository for Molecular Brain Neoplasia Data. As shown in Figure 1E, within 181 GBM patients, 159 cases of them showed upregulated or intermediate expression of NHE1. Most importantly, there are survival advantages for GBM patients with downregulated NHE1 (n = 22) versus intermediate (n = 150) versus upregulated NHE1 expression (n = 9; http://rembrandt-db.nci.nih.gov). Taken together, these data illustrate abundant GC expression of NHE1 at messenger RNA (mRNA) and protein levels, suggesting a possible significant role in glioma biology.
NHE1 activity maintains alkaline pHi of glioma cells
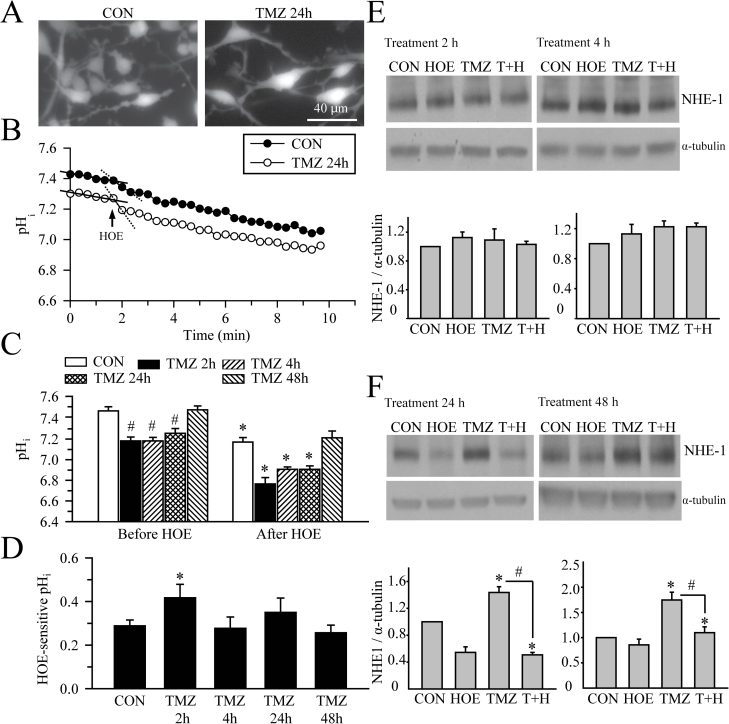
The resting pHi level of GC#22 cells was determined by the H+-sensitive dye 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (Figure 2A). GC#22 cells displayed a slow, continuous drift of baseline pHi, which is due to dye leakage after photobleaching, a phenomenon that has been reported previously (22–24). Despite of the dye leakage-mediated baseline drift, we were able to detect significant intracellular acidification after addition of HOE-642. After fitting a linear regression to the baseline pHi and pHi after addition of HOE-642 with a method as described by Altamirano et al. (25) to correct the drifting effects, it was clearly shown that the slope of pHi decrease caused by HOE-642 treatment was significantly steeper than the baseline drifting slope (solid and dash lines in Figure 2B, −0.031 ± 0.004 versus −0.013 ± 0.007, n = 9, P = 0.043 by student’s t-test).
Fig. 2.
NHE1 maintains alkaline pHi of GC. (A) 2′,7′-Bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester-loaded GC#22 cells show similar morphology under control condition or after TMZ treatment (100 µM, 24h). (B) Representative traces of pHi change in response of 1 µM HOE-642 in control or TMZ-treated GC#22. Addition of HOE-642 at 2min of the recording was indicated. Solid lines showed the slope of pHi decrease caused by HOE-642 treatment. It was significantly steeper than the baseline drifting slope (dash lines), n = 9, P < 0.05). (C) Summary of resting pHi under baseline (before HOE-642) or after HOE-642 application (8min, 1 µM) in control or TMZ-treated GC#22 cells. The TMZ-treated cells were incubated with 100 µM TMZ for 2–48h. pHi were calculated from 6–12 cells on each coverslip/culture. n = 3–5 cultures. Data are expressed as mean ± SEM. # P < 0.05 versus CON. *P < 0.05 versus before HOE. (D) Summary of HOE-642-sensitive pHi in GC#22 cells. HOE-642-sensitive pHi was calculated as described in Methods. Data are mean ± SEM. n = 3–5 cultures. *P < 0.05 versus CON. (E) Representative immunoblots for expression of NHE1 in GC#22 after exposure to CON, 1 µM HOE-642 (HOE), 100 µM TMZ or TMZ plus HOE-642 (T+H), for 2 or 4h. Expression of NHE1 protein was first normalized to α-tubulin in each condition. Relative expression level of NHE1 in each group was then normalized to the control group. Data are mean ± SEM. n = 4. *P < 0.05 versus CON, # P < 0.05 versus TMZ. (F) Representative immunoblots for expression of NHE1 in GC#22 after exposure to CON, 1 µM HOE-642 (HOE), 100 µM TMZ or TMZ plus HOE-642 (T+H), for 24 or 48h. Data are mean ± SEM. n = 4. *P < 0.05 versus CON, # P < 0.05 versus TMZ.
GC#22 cells display alkaline pHi (7.46±0.04) compared with normal astrocytes (Figure 2C), which is consistent with previous findings in C6, U118, U87 and U251 glioma cell lines (6). Pharmacological inhibition of NHE1 with HOE-642 acidified cells (7.17±0.04, n = 3–4, P < 0.05; Figure 2C). Interestingly, exposure of GC to TMZ treatment (100 µM) for 2–24h caused a transient decrease in pHi (7.18–7.26) that recovered to basal levels after 48h of TMZ treatment (Figure 2B). TMZ-treated GC (2–48h) are further acidified by blocking NHE1 with the potent inhibitor HOE-642 (Figure 2B and D). These findings suggest that TMZ treatment triggers a transient acidosis. NHE1 function remains intact after TMZ treatment to rescue and maintain GC H+ homeostasis.
We also determined whether H+-ATPase plays a role in GC H+ extrusion. Inhibition of the H+ pump with bafilomycin did not cause significant decrease in pHi under control condition or after 2–24h treatment of TMZ (data not shown), confirming the dominant role of NHE1 in the regulating pHi in GC cells, especially in the presence of TMZ.
We speculated that GC may upregulate NHE1 protein expression to counteract TMZ-induced acidosis. Figure 2E shows that TMZ treatment for 2h or 4h did not change NHE1 protein expression. However, TMZ treatment for 24h induced 1.44±0.17 fold increase in NHE1 protein level (P < 0.05 versus control). By 48h treatment, TMZ-treated GC exhibited 1.73±0.25 fold increase in NHE1 protein level (P < 0.05 versus control; Figure 2F). Interestingly, increased NHE1 expression was not detected in GC cells treated with TMZ plus HOE-642. These findings suggest that GC can acquire overexpression of NHE1 protein to counteract TMZ-mediated intracellular acidosis.
Upregulation of NHE1 protein stimulates GC migration
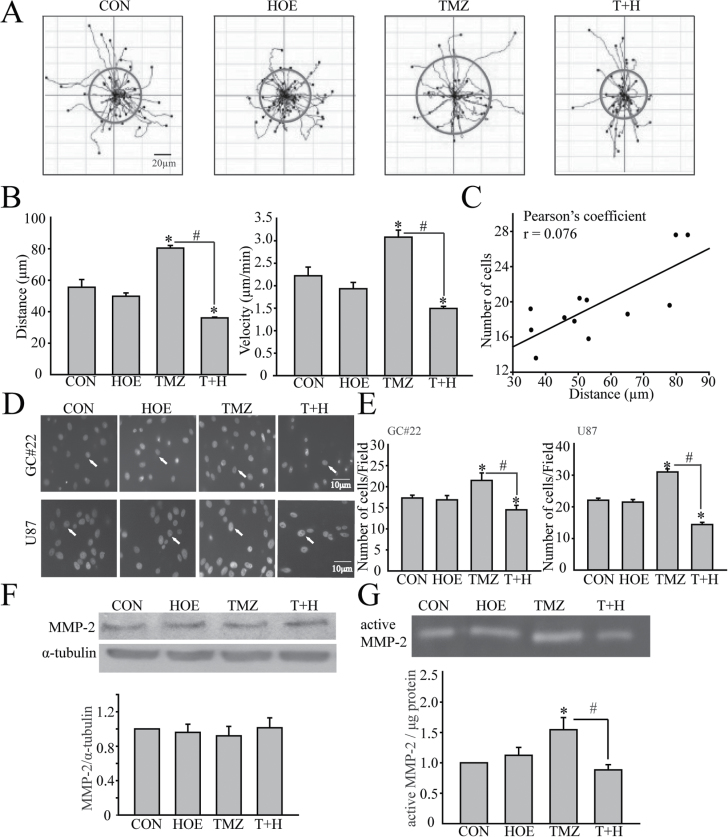
We investigated whether increased NHE1 protein expression and function affects glioma migration. Figure 3A illustrates the random moving traces of GC, the red circles represent translocation of cells within 5h time period. The motility of GC#22 appeared to be stimulated by TMZ but was abolished by NHE1 inhibition with HOE-642. The summarized data in Figure 3B demonstrated that GC#22 exhibited a low basal motility under control conditions (travel distance of 55.52±8.52 μm in 5h). HOE-642 treatment had no effects on the basal motility (neither on the total travel distance or the speed). Interestingly, in the presence of TMZ, GC#22 cell mobility was increased to 80.44±2.82 μm in 5h, (P < 0.05 versus CON). The mobility rate in TMZ-treated GC was increased from 2.22 to 3.08 μm/min (P < 0.05 versus CON). Most importantly, NHE1 inhibition with HOE-642 abolished TMZ-associated motility (P < 0.05 versus T+H).
Fig. 3.
NHE1 activity in GC migration and invasion. (A) Random moving traces of GC#22 in the 300min recording period were recorded under four different conditions, CON (Dulbecco’s modified Eagle’s medium + 10% fetal bovine serum), HOE (1 µM HOE-642), TMZ (100 µM) or T+H (100 µM TMZ plus 1 µM HOE-642). The red circles show the average translocation of cells of each group. (B) Summary data of glioma motility. Accumulated distance and speed of GC movement during 0–300min were calculated in each condition. Data are mean ± SEM. n = 4. *P < 0.05 versus CON. # P < 0.05 versus T+H. (C) Pearson’s correlation analysis of the correlation between cell migration distance and migrated cell numbers. (D) Serum-induced microchemotaxis of GC#22 (upper) and U87 (lower) in the Boyden Chamber (8 µm pore) was recorded for 5h under four different treatment conditions as described above. Arrow: 4′,6-diamidino-2-phenylindole-stained nuclei of the translocated cells. (E) Summary data of numbers of migrated cells in four different treatment groups. Data are mean ± SEM. n = 7. *P < 0.05 versus CON. # P < 0.05 versus T+H. (F) Representative immunoblots for expression of MMP2 in GC#22 after exposure to CON, 1 µM HOE-642 (HOE), 100 µM TMZ or TMZ plus HOE-642 (T+H), for 48h. Summary data were shown. Expression of MMP2 protein was first normalized to α-tubulin in each condition. Relative expression level of MMP2 in each group was then normalized to the control group. Data are mean ± SEM. n = 3. (G) Gelatinase zymography exhibiting MMP2 activity under four different treatment conditions. Summary data of MMP2 activity in different treatment groups were shown. Data are mean ± SEM. n = 4. *P < 0.05 versus CON. # P < 0.05 versus TMZ.
To further establish the roles of NHE1 in GC migration, we examined migration behaviors of GC#22 in the transwell migration assay (Figure 3D). Consistent with their motility profiles in live cell imaging, GC#22 exhibited lower basal migratory ability through the 8 µm transwell membrane under control conditions (17.3±1.8 cells/field, Figure 3E). NHE1 inhibition did not affect the basal level of GC#22 migration. However, the number of GC#22 migrated cells increased after TMZ addition (21.5±4.7 cells/field, P < 0.05). NHE1 inhibition with HOE-642 treatment significantly attenuated TMZ-associated GC#22 migration (P < 0.05). Furthermore, the Pearson’s correlation analysis revealed a positive correlation between cell migration distance and migrated cell numbers (Pearson’s r of 0.767, Figure 3C).
Association between NHE1 function and matrix metalloproteinase activation in glioma mobility
The above findings led us to study on how NHE1 activity regulates glioma mobility in response to TMZ treatment. Activation of several proteases, including matrix metalloproteinases (MMP2 and MMP9), have been shown to be an essential step in tumor invasion and metastasis (26). Therefore, we tested whether activities of MMP2 and MMP9 are altered in GC after TMZ treatment. Gelatinase zymography with high sensitivity for detecting gelatinolytic enzymatic activity was utilized to examine changes of active forms of MMP2 and MMP9. Gelatinase zymography in Figure 3G showed basal level of MMP2 activity in GC#22 cells. After 48h of TMZ treatment, MMP2 activity was increased significantly. In contrast, the TMZ-mediated stimulation of MMP2 activity was absent in the presence of HOE-642. There were no changes in MMP2 protein expression (Figure 3F). On the other hand, no changes of MMP9 activity were detected under all four different treatment conditions (Supplementary Figure 1, available at Carcinogenesis Online). These data clearly suggest that NHE1 activity selectively alters MMP2 activity.
Direct interactions between NHE1 and ezrin are involved in glioma migration
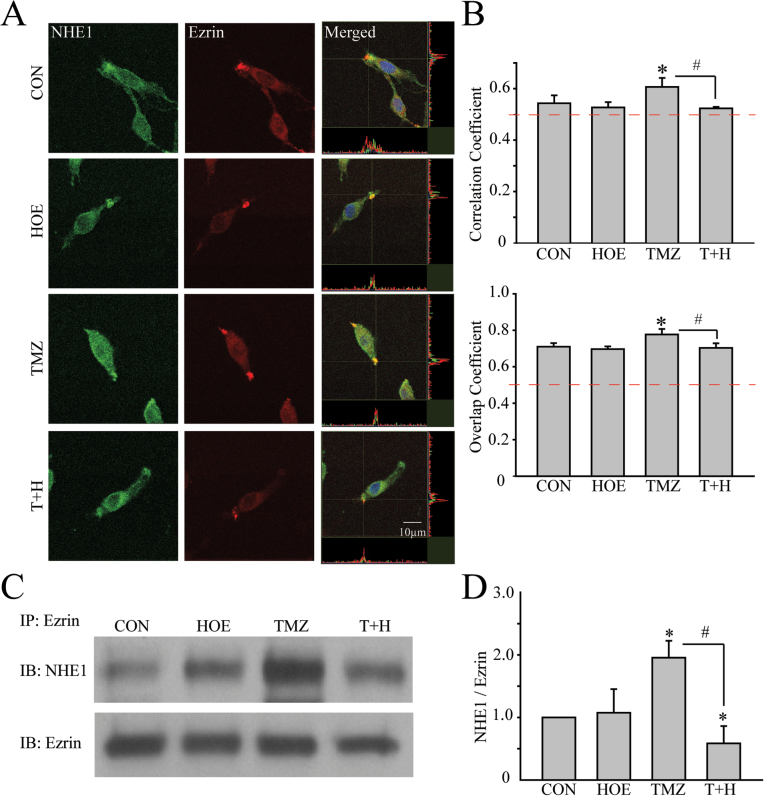
ERM proteins play an important role in cancer migration and invasion and interact with NHE1 protein in migration (27). We speculate that increased NHE1 protein expression may alter its interaction with the ERM complex and promote glioma migration. Immunofluorescence visualization showed that NHE1 protein was abundantly expressed in GC plasma membrane and cytosol under control conditions. In the lamellipodia, NHE1 is partially colocalized with ezrin, reflected by the overlap of green and red immunostaining signals and the high values in Figure 4A and B. Upon TMZ treatment for 48h, NHE1 expression was elevated in lamellipodia (Figure 4A). Increased colocalization of NHE1 and ezrin was detected with correlation coefficient of 0.61±0.04 and overlap coefficient of 0.77±0.03 in the TMZ-treated cells (Figure 4B).
Fig. 4.
Direct interactions between NHE1 and ezrin in GC. (A) Representative images showing expression of NHE1 (green) and ezrin (red) in GC#22 under four different conditions. Cells were exposed to CON, 1 µM HOE-642 (HOE), 100 µM TMZ or 100 µM TMZ plus 1 µM HOE-642 (T+H) for 48h. Immunosignals of NHE1 and ezrin proteins were colocalized at the lamellipodia (merged images). Colocalization of NHE1 and ezrin was analyzed using Image J software with the Colocalization Indices plugin (50). The Pearson’s correlation coefficient and overlap coefficient were used as indices of the frequency of colocalization between NHE1 and ezrin as described previously (16). Pearson correlation coefficient was calculated using online statistics software (http://www.wessa.net/, Free Statistics Software, Office for Research Development and Education, version 1.1.23-r7). (B) Summary data of correlation coefficient and overlap coefficient in four different treatment groups. Data are mean ± SEM. n = 5. *P < 0.05 versus CON. # P < 0.05 versus TMZ. (C) Representative immunoblots showing coimmunoprecipitation of ezrin and NHE1 protein was detected in GC#22 using anti-ezrin antibody. (D) Summary data of immunoprecipitation. Expression of NHE1 protein was first normalized by ezrin in each sample. Relative expression level in each group was normalized to CON. Data are mean ± SEM. n = 4. *P < 0.05 versus CON. # P < 0.05 versus T+H.
To further establish interactions between NHE1 and ERM proteins, we performed immunoprecipitation in GC#22 cells in which NHE1 protein was pulled down in the immunoprecipitation (IP) using the anti-ezrin antibody. As shown in Figure 4C, NHE1 protein in the IP fractions was significantly increased in the TMZ-treated cells (48h, Figure 4C). Most interestingly, this increase was abolished in cells treated with TMZ plus HOE-642 (48h, P < 0.05, Figure 4C and D). Such increased interactions were also detected in cells treated with TMZ for 5h (Supplementary Figure 2, available at Carcinogenesis Online). Taken together, these findings suggest that there is an increased interaction between NHE1 and ezrin in GCs, which may promote GC migration.
Elevated NHE1 protein expression increases GC’s tolerance against TMZ-mediated apoptosis
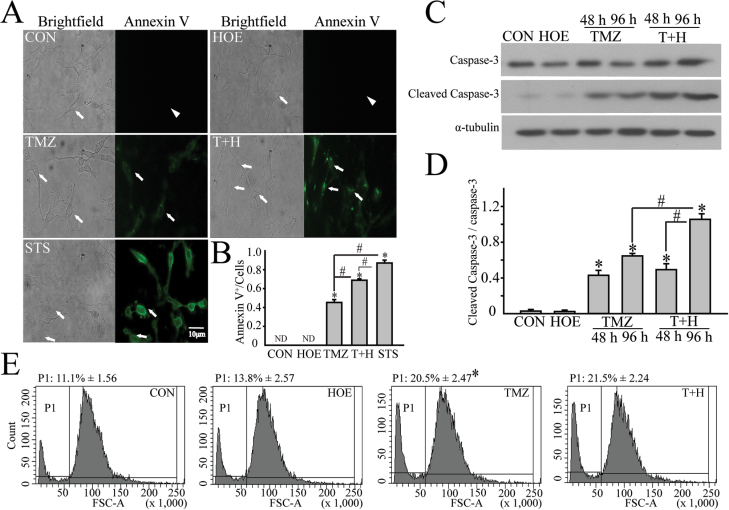
NHE1 was shown to play a role in survival of U87 GBM cell line (28). We speculate that increased NHE1 can increase GC resistance to TMZ-mediated apoptosis. TMZ undergoes spontaneous intracellular conversion into a potent methylating agent of nucleobases, results in the formation of nicks in the DNA and triggers apoptosis (29). Early GC#22 apoptosis was induced by a low concentration of TMZ (50 μM) and detected by Annexin V staining. As shown in Figure 5A, no apoptosis was detected in control or HOE-642-treated cells. TMZ (50 µM, 48h) alone induced a moderate apoptosis in GC#22 (arrow, Figure 5A), with 43±1.9% of GC stained positive for Annexin V. Interestingly, blocking NHE1 activity with HOE-642 further increased apoptosis in TMZ-treated GC#22 cells (71±1.3%, P < 0.05, Figure 5A), which is similar to the high apoptotic cell death rate (84±2.1%) in the staurosporine-treated positive control study (4 μM, 1h). These data suggest that inhibition of NHE1 activity can sensitize GC to apoptosis.
Fig. 5.
Inhibition of NHE1 potentiates TMZ-mediated apoptosis of GC. (A) Annexin V positive cells under different treatment conditions. GC#22 were exposed to control medium (Dulbecco’s modified Eagle’s medium + 10% fetal bovine serum), HOE-642(1 µM), TMZ (50 µM), or T+H (50 µM TMZ plus 1 µM HOE-642) for 48h. STS (4 µM, for 1h) was used as positive control. Arrow, Annexin V positive cells (Annexin V+). Arrowhead: Annexin V negative cells (Annexin V). (B) Summary data of Annexin V+ cells. Data are expressed as mean ± SD. *P < 0.05 versus CON. # P < 0.05 versus T+H. (C) Representative immunoblots of caspase-3 protein expression in GC#22 under four different treatment conditions. (D) Summary data of immunoblotting. Relative expression level of each protein was normalized to α-tubulin. Data are mean ± SEM. n = 8. *P < 0.05 versus CON. # P < 0.05 versus 96h T+H. (E) Flow cytometry analysis of cell shrinkage under different treatment conditions for 48h. P1: population of shrunken cells. Data are mean ± SEM. n = 3. *P < 0.05 versus CON. STS, staurosporine.
We further examined whether NHE1 inhibition can enhance TMZ-mediated activation of downstream apoptosis pathways, including extrinsic apoptotic pathway initiator caspase-3. GC#22 were exposed to CON, 1 µM HOE-642, 100 µM TMZ or 100 µM TMZ plus 1 µM HOE-642. Time-dependent cleavage of caspase-3 was evaluated at 48 or 96h. As shown in Figure 5B, neither control nor HOE-642 had a significant effect on caspase-3 activation. In contrast, TMZ triggered activation of caspase-3 and increased cleaved caspase-3 level (17kDa) after 48 or 96h treatment. In contrast, NHE1 inhibition accelerated TMZ-mediated apoptosis and significantly sensitized GC to apoptosis at 96h (Figure 5C and D).
NHE1 activation may promote survival and counteract apoptosis-induced apoptotic volume decrease by increasing Na+ influx and cell volume (30). We conducted flow cytometry analysis to examine cell size changes with the forward scatter analysis. Figure 5E shows that TMZ-treated GC#22 cells (100 µM, TMZ, 48h) exhibited increased cell population with smaller cell size (shrunken cell population in P1, 20.5 ± 2.5% versus 11.1 ± 1.6 % in controls, P < 0.05). However, combined treatment of TMZ plus HOE-642 did not further increase apoptotic volume decrease in GC#22. These data imply that protection by NHE1 against apoptotic volume decrease may not be a major defending mechanism for GC to counteract the TMZ-mediated apoptosis.
Stimulation of NHE1 protein regulates ERK activation in GC
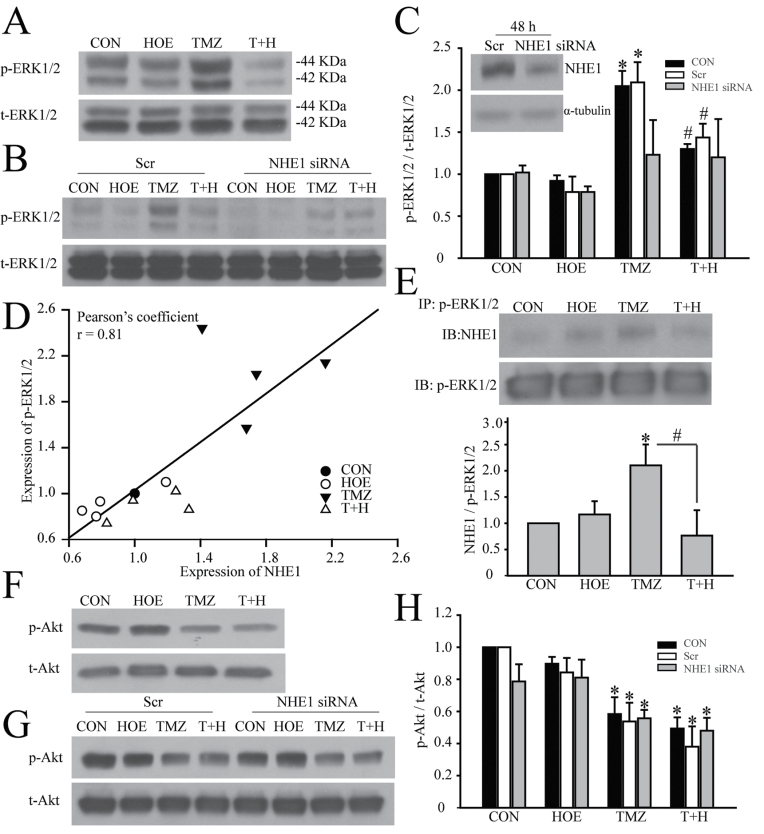
It was reported that NHE1 activation enhances ERK1/2 phosphorylation (31). In this study, we further investigated whether NHE1 could affect cellular protection in GC via regulating ERK activation. Figure 6A shows TMZ significantly stimulating ERK activation (44kDa and 42kDa). In contrast, NHE1 inhibition with HOE-642 abolished TMZ-mediated ERK activation in GC (Figure 6A). Furthermore, the Pearson’s correlation analysis revealed a positive correlation between NHE1 expression and ERK activation under four different treatment conditions (Pearson’s r of 0.81, Figure 6D). Alternatively, siRNA-mediated knockdown was also utilized to selectively reduce NHE1 protein expression and investigated its impact on activation of ERK. Compared with scrambled siRNA-treated cells, expression of NHE1 in the NHE1 siRNA-treated cells for 48h was reduced by ~50% (48.3±5.3% scrambled siRNA, P < 0.05, Figure 6C). Exposure of the scrambled siRNA-treated GC to TMZ for 48h triggered a significant activation of ERK signaling pathway, reflected by an increase in expression of p-ERK1/2 (44kDa and 42kDa) in Figure 6B. Reduced p-ERK1/2 expression was detected in NHE1 siRNA-treated GC#22 cells under all four conditions (Figure 6B). Most importantly, TMZ failed to induce elevation of p-ERK1/2 in NHE1 siRNA-treated GC cells (Figure 6B). To further establish interactions between NHE1 and p-ERK1/2 protein, we performed immunoprecipitation in GC#22 cells in which NHE1 protein was pulled down in the IP using the anti-p-ERK1/2 antibody. As shown in Figure 6E, NHE1 protein in the IP fractions was significantly increased in the TMZ-treated cells (Figure 6E). Most interestingly, this increase was abolished in cells treated with TMZ plus HOE-642 (P < 0.05, Figure 6E). These findings further illustrated the interactions between NHE1 and ERK1/2 in GCs. Taken together, these findings strongly suggest that NHE1 functions in regulating ERK activation and, especially, is required for the TMZ-mediated ERK stimulation in GC. Therefore, NHE1 could promote GC survival by stimulating ERK signaling pathway to escape TMZ-induced apoptosis.
Fig. 6.
Pharmacological inhibition or siRNA-mediated knockdown of NHE1 decreases ERK signal activation. (A) Representative immunoblots of p-ERK protein expression in GC#22 under four different treatment conditions. (B) Representative immunoblots showing siRNA-induced knockdown of NHE1 selectively reduces p-ERK expression. GC#22 were treated with scrambled siRNA or NHE1 siRNA for 48h and followed with control, 1 µM HOE-642, 100 µM TMZ or 100 µM TMZ plus 1 µM HOE-642 (T+H) for another 48h. The level of p-ERK protein expression was normalized to t-ERK. (C) Representative immunoblots showing effects of NHE1 siRNA treatment on expression of NHE1. GC#22 were treated with scrambled siRNA and NHE1 siRNA for 48h. Summary data of immunoblotting. Expression of p-ERK protein was first normalized to t-ERK in each condition. Relative expression level of p-ERK in each group was then normalized to the control group. Data are mean ± SEM. n = 5. *P < 0.05 versus CON. # P < 0.05 versus 48h T+H. (D) Pearson’s correlation analysis of the correlation between NHE1 and p-ERK expression under four different treatment conditions. (E) Representative immunoblots showing coimmunoprecipitation of p-ERK1/2 and NHE1 protein was detected in GC#22 using anti-p-ERK1/2 antibody. Expression of NHE1 protein was first normalized to p-ERK1/2 in each sample. Relative expression level in each group was normalized to CON. Data are mean ± SEM. n = 4. *P < 0.05 versus CON. # P < 0.05 versus T+H. (F) Representative immunoblots of p-Akt protein expression in GC#22 under four different treatment conditions. (G) Representative immunoblots showing p-Akt expression under siRNA-induced knockdown of NHE1 conditions. GC#22 were treated with scrambled siRNA or NHE1 siRNA for 48h and followed with control, 1 µM HOE-642, 100 µM TMZ or 100 µM TMZ plus 1 µM HOE-642 (T+H) for another 48h. The level of p-Akt protein expression was normalized to t-Akt. (H) Summary data of immunoblotting. Relative expression level of each protein was normalized to t-Akt. Data are mean ± SEM. n = 4. *P < 0.05 versus CON.
NHE1 activation could affect GC survival by regulating Akt activation because direct interactions between NHE1 and ERM can recruit phosphoinositide 3-kinase and Akt to regulate cell survival (32). We further investigated if this pathway is activated in GC. Shown in Figure 6F and G TMZ treatment significantly reduced p-Akt expression but combined TMZ and NHE1 inhibition did not further decrease the Akt activation. These data imply that NHE1 activation did not facilitate GC cytoprotection through regulation of Akt signaling.
Discussion
High NHE1 protein expression maintains alkaline pHi in GC
High NHE1 activity plays an essential role in developing the transformed phenotype of cancer cells during tumorigenesis (10). NHE1 activation in MDA-MB-435 breast cancer cells led to morphological and cytoskeletal changes with increased chemotaxis and cell invasion (33). Increased NHE1 expression in hepatocellular carcinoma was associated with increased tumor size, enhanced venous invasion and advanced tumor stages (34). In this study, we detected abundant NHE1 protein expression only in glioma cells, but not in control human NSCs and HAs. Analysis of a GBM tissue array revealed that >70% of GBM specimens showed moderate to strong NHE1 protein expression. The Rembrandt brain tumor database illustrates that the patients with higher NHE1 mRNA expression had shorter survival rate.
In 1920s, Warburg determined that cancer tissue consumes 10-fold more glucose than can be accounted for by respiration and produces two orders of magnitude more lactic acid than the normal tissue (35). Despite of the increased intracellular lactate production, cancer cells are still able to maintain a higher pHi, compared with normal cells. Activation of H+ extruder NHE1 has been proposed as a key player in the elevation of the pHi in the cancer cell, including glioma (36).
A major physiological role of NHE1 is to regulate and maintain the physiological pHi (6.9–7.2) in normal cells (37). Acidification of the intracellular milieu in normal cells activates NHE1-mediated electroneutral exchange of H+ extrusion and Na+ influx. However, in this study, we detected an alkaline resting pHi in GC#22 (7.46±0.04), which is consistent with previous reports on alkaline pHi of rat and human glioma cell lines (C6, U87, U118 or U251) (6). The alkaline pHi in GC#22 largely results from NHE1-dependent H+ extrusion because NHE1 inhibition with HOE-642 significantly acidified GC#22 cells. We also found that H+-ATPase activity does not play a major role in maintaining basal pHi in GC cells (data not shown). Thus, NHE1 is a dominant mechanism in regulating GC baseline pHi.
One of the basic features of NHE1 regulation is the exquisite regulation of pHi through its internal allosteric H+ binding regulatory site in the transmembrane domains (38). Therefore, intracellular acidosis in tumor cells can increase the binding affinity of NHE1 for intracellular H+ and changes the pH set point higher (more alkaline) than the resting pHi (39).
GC acquires NHE1 protein upregulation to counteract TMZ-induced acidosis
In this study, TMZ treatment caused a transient acidification in GC cells for 2–24h, which recovered by 48h. Intracellular acidification is commonly reported in apoptotic cells (40). This acidification may result from mitochondrial dysfunction, loss of intracellular adenosine triphosphate, and subsequent inhibition of alkalinizing mechanisms such as inhibition of NHE1 or H+-ATPase activity (40–42). In our study, HOE-642-sensitive pHi increased after 2h of TMZ treatment, indicating increased NHE1 activity. Blockade of NHE1 activity with HOE-642 further acidified the TMZ-treated GC cells, implying sustained NHE1 activation in GC. Interestingly, we detected concurrent recovery of pHi and increased NHE1 protein expression in GC cells after 48h TMZ treatment. These data suggest that GC upregulate NHE1 to maintain favorable pHi to counteract the TMZ-associated apoptotic acidosis.
Elevated NHE1 activation promotes GC migration
In this study, increased GC mobility correlated with transwell migration under different treatment conditions. The Pearson’s correlation analysis also revealed a positive correlation between cell migration distance and migrated cell numbers (Pearson’s r of 0.767). NHE1 inhibition with HOE-642 abolished the increase in GC mobility and transwell migration. NHE1 may regulate GC migration through several mechanisms. First, NHE1 can play a role in cell migration (33,43) via its function in cytoskeletal stability and plasma membrane anchoring because ERM proteins bind directly to the membrane-proximal NHE1 cytoplasmic domain (32). We show that NHE1 colocalized with ezrin in GC lamellipodia. Our immunoprecipitation data provided further evidence on interactions between NHE1 and ezrin proteins in GC. We found concurrent increase of NHE1 protein expression and increased interactions with ezrin protein in the same IP fractions of GCs in response to TMZ-mediated stress.
The MMPs are a family of zinc-containing proteases that play an important role in tumor metastasis via extracellular matrix degradation (44). MMP2 and MMP9 are important in tumor invasion and metastasis because of their specificity for type IV collagen (45). In our study, no changes in expression of MMP2 and MMP9 proteins were detected under four treatment conditions. However, TMZ triggered significant activation of MMP2. NHE1 inhibition with HOE-642 was associated with blockade of MMP2 activation. The mechanisms underlying NHE1-associated MMP2 activation remain to be determined. In breast cancer cells, NHE1 activity increases expression of MMP2 stimulator membrane type 1 MMP via ERK1/2 and p38 mitogen-activated protein kinase signaling pathways (46). NHE1 and ERK1/2 signaling activations are also involved in fibronectin-induced enhancement of MMP2 and F-actin expression and cell migration (31). We detected significant elevation of membrane type 1 MMP protein expression in the TMZ-treated samples, but this change was abolished by HOE-642 (Supplementary Figure 3, available at Carcinogenesis Online). In light of these findings, future studies are required to further investigate roles of NHE1 activation in ERK activation and membrane type 1 MMP expression.
NHE1 activation is associated with prosurvival ERK signal pathway stimulation and counteracts TMZ-mediated apoptosis
In our study, a low concentration of TMZ (50 μM, 4h) induced early apoptosis with increased Annexin V staining. Interestingly, blocking NHE1 activity with HOE-642 enhanced early GC apoptosis. Combined TMZ and HOE-642 treatments further accelerated caspase-3 activation after 48 or 96h. These data imply that NHE1 plays a role in sensitizing GC to apoptosis. Recent studies suggest that NHE1 activity is required for ERK activation in ischemia-reperfusion injury (47). In pancreatic cancer, inhibiting ERK phosphorylation results in cell cycle arrest and apoptosis (48). Here, we report that ERK activation in GC was abolished when NHE1 activity was inhibited by HOE-642 or siRNA. We further found that NHE1 expression is positively correlated to ERK activity. Therefore, NHE1 inhibition may suppress the ERK activity and enhance TMZ-mediated apoptosis in GC.
Lastly, NHE1 may also play a role in regulating apoptosis by altering pHi. pHi in the acidic range is required for death effectors, endonucleases, caspases and cathepsins (49). The major role of NHE1 against apoptosis is limiting intracellular acidification during the early event of cell death (49). The increased GC sensitivity to TMZ plus HOE-642-induced apoptosis could be due to acidified pHi, which could further trigger caspase activation. On the other hand, NHE1-dependent regulatory volume increase did not alter apoptotic volume decrease after TMZ treatment. This suggests that NHE1-mediated regulatory volume increase does not contribute to GC resistance against TMZ-apoptosis.
In summary (Supplementary Figure 4, available at Carcinogenesis Online), in GBM cells, NHE1 is the major H+ extrusion pathway to regulate pHi under physiological condition and in response to TMZ-mediated apoptosis. First, NHE1 activity maintains alkaline pHi and acidic extracellular microenvironment, which are favorable for MMP2 activation. TMZ increases NHE1 expression and the NHE1-ezrin interactions, thereby stimulating cell cytoskeletal rearrangements and promoting migration. NHE1 inhibition attenuated glioma cell migration by reducing MMP2 activity. Moreover, NHE1 activation also stimulated ERK1/2 activity. Therefore, inhibition of NHE1 activity amplified TMZ-induced cytotoxicity. Taken together, this study suggests potential utility of a combined therapeutic strategy of TMZ and NHE1 inhibition for GBM.
Supplementary material
Supplementary Figures 1–4 can be found at http://carcin.oxfordjournals.org/.
Funding
National Institutes of Health (R01NS75995 to D.S., J.S.K., K.B.P.; R01NS48216 to D.S.; T32 GM008692 to K.B.P.; U L1RR025011 and P30CA014520); UW Carbone Cancer Center; Headrush Brain Tumor Research Professorship; Roger Loff Memorial Fund “Farming against Brain Cancer” to J.S.K.; the �Program for New Century Excellent Talents In University� from the Ministry of Education, China (NCET-09-0131) and China National Natural Scientific Fund (30872650 to S.H.).
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations:
- CON
control medium
- ERK
extracellular signal-regulated kinase
- ERM
ezrin, radixin and moesin
- GBM
glioblastoma multiforme
- GC
glioma cells
- IP
immunoprecipitation
- MMP
matrix metalloproteinases
- NHE1
sodium–hydrogen exchanger isoform 1
- NSC
neural stem cells
- SDS
sodium dodecyl sulfate
- siRNA
small interfering RNA
- TMZ
temozolomide.
References
- 1. Denysenko T., et al. (2010). Glioblastoma cancer stem cells: heterogeneity, microenvironment and related therapeutic strategies. Cell Biochem. Funct., 28, 343–351 [DOI] [PubMed] [Google Scholar]
- 2. Dirks P.B. (2010). Brain tumor stem cells: the cancer stem cell hypothesis writ large. Mol. Oncol., 4, 420–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stupp R., et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. (2005). Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med., 352, 987–996 [DOI] [PubMed] [Google Scholar]
- 4. Harguindey S., et al. (2005). The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin–one single nature. Biochim. Biophys. Acta, 1756, 1–24 [DOI] [PubMed] [Google Scholar]
- 5. Gillies R.J., et al. (2002). MRI of the tumor microenvironment. J. Magn. Reson. Imaging, 16, 430–450 [DOI] [PubMed] [Google Scholar]
- 6. McLean L.A., et al. (2000). Malignant gliomas display altered pH regulation by NHE1 compared with nontransformed astrocytes. Am. J. Physiol. Cell Physiol., 278, C676–C688 [DOI] [PubMed] [Google Scholar]
- 7. Cardone R.A., et al. (2005). The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer, 5, 786–795 [DOI] [PubMed] [Google Scholar]
- 8. Heddleston J.M., et al. (2012). Hypoxia-induced mixed-lineage leukemia 1 regulates glioma stem cell tumorigenic potential. Cell Death Differ., 19, 428–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glunde K., et al. (2002). Na(+)/H(+) exchange subtype 1 inhibition during extracellular acidification and hypoxia in glioma cells. J. Neurochem., 80, 36–44 [DOI] [PubMed] [Google Scholar]
- 10. Reshkin S.J., et al. (2000). Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J., 14, 2185–2197 [DOI] [PubMed] [Google Scholar]
- 11. Lauritzen G., et al. (2012). The Na+/H+ exchanger NHE1, but not the Na+, HCO3(-) cotransporter NBCn1, regulates motility of MCF7 breast cancer cells expressing constitutively active ErbB2. Cancer Lett., 317, 172–183 [DOI] [PubMed] [Google Scholar]
- 12. Clark P.A., et al. (2012). Activation of multiple ERBB family receptors mediates glioblastoma cancer stem-like cell resistance to EGFR-targeted inhibition. Neoplasia, 14, 420–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zorniak M., et al. (2012). Differential expression of 2’,3’-cyclic-nucleotide 3’-phosphodiesterase and neural lineage markers correlate with glioblastoma xenograft infiltration and patient survival. Clin. Cancer Res., 18, 3628–3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Svendsen C.N., et al. (1998). A new method for the rapid and long term growth of human neural precursor cells. J. Neurosci. Methods, 85, 141–152 [DOI] [PubMed] [Google Scholar]
- 15. Zhu W., et al. (2014). WNK1-OSR1 kinase-mediated phospho-activation of Na+-K+-2Cl- cotransporter facilitates glioma migration. Mol. Cancer, 13, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shi Y., et al. (2013). Stimulation of na(+)/h(+) exchanger isoform 1 promotes microglial migration. PLoS One, 8, e74201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boyarsky G., et al. (1993). Intracellular pH regulation in single cultured astrocytes from rat forebrain. Glia, 8, 241–248 [DOI] [PubMed] [Google Scholar]
- 18. Algharabil J., et al. (2012). Inhibition of Na(+)-K(+)-2Cl(-) cotransporter isoform 1 accelerates temozolomide-mediated apoptosis in glioblastoma cancer cells. Cell. Physiol. Biochem., 30, 33–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karki P., et al. (2011). B-Raf associates with and activates the NHE1 isoform of the Na+/H+ exchanger. J. Biol. Chem., 286, 13096–13105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lagana A., et al. (2000). Regulation of the formation of tumor cell pseudopodia by the Na(+)/H(+) exchanger NHE1. J. Cell Sci., 113, 3649–3662 [DOI] [PubMed] [Google Scholar]
- 21. Chanana V., et al. (2012). The Na+/H+ exchanger-1 as a new molecular target in stroke interventions. In Balestrino D.M. (ed) Advances in the Preclinical Study of Ischemic Stroke. InTech, Rijeka, Croatia, pp. 497–510 [Google Scholar]
- 22. O’Donnell B.R., et al. (1994). Influence of pH on calcium influx during hypoxia in rat cortical brain slices. Stroke., 25, 171–177 [DOI] [PubMed] [Google Scholar]
- 23. Rochon P., et al. (2007). Evaluation of BCECF fluorescence ratio imaging to properly measure gastric intramucosal pH variations in vivo . J. Biomed. Opt., 12, 064014. [DOI] [PubMed] [Google Scholar]
- 24. Shahidullah M., et al. (2009). Responses of sodium-hydrogen exchange to nitric oxide in porcine cultured nonpigmented ciliary epithelium. Invest. Ophthalmol. Vis. Sci., 50, 5851–5858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Altamirano J., et al. (1998). Regulatory volume decrease and intracellular Ca2+ in murine neuroblastoma cells studied with fluorescent probes. J. Gen. Physiol., 112, 145–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Könnecke H., et al. (2013). The role of microglia and matrix metalloproteinases involvement in neuroinflammation and gliomas. Clin. Dev. Immunol., 2013, 914104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adada M., et al. (2013). Sphingolipid regulation of ezrin, radixin, and moesin proteins family: implications for cell dynamics. Biochim. Biophys. Acta, 13, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harley W., et al. (2010). Dual inhibition of sodium-mediated proton and calcium efflux triggers non-apoptotic cell death in malignant gliomas. Brain Res., 1363, 159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Friedman H.S., et al. (2000). Temozolomide and treatment of malignant glioma. Clin. Cancer Res., 6, 2585–2597 [PubMed] [Google Scholar]
- 30. Maeno E., et al. (2006). Dysfunction of regulatory volume increase is a key component of apoptosis. FEBS Lett., 580, 6513–6517 [DOI] [PubMed] [Google Scholar]
- 31. Park J.H., et al. (2012). Fibronectin stimulates migration through lipid raft dependent NHE-1 activation in mouse embryonic stem cells: involvement of RhoA, Ca(2+)/CaM, and ERK. Biochim. Biophys. Acta, 1820, 1618–1627 [DOI] [PubMed] [Google Scholar]
- 32. Wu K.L., et al. (2004). The NHE1 Na+/H+ exchanger recruits ezrin/radixin/moesin proteins to regulate Akt-dependent cell survival. J. Biol. Chem., 279, 26280–26286 [DOI] [PubMed] [Google Scholar]
- 33. Paradiso A., et al. (2004). The Na+-H+ exchanger-1 induces cytoskeletal changes involving reciprocal RhoA and Rac1 signaling, resulting in motility and invasion in MDA-MB-435 cells. Breast Cancer Res., 6, R616–R628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schneider L., et al. (2009). The Na+/H+ exchanger NHE1 is required for directional migration stimulated via PDGFR-alpha in the primary cilium. J. Cell Biol., 185, 163–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jin W.N., et al. (2011). [Inhibition of NHE1 promotes hypoxia-induced differentiation of K562 leukemic cells]. Zhongguo Shi Yan Xue Ye Xue Za Zhi, 19, 661–665 [PubMed] [Google Scholar]
- 36. López-Lázaro M. (2008). The warburg effect: why and how do cancer cells activate glycolysis in the presence of oxygen? Anticancer. Agents Med. Chem., 8, 305–312 [DOI] [PubMed] [Google Scholar]
- 37. Loo S.Y., et al. (2012). NHE-1: a promising target for novel anti-cancer therapeutics. Curr. Pharm. Des., 18, 1372–1382 [DOI] [PubMed] [Google Scholar]
- 38. Putney L.K., et al. (2002). The changing face of the Na+/H+ exchanger, NHE1: structure, regulation, and cellular actions. Annu. Rev. Pharmacol. Toxicol., 42, 527–552 [DOI] [PubMed] [Google Scholar]
- 39. Reshkin S.J., et al. (2013). Na+-H+ exchanger, pH regulation and cancer. Recent Pat. Anticancer. Drug Discov., 8, 85–99 [DOI] [PubMed] [Google Scholar]
- 40. Lagadic-Gossmann D., et al. (2004). Alterations of intracellular pH homeostasis in apoptosis: origins and roles. Cell Death Differ., 11, 953–961 [DOI] [PubMed] [Google Scholar]
- 41. Thangaraju M., et al. (1999). Regulation of acidification and apoptosis by SHP-1 and Bcl-2. J. Biol. Chem., 274, 29549–29557 [DOI] [PubMed] [Google Scholar]
- 42. Thangaraju M., et al. (1999). Interdependent regulation of intracellular acidification and SHP-1 in apoptosis. Cancer Res., 59, 1649–1654 [PubMed] [Google Scholar]
- 43. McHardy L.M., et al. (2004). The tumor invasion inhibitor dihydromotuporamine C activates RHO, remodels stress fibers and focal adhesions, and stimulates sodium-proton exchange. Cancer Res., 64, 1468–1474 [DOI] [PubMed] [Google Scholar]
- 44. Lin Y., et al. (2011). NHE1 mediates migration and invasion of HeLa cells via regulating the expression and localization of MT1-MMP. Cell Biochem. Funct., 30, 41–46 [DOI] [PubMed] [Google Scholar]
- 45. Komatsu K., et al. (2004). Expression and quantitative analysis of matrix metalloproteinase-2 and -9 in human gliomas. Brain Tumor Pathol., 21, 105–112 [DOI] [PubMed] [Google Scholar]
- 46. Lin Y., et al. (2011). NHE1 mediates MDA-MB-231 cells invasion through the regulation of MT1-MMP. Exp. Cell Res., 317, 2031–2040 [DOI] [PubMed] [Google Scholar]
- 47. Coccaro E., et al. (2009). Phenylephrine and sustained acidosis activate the neonatal rat cardiomyocyte Na+/H+ exchanger through phosphorylation of amino acids Ser770 and Ser771. Am. J. Physiol. Heart Circ. Physiol., 297, H846–H858 [DOI] [PubMed] [Google Scholar]
- 48. Roy S.K., et al. (2010). Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J. Mol. Signal., 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Matsuyama S., et al. (2000). Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat. Cell Biol., 2, 318–325 [DOI] [PubMed] [Google Scholar]
- 50. Nakamura K., et al. (2007). Transiently increased colocalization of vesicular glutamate transporters 1 and 2 at single axon terminals during postnatal development of mouse neocortex: a quantitative analysis with correlation coefficient. Eur. J. Neurosci., 26, 3054–3067 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.