Abstract
DNA adducts are a measure of internal exposure to genotoxicants. However, the measurement of DNA adducts in molecular epidemiology studies often is precluded by the lack of fresh tissue. In contrast, formalin-fixed paraffin-embedded (FFPE) tissues frequently are accessible, although technical challenges remain in retrieval of high quality DNA suitable for biomonitoring of adducts. Aristolochic acids (AA) are human carcinogens found in Aristolochia plants, some of which have been used in the preparation of traditional Chinese herbal medicines. We previously established a method to measure DNA adducts of AA in FFPE tissue. In this study, we examine additional features of formalin fixation that could impact the quantity and quality of DNA and report on the recovery of AA-DNA adducts in mice exposed to AA. The yield of DNA isolated from tissues fixed with formalin decreased over 1 week; however, the levels of AA-DNA adducts were similar to those in fresh frozen tissue. Moreover, DNA from FFPE tissue served as a template for PCR amplification, yielding sequence data of comparable quality to DNA obtained from fresh frozen tissue. The estimates of AA-DNA adducts measured in freshly frozen tissue and matching FFPE tissue blocks of human kidney stored for 9 years showed good concordance. Thus, DNA isolated from FFPE tissues may be used to biomonitor DNA adducts and to amplify genes used for mutational analysis, providing clues regarding the origin of human cancers for which an environmental cause is suspected.
Introduction
DNA adducts represent a measure of internal exposure to genotoxins and an important end point for cross-species extrapolation of the biologically effective dose of chemical carcinogens in human risk assessment analysis (1,2). The identification of DNA adducts often is the first step in elucidating the potential role of a genotoxic chemical in the etiology of human cancer (3). However, fresh frozen human tissues usually are unavailable for biomonitoring studies, whereas formalin-fixed paraffin-embedded (FFPE) tissues, especially from patients with a clinical diagnosis of cancer, are regularly archived and frequently available.
Formalin fixation, followed by paraffin embedding of tissues has been used as the standard preservation technique for more than a century in laboratories worldwide (4). Although several classes of carcinogen DNA adducts have been screened, using immunohistochemical (IHC) methods, in FFPE tissues (5–7), an important drawback of this method is that the specificity of the antibodies, even monoclonal antibodies, for DNA adducts, is uncertain and cross-reactivity with structurally related DNA lesions can occur, leading to errors in identification and quantification. Mass spectrometry is more specific and sensitive than IHC and can be used to quantify DNA adducts in human tissues (8). However, the difficulty in retrieving DNA without cross-links that is fully digestible by nucleases to form single deoxyribonucleosides containing a chemical carcinogen has precluded the employment of mass spectrometry for DNA adduct measurements in FFPE tissues.
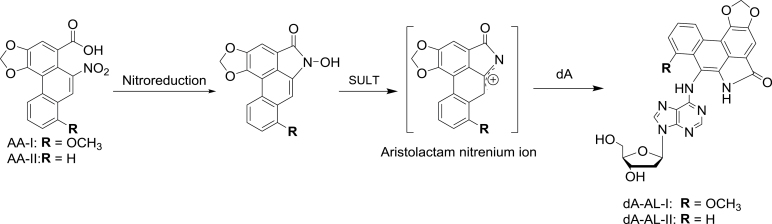
We recently reported that the cross-links of DNA in FFPE tissue can be quantitatively reversed by employing a commercial kit developed for retrieval of DNA (9). The potential to employ this method for measurement of DNA adducts was investigated with 8-methoxy-6- nitrophenanthro-[3,4-d]-1,3-dioxole-5-carboxylic acid (AA-I), a component of Aristolochia herbs and a potent human urothelial carcinogen (10–12). The metabolic intermediate of AA-I reacts with DNA to form the aristolactam (AL-I)-DNA adduct 7-(deoxyadenosin-N 6-yl) aristolactam I (dA-AL-I) (Figure 1) (13). This DNA lesion is believed to be responsible for the induction of otherwise rare A:T → T:A transversions in the TP53 tumor suppressor gene in urothelial carcinomas of patients with AA-induced urothelial carcinomas of the upper urinary tract (11,14,15).The DNA retrieval procedure employed allowed us to quantitatively measure dA-AL-l by ultraperformance-liquid chromatography-electrospray ionization-multistage scan mass spectrometry (UPLC-ESI-MS/MSn) (9,16). The level of adducts in FFPE human kidney were comparable with those determined in matching freshly frozen tissues, when assayed by quantitative UPLC/MS (9).
Fig. 1.
Bioactivation of AA-I and formation of dA-AL-I. SULT, sulfotransferases.
Further validation of the FFPE method will be required before this analytical procedure can be routinely employed in molecular epidemiology studies. For example, the time of formalin fixation of tissues may vary in the clinical setting and adversely affect the recovery of high quality and fully digestible DNA, or affect the structural integrity of the DNA lesion (17). The ability to recover DNA and to quantify carcinogen DNA adducts in tissues preserved with formalin for variable periods of time prior to paraffin embedding is required to establish a robust DNA adduct biomarker. In this study, we have investigated the effect of time of formalin fixation on the quantity and quality of DNA, and the estimates in the level of AA-DNA adducts in FFPE tissue. We also examined the suitability of the DNA to serve as a template for PCR amplification of 100–500 bp fragments. The same DNA sample of FFPE tissue can be used for quantitative measurements of DNA adducts and PCR amplification of genes, which can be sequenced to establish mutations induced by genotoxicants. Our DNA retrieval method permits the recovery and quantitative measurement of the dA-AL-I adduct in human FFPE tissues archived for over 9 years.
Materials and methods
Materials
AA-I was provided by Dr H.Priestap, Department of Biological Sciences, Florida International University. dA-AL-I was provided by Dr F.Johnson and Dr R.Bonala, Department of Pharmacological Science, Stony Brook University, Stony Brook, New York. [15N5]-dA-AL-I (isotopic purity = 99%) was synthesized as described previously (16). DNase I (Type IV, from bovine pancreas), alkaline phosphatase (from Escherichia coli), nuclease P1 (from Penicillium citrinum), RNase A and RNase T1 were purchased from Sigma–Aldrich (St Louis, MO). Phosphodiesterase I (from Crotalus adamanteus venom) was purchased from Worthington Biochemicals Corp. (Newark, NJ). Formalin cups containing 10% neutral-buffered formalin (20 ml) were purchased from Richard Allen Scientific through Thermo Fisher Scientific (Kalamazoo, MI). All solvents were Optima LC/MS brand from Fisher Scientific (Fair Lawn, NJ). ZR FFPE DNA MiniPrep kit was from Zymo Research (Irvine, CA). Microliter CapLC vials with silylated inserts were from Microliter Analytical Supplies (Suwanee, GA).
Animal studies
The guidelines established by the National Institutes of Health Office of Laboratory Animal Welfare were adhered to for the use of animals with housing conditions described previously (9). All protocols were reviewed and approved by the Stony Brook University Institutional Animal Care and Use Committee. Male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME), 8−10 weeks old (n = 4 per group), were dosed (intraperitoneally) with AA-I (0.1mg/kg, in phosphate-buffered saline). After 20h, mice were euthanized by asphyxiation with CO2, followed by cervical dislocation. For freshly frozen tissues, the whole livers and kidneys were excised and stored at −80°C. Organs from a second set of animals were immersed in 10% neutral-buffered formalin (20 ml) for 24, 48, 72 and 168h at room temperature. A sagittal section of the kidney was cut prior to fixation, whereas the entire liver was fixed in formalin. Thereafter, the tissues were placed in a tissue processor, and serial washes of increasing amounts of ethanol in water, followed by washing with xylene at 42°C, prior to embedding in paraffin, at the Histology Core Facility at Stony Brook University (9). The FFPE tissue blocks were stored at ambient temperature.
Human tissue samples
The research protocol was reviewed and approved by the Institutional Review Boards at Stony Brook University, the Wadsworth Center and the School of Medicine, University of Zagreb. Kidney samples, containing both renal cortex and medulla, were obtained from individuals residing in endemic regions of Croatia and Serbia who were shown to have AA nephropathy and underwent nephroureterectomy for urothelial cancers of the upper urinary tract (18). Portions of the excised kidney were immediately frozen and stored at −80°C or fixed in neutral-buffered formalin employing processing conditions described previously (9). The human FFPE tissue blocks were prepared between 2004 and 2009 and stored at ambient temperature. DNA was obtained from 10 μm thick sections cut from FFPE tissue blocks with a rotary microtome, and stored in Eppendorf tubes at room temperature for up to 2 months prior to analysis of the DNA adducts.
DNA retrieval from freshly frozen mouse tissue
The tissues were thawed in a water bath at 4°C, and then immediately homogenized in 50mM Tris–HCl buffer (pH 8.0) containing 10mM ethylenediaminetetraacetic acid (TE buffer, 3 ml per gram tissue) with a Potter-Elvehjem homogenizer. The homogenate was centrifuged at 3000g for 10min at 4°C. The genomic DNA in the nuclear pellet (equivalent of 50mg of wet tissue weight) was isolated by CHCl3/phenol extraction as described (9,16).
Deparaffinization, rehydration and isolation of DNA from mouse FFPE tissues and human kidney tissue sections
The mouse liver or kidney were dissected from the paraffin block, and the bulk of the paraffin removed with a scalpel. The tissues were submerged in p-xylene, and then underwent rehydration, followed by homogenization, and centrifugation at 3000g (9). The nuclear pellet, using the equivalent of 20−25mg of dry weight of deparaffinized tissue, was processed with the ZR FFPE DNA MiniPrep kit (Zymo Research) according to the manufacturer’s instructions with minor modifications (9). For human kidney, two 10 μm sections, each with a surface area of 0.9−1.8cm2, were placed in an Eppendorf tube and washed with p-xylene (1.5 ml), followed by ethanol/water (95/5%). The tissue was air-dried for 30min and then resuspended in TE buffer (0.1 ml). The samples were digested with proteinase K for 4h at 55°C in lysis buffer. A complete description of the retrieval of DNA from FFPE tissue is provided as Supplementary Protocol S1, available at Carcinogenesis Online. To estimate the size distribution of the recovered DNA, 250ng of each DNA was electrophoresed through a 1% agarose gel in 1× TAE buffer and 0.5 µg/ml ethidium bromide. Each gel contained multiple lanes loaded with a 100bp ladder (New England BioLabs, Beverly, MA). The gels were photographed and staining intensity in each lane measured with ImageJ software. The molecular weight of DNA was estimated from standard curves constructed from the migration of authentic standards.
Enzymatic digestion of genomic DNA
DNA (2–5 µg) was spiked with [15N5]-dA-AL-I at a level of 5 adducts per 108 DNA bases and incubated with DNase I for 1.5h, followed by incubation with nuclease P1 for 3h, and then with spleen phosphodiesterase I and alkaline phosphatase for 18h at 37°C. The DNA digest was dried by vacuum centrifugation and dissolved in 35 µl of 1:1 H2O:DMSO (9).
Measurement of AL-DNA adducts by UPLC-ESI/MS3
Adduct quantification, utilizing the stable isotope dilution method, was performed with a NanoAcquity UPLC system (Waters Corporation, Milford, MA) interfaced with an Advance CaptiveSpray source from Michrom Bioresource (Auburn, CA) and an ion-trap mass spectrometer (LTQ Velos; Thermo Fisher, San Jose, CA). A Waters Symmetry trap column (180 μm × 20mm, 5 μm particle size) was employed for online enrichment of the DNA adducts. A C18AQ (0.3 × 150mm, 3 μm particle size; Michrom Bioresources) was used for chromatography. The DNA adducts were measured in the positive ionization mode at the MS3 scan stage. The chromatographic and mass spectra acquisition parameters were described previously (9,16).
Enzymatic digestion of AL-DNA and 32P-postlabeling/PAGE analysis of fresh frozen samples
Human DNA digests (5 or 20 μg) were incubated with 20 μCi of [γ-32P]-ATP (6000 Ci/mmol) and 10 units of 3′-phosphatase-free OptiKinase at 37°C, as described previously (19). The 32P-labeled adducts were resolved by electrophoresis on a nondenaturing 30% polyacrylamide gel, and AL-DNA adducts were measured using a β-phosphorimager with Image QuaNT v5.2 software (Molecular Dynamics, Piscataway, NJ).
PCR amplification
Primer Sequences: mRPRM-A: TCGTCC CGCGCTACAGCCACTCTGG; mRPRM-B: CGTTGCTCGGTCCGTGATGGTGAGG; mALAD-B: CTTGAT GGCCTCAACT CGT CCGTCC; mALAD-C: AGCGGTTGGCAGAGGTGG CACTGGC; mPLA2G2A-A: GGG AGC AGAGAGA TGCCATGTGGAG; mPLA2G2A-B: TGG CTGGAAACCA CTGGGAC ACT GAGG. The mALAD-B/C primer pair amplifies a 184 bp fragment from mouse DNA, the mPLA2G2A-A/B primer pair amplifies a 327 bp fragment and the mRPRM-A/B primer pair amplifies a 501bp fragment. Each amplicon size is for C57BL/6J mouse DNA. Twenty-five nanograms of mouse DNA was amplified in a 20 μl reaction containing 0.5 µM primers, 0.2mM dNTPs, 1.5mM MgCl2, 50mM KCl, 0.001% gelatin, 10mM Tris–HCl, pH 8.3 and 1.25 units of recombinant Taq Polymerase (Sigma). Reactions conditions included an initial denaturation step of 2 min at 94°C followed by 40 cycles of 94°C for 20 s, 65°C for 30 s and 72°C for 90 s. Reaction products were separated by electrophoresis in a 1.2% agarose gel in 1× TAE buffer and 0.5 µg/ml ethidium bromide. Prior to sequencing the PCR primers and excess nucleotides were degraded by addition of 0.25U shrimp alkaline phosphatase (Fermentas, Waltham, MA) and 0.5U E. coli exonuclease I (New England Biolabs) followed by 15 min at 37°C. Enzymes were denatured by incubating for 30min at 85°C. One microliter of PCR product and 3.2 pmol of sequencing primer were submitted to the Stony Brook University DNA sequencing core for standard Sanger dye-termination sequencing. The sequencing primers used were the same primers used for PCR amplification. Sequence quality scores used to compare PCR products were provided within the .abi files provided by the sequencing core.
Statistical methods
The t-test or one-way analysis of variance followed by Dunnett’s multiple comparisons test were performed using GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla, CA. A P value <0.05 was considered statistically significant.
Results
Recovery of DNA as a function of duration of formalin fixation
Genomic DNA was isolated by a silica-based fractionation method, employing the ZR FFPE Miniprep™ Kit and its proprietary reagents were critical for the quantitative removal of the DNA cross-links (9). The average yield of DNA recovered from freshly frozen mouse liver and kidney was ~150 µg per 100mg tissue (wet weight), whereas the yield of DNA in tissues preserved with formalin decreased over the time during formalin fixation (Table I). Thus, the quantity of DNA recovered from tissues preserved in formalin for 1 week was ~30% of the amount recovered from frozen tissue, indicative of irreversible cross-linking between DNA and protein. Similar declines in the recovery of DNA from FFPE tissue, using other methods of DNA retrieval, have been reported (17,20,21).
Table I.
The yield of DNA and dA-AL-I adduct levels recovered from freshly frozen and formalin-fixed mouse liver and kidney as a function of time of formalin fixation (μg DNA per 100mg tissue)
| Duration of formalin fixation (days) | Liver | Kidney | ||
|---|---|---|---|---|
| μg DNA/100mg tissue | dA-AL-I adducts per 108 bases | μg DNA/100mg tissue | dA-AL-I adducts per 108 bases | |
| 0 | 145.3±29.5 | 1.4±0.4 | 156.3±48.2 | 65.8±18.8 |
| 1 | 81.2±21.1*** | 1.4±0.3 | 111.7±25.6 | 61.8±1.9 |
| 2 | 73.3±5.4*** | 1.6±0.3 | 82.1±17.9** | 60.3±4.4 |
| 3 | 56.9±15.8*** | 1.3±0.2 | 50.3±13.2*** | 55.1±11.2 |
| 7 | 46.7±13.1*** | 1.5±0.4 | 49.2±15.7*** | 43.3±14.6 |
The mean and standard deviation values obtained from four animals. The one-way analysis of variance for mean DNA levels in liver or kidney was P < 0.0002; Dunnett’s multiple comparisons test for mean DNA levels for day 0 versus other days: **P < 0.006, ***P < 0.0003. The one-way analysis of variance for the mean dA-AL-I adduct levels in liver and kidney are, respectively: P = 0.749 and P = 0.129; Dunnett’s multiple comparisons test for mean dA-AL-I adduct levels in kidney for day 0 versus day 7 is P = 0.057.
Evaluation of DNA quality in FFPE tissues
The quality, purity and integrity of DNA recovered from fresh frozen and FFPE tissues were evaluated by several different techniques. Ultraviolet spectroscopy was employed to characterize the purity of the DNA. The A260/A280 ratio was used to assess the contamination of DNA with RNA and/or protein. The values of the A260/A280 measurements (mean ± SD of N animals) for DNA isolated by phenol chloroform extraction of fresh tissues were: 1.84±0.04 (n = 12 animals) for liver and 1.81±0.04 (n = 4 animals) for kidney. The A260/A280 values of DNA isolated from FFPE tissue by the ZR FFPE Miniprep™ Kit were comparable with the values from fresh frozen tissue. The A260/A280 values of DNA isolated from FFPE liver and kidney following fixation in formalin for 7 days were, respectively, 1.81±0.02 (n = 8 animals) and 1.85±0.02 (n = 8 animals). An A260/A280 ratio >1.80 signifies high quality DNA (22).
The efficacy of removal of cross-links and subseqent digestibility of DNA from FFPE tissues were established by treatment with nucleases to form deoxyribonucleosides, measured by high-performance liquid chromatography with ultraviolet detection (9). Incomplete removal of cross-links impedes the efficacy of nucleases, resulting in the appearance of oligomers and an underestimation of deoxyribonucleosides (9,23). DNA isolated from freshly frozen tissue samples by the CHCl3/phenol method, undergoes complete digestion to deoxyribonucleosides, and the level of RNA contamination, based on the detection of guanosine and adenosine, is <1% (9). DNA processed from FFPE tissue with ZR FFPE Miniprep™ Kits was efficiently digested by nucleases, irrespective of the time of fixation with formalin. Representative high-performance liquid chromatography ultraviolet chromatograms of the digested DNA from fresh frozen and tissues fixed with formalin for 7 days are shown in Supplementary Figure S1, available at Carcinogenesis Online. Contamination with RNA is <1%.
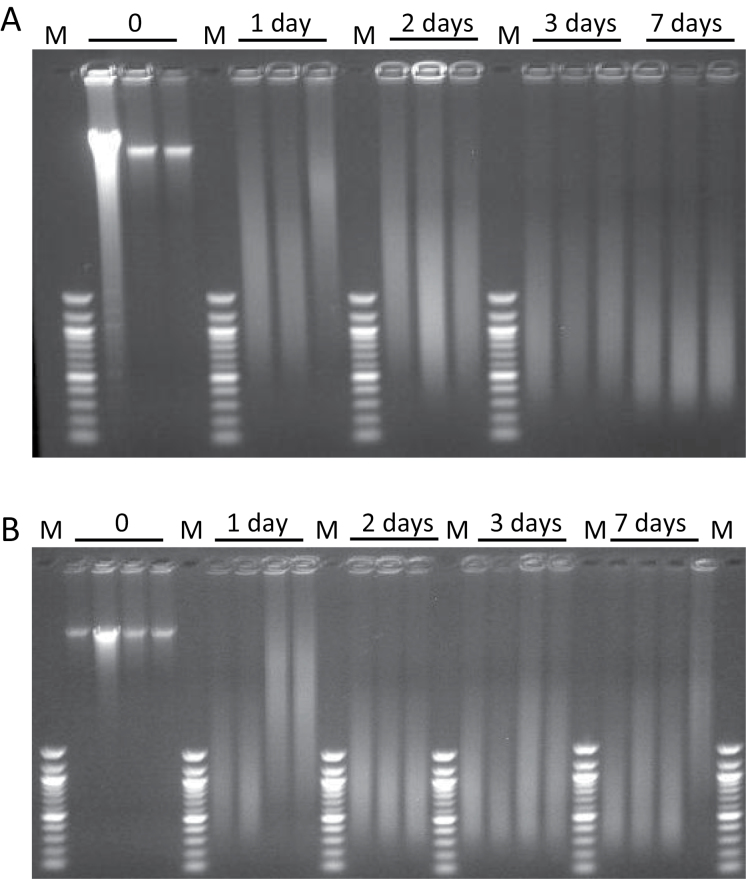
The integrity of DNA from freshly frozen and FFPE tissue was examined by agarose gel electrophoresis (Figure 2). DNA isolated from FFPE tissue contains a higher content of double-stranded DNA of low molecular weight than DNA isolated from freshly frozen tissue. Treatment of cells with formalin is known to cause DNA strand breakage (17). Moreover, isopropanol was added to the tissue lysis buffer to increase the yield of DNA from FFPE tissue, resulting in the recovery of low molecular weight double-stranded DNA (~50bp) and single-stranded DNA, in addition to high molecular weight DNA (>500bp). The lysis buffer without isopropanol preferentially recovers high molecular weight DNA (>500bp)from FFPE tissue, but with a 5-fold lower amount of DNA; a quantity insufficient for DNA adduct measurements (B.H.Yun, unpublished observations). As expected, DNA isolated from fresh frozen, unfixed tissue was mainly high molecular weight (>15 kb) and migrated slowly in the agarose gel. In contrast, DNA isolated from FFPE tissue was fragmented and possessed a broad range of sizes, evident after electrophoresis (Figure 2). For comparison, the images of the gels were analyzed to determine the size range of the DNA and the size of peak-staining intensity. The peak-staining size of the recovered DNA was progressively reduced with increasing time in formalin. After 1 day in formalin the mean length was 3.8kb, after 2 days was reduced to 1.3kb, after 3 days 0.88 kb and after 1 week the average size was 0.63kb.
Fig. 2.
Agarose gel electrophoresis of fresh frozen and FFPE DNA. Each lane contains DNA from a separate mouse. Above the gel is indicated the time the tissue was fixed in formalin prior to processing. Size markers (M) range from 100 to 1517bp (100bp ladder). This size range was used to estimate the size of the DNA in the region critical for most sequence-oriented applications. (A) Contains DNAs isolated from mouse kidney. (B) Contains DNAs isolated from mouse liver.
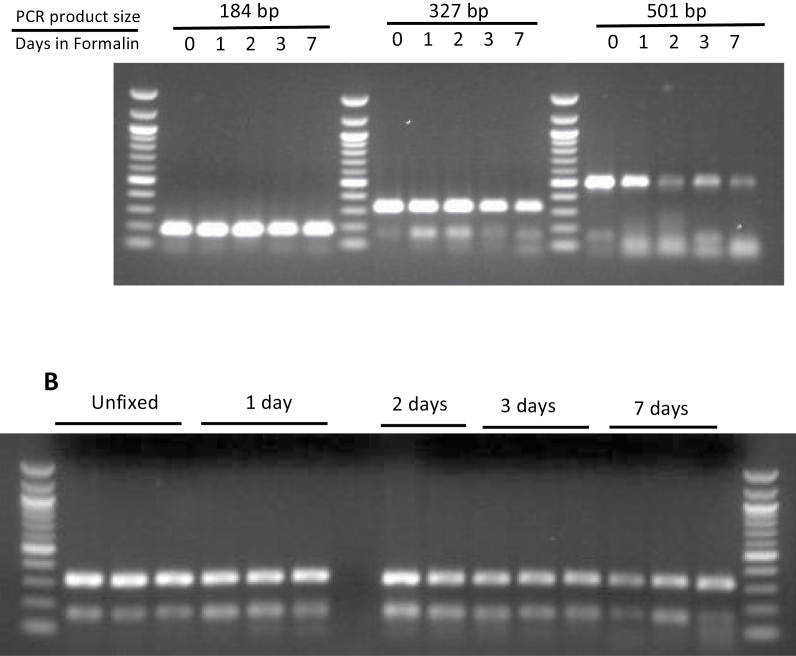
The DNA retrieved from fresh frozen and FFPE tissue was examined for its ability to serve as a template for PCR amplification of fragments ranging in the size range of 100–500 bp (Figure 3). Regardless of fixation time in formalin, the 184bp and 327bp fragments were readily amplified. However, the yield of a 501bp PCR fragment was reduced in DNA from tissue fixed longer than 1 day in formalin. To confirm the fidelity of the PCR amplification, we sequenced PCR products shown in Figure 3B. The quality of the templates obtained from FFPE was similar to that obtained from DNA from unfixed tissue regardless of fixation time in formalin (Supplementary information, Figure S3, available at Carcinogenesis Online).
Fig. 3.
Agarose gel electrophoresis of PCR amplification products from fresh frozen and FFPE DNA after 40 cycles of amplification. The size of standards range from 100 to 1517bp. (A) PCR products from liver DNA. Above the gel is indicated the expected size of the PCR product and the fixation time in formalin prior to tissue processing. (B) Yield of 327bp PCR product from kidney DNA. Each lane contains DNA from a separate mouse. Above the gel is indicated the fixation time in formalin prior to tissue processing.
dA-AL-I adducts in fresh and FFPE mouse tissue
Estimates of the level of dA-AL-I adduct in DNA of kidney and liver are reported in Table I, and representative UPLC-ESI/MS3 chromatograms of the adducts are shown in Supplementary Figure S2, available at Carcinogenesis Online. Although recovery of DNA diminished during the time of formalin fixation (Table I), the level of dA-AL-I in DNA of liver was constant over the 7 days of preservation with formalin and comparable with values of dA-AL-I present in DNA of freshly frozen liver (Table I). The estimate of dA-AL-I in DNA of FFPE kidney was comparable with that level measured in DNA of freshly frozen kidney of tissue, when exposure to formalin was extended for up to 72 h and began to decline in kidney fixed with formalin for 7 days (adjusted P value 0.057, Dunnett’s multiple comparison test of mean adduct level day 0 versus day 7).
Estimates of the level of dA-AL-I from freshly frozen and FFPE kidney tissue of patients with upper urothelial tract cancer
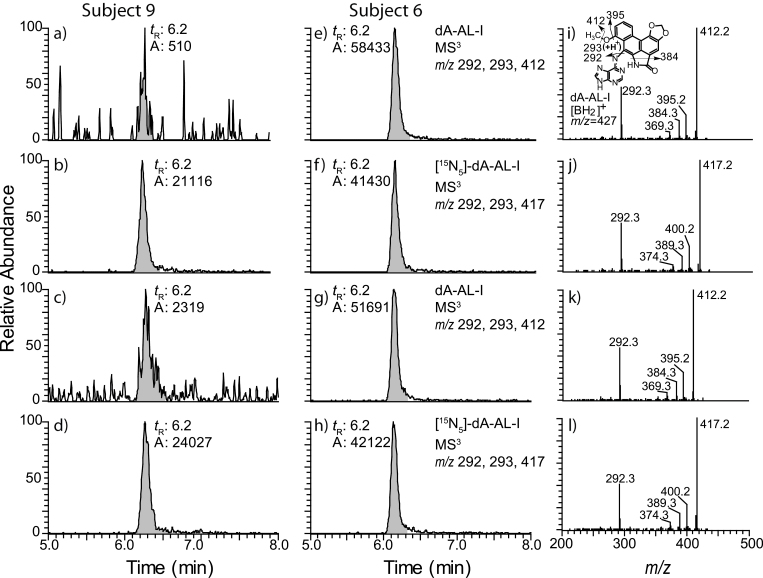
The levels of dA-AL-I were estimated, by UPLC-ESI/MS3 and 32P-postlabeling, in FFPE and matching freshly frozen tissue of 15 Croatian and Serbian patients from the Balkan endemic regions who underwent nephroureterectomy. Some blocks of FFPE tissue had been stored at room temperature for up to 9 years prior to analysis. The yield of DNA and surface area of 10 µm sections cut from paraffin-embedded blocks are reported with the measurements of dA-AL-I adduct levels (Table II), and representative UPLC-ESI/MS3 chromatograms and product ion spectra of dA-AL-I are presented in Figure 4. The dA adduct derived from 6-nitrophenathrene-[3,4-d]-1,3-dioxolo-5-carboxylic acid (AA-II), 7-(deoxyadenosin-N 6-yl)-aristolactam II (dA-AL-II), was not detected in any of the human samples (B.Hwa Yun, unpublished observations). Estimates of the dA-AL-I levels obtained by 32P-postlabeling and UPLC-ESI/MS3 are fairly consistent. In several samples, dA-AL-I was not detected by 32P-postlabeling although adduct levels remained above the limit of quantification (3 adducts per 109 DNA bases) when measured with UPLC-ESI/MS3. We reported previously that the UPLC-ESI/MS3 method is more sensitive than 32P-postlabeling (9,16). We conclude from our studies that dA-AL-I is stable in archived FFPE human tissues and can be accurately determined after many years of storage.
Table II.
Estimates of the level of dA-AL-I in frozen and FFPE kidney tissue of human patients with upper urothelial tract cancer
| Subject | Tissue | 32P-postlabeling | LC-MS/MS3 | DNA yielda (μg) | Surface area (cm2)b | Year, procured |
|---|---|---|---|---|---|---|
| (adducts per 108 DNA bases) | ||||||
| 1 | FR | 3.2 | 2.7±0.1 | |||
| FFPE | 1.8 | 2.1 | 1.6 | 2007 | ||
| 2 | FR | 2.2 | 3.9±0.1 | |||
| FFPE | 3.8±0.1 | 6.8 | 1.5 | 2008 | ||
| 3 | FR | 1.1 | 2.2±0.0 | |||
| FFPE | 3.5 | 3.5 | 2.1 | 2008 | ||
| 4 | FR | 0.9/0.7 | 1.2±0.0 | |||
| FFPE | 1.0±0.0 | 4.5 | 1.5 | n.a. | ||
| 5 | FR | 0.9/0.7 | 3.5±0.1 | |||
| FFPE | 2.4±0.0 | 6.0 | 2.1 | n.a. | ||
| 6 | FR | 6.5 | 7.0±0.2 | |||
| FFPE | 5.4±0.8 | 4.8 | 1.8 | 2008 | ||
| 7 | FR | BLDd | 1.0±0.1 | |||
| FFPE | 1.6±0.1 | 5.9 | 1.2 | 2007 | ||
| 8 | FR | BLDd | 0.5±0.0 | |||
| FFPE | 0.7±0.1 | 4.1 | 1.2 | 2009 | ||
| 9 | FR | BLDd | BLDe | |||
| FFPE | 0.48 | 3.3 | 1.0 | n.a. | ||
| 10 | FR | BLDd | 0.4±0.0 | |||
| FFPE | 1.0 | 1.4 | 1.4 | n.a. | ||
| 11 | FR | BLDd | BLDe | |||
| FFPE | 0.3±0.0 | 6.5 | 2.1 | n.a. | ||
| 12 | FR | 2.3/8.8 | n.a. | |||
| FFPE-A | 2.6±0.1 | 3.0 | 2.2 | 2004 | ||
| FFPE-B | 3.9±0.0 | 3.8 | 2.2 | 2004 | ||
| 13 | FR | 2.7 | n.a. | |||
| FFPE-A | 2.2±0.0 | 3.9 | 1.3 | 2008 | ||
| FFPE-B | 1.9±0.0 | 5.3 | 2.0 | 2008 | ||
| 14 | FR | BLDd | n.a. | |||
| FFPE-A | 0.3±0.0 | 4.2 | 1.4 | 2008 | ||
| FFPE-B | 0.2±0.0 | 4.2 | 1.8 | 2008 | ||
| 15 | FR | 4.3/8.8 | n.a. | |||
| FFPE-A | 1.6±0.2 | 5.7 | 2.3 | 2008 | ||
| FFPE-B | 1.5±0.1 | 7.2 | 1.7 | 2008 | ||
BLD, Below limit of detection; FR, freshly frozen tissues.
aMicrogram DNA per two 10 µm section cuts.
bSurface area FFPE tissues in paraffin block.
cn.a., not analyzed or year of procurement of FFPE tissue is unknown.
dBLD (1 adduct per 108 DNA bases using 32P-postlabeling method).
eBLD (1 adduct per 109 DNA bases in UPLC-MS/MS3).
Fig. 4.
UPLC-ESI/MS3 reconstructed ion chromatograms and product ion spectra of dA-AL-I at the MS3 scan stage in human freshly frozen and FFPE kidney DNA spiked with [15N5]-dA-AL-I at a level of 5 adducts per 108 DNA bases (b, d, f and h). The chromatograms were reconstructed with dA-AL-I ( m/z 292, 293, 412) and [15N5]-dA-AL-I ( m/z 292, 293, 417). The chromatograms of freshly frozen kidney tissues (a and e) and their matching FFPE kidney tissues (c and g) are shown. Subject 9 harbored dA-AL-l at <0.3 adducts per 108 bases and subject 6 contained dA-AL-I at a level ranging between 5.0 and 6.5 adducts per 108 bases. Product ion spectra were acquired for dA-AL-I in frozen (i) and FFPE tissue (k), and for the internal standard [15N5]-dA-AL-I (j, l). The structure of the aglycone [BH2]+ adduct of dA-AL-I and proposed mechanism of fragmentation are presented.
Discussion
IHC has been the technique of choice to screen for DNA adducts in FFPE tissues for the past two decades (5–7). However, this method has several limitations. The relative lack of specificity of antibodies for DNA adducts may lead to errors in identification and quantification and antibodies may cross-react with other DNA lesions. Moreover, relatively harsh conditions involving exposure of DNA to acidic or alkaline pH conditions, which, in IHC, are employed to denature DNA and to increase the access of antibodies to the adduct and certain DNA adducts may be unstable under these conditions. Lastly, the development and characterization of antibodies is time consuming and costly, and the panel of antibodies currently available for screening adducts is limited.
A quantitative mass spectrometric method has been long sought to examine, in FFPE tissues, human exposure to chemicals that damage DNA. The DNA retrieval methodology reported here can be used to measure dA-AL-I adducts in rodent tissues fixed for up to 7 days in formalin, yielding values similar to those of DNA adducts in matched freshly frozen tissues. dA-AL-I adducts present in human kidney tissues fixed with formalin and embedded in paraffin for up to 9 years are recovered in high yield and their levels are comparable with those of dA-AL-I adducts in matching freshly frozen tissues. The employment of stable, isotopically labeled internal standards, combined with the excellent sensitivity of the ion trap MS at the MS3 scan stage, permits precise measurement of the dA-AL-I adduct in human FFPE kidney at a limit of quantitation value of 3 adducts per 109 DNA bases using only 2 µg of DNA; a quantity of DNA recovered from two tissue section cuts of FFPE blocks of 10 µm thickness. Moreover, the high-quality product-ion spectrum obtained from the aglycone adduct at the MS3 scan stage, provides extensive spectral data to corroborate the identity of dA-AL-I.
Interpretations of the biological significance of DNA adducts in human tissue samples should be made with caution. Tumor formation is a multistage process that occurs over a period of years. Thus, the measurement of DNA adducts in patients with a clinical diagnosis of cancer may not accurately reflect levels of chemicals that initiated the process of malignant tumor formation. However, tobacco smoking, diet, and environmental pollution represent long-term exposures, and levels of adducts derived from such exposures are likely to correlate with those that existed during tumor progression. Furthermore, AA is atypical among environmental carcinogens which form adducts that are effectively repaired inasmuch as the dA-AL-I adduct is highly resistant to global genomic repair, evidenced by its detection decades after exposure has ceased (18,24). Thus, measurements of dA-AL-I in FFPE kidney likely represents long-term accumulation of adducts following exposure to AA-I.
DNA retrieved from FFPE tissue can be used both to biomonitor DNA adducts of AA-I and to amplify genes with high fidelity. Massively parallel sequencing methods routinely shear input DNA to 200–300bp in length. Even after 1 week in formalin, the recovered DNA exceeds this size and can be readily amplified to yield high-quality templates for DNA sequencing. Our previous studies have shown that sequencing of the TP53 gene in urothelial carcinomas of patients with AA-induced urothelial carcinomas of the upper urinary tract reveals characteristic A:T → T:A transversions (10,11,15). Thus, the ability to screen for both DNA adducts and mutations in FFPE tissues may provide clues about the origin of cancers induced by environmental carcinogens.
In conclusion, the methodology reported here enhances significantly the value of AL-DNA adduct analysis as a primary biomarker of exposure in studies of AA-induced upper tract urothelial carcinoma. Although for centuries, Aristolochia herbs have been used for medicinal purposes throughout the world, recognition that AA can cause urothelial cancer and nephropathy is relatively recent (12). The ability to quantify AL-DNA adducts in archived, FFPE specimens makes it possible to identify conveniently individuals and populations at risk (10). Currently, we are exploring the usefulness of DNA retrieved from FFPE for the identification, by mass spectrometry, of DNA adducts formed by other environmental carcinogens in human tissues.
Supplementary material
Supplementary Tables S1 and S2 and Figures S1–S3 can be found at http://carcin.oxfordjournals.org/
Funding
National Institute of Environmental Health Sciences (R01 ES019564 to R.J.T., ES04068 to A.P.G.); Henry and Marsha Laufer (A.P.G.); Ministry of Science of Republic of Croatia (108-0000000-0329); Croatian Foundation for Science (04/38 to B.J.). T.A.R. was the recipient of a Zickler Translational Research Scholar Award funded by the Zickler Family Foundation.
Conflict of Interest Statement: The authors declare no competing financial interest.
Supplementary Material
Glossary
Abbreviations:
- AA-I
aristolochic acid I
- AL
aristolactam
- dA-AL-I
7-(deoxyadenosin-N 6-yl) aristolactam I
- FFPE
formalin-fixed paraffin-embedded
- IHC
immunohistochemistry
- UPLC-ESI-MS/MSn
ultraperformance liquid chromatography-electrospray ionization-multistage scan mass spectrometry.
References
- 1. Himmelstein M.W., et al. (2009). Creating context for the use of DNA adduct data in cancer risk assessment: II. Overview of methods of identification and quantitation of DNA damage. Crit. Rev. Toxicol., 39, 679–694 [DOI] [PubMed] [Google Scholar]
- 2. Jarabek A.M., et al. (2009). Creating context for the use of DNA adduct data in cancer risk assessment: I. Data organization. Crit. Rev. Toxicol., 39, 659–678 [DOI] [PubMed] [Google Scholar]
- 3. Loeb L.A., et al. (2008). Advances in chemical carcinogenesis: a historical review and prospective. Cancer Res., 68, 6863–6872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nirmalan N.J., et al. (2008). Mining the archival formalin-fixed paraffin-embedded tissue proteome: opportunities and challenges. Mol. Biosyst., 4, 712–720 [DOI] [PubMed] [Google Scholar]
- 5. Santella R.M. (1999). Immunological methods for detection of carcinogen-DNA damage in humans. Cancer Epidemiol. Biomarkers Prev., 8, 733–739 [PubMed] [Google Scholar]
- 6. Poirier M.C., et al. (2000). Carcinogen macromolecular adducts and their measurement. Carcinogenesis, 21, 353–359 [DOI] [PubMed] [Google Scholar]
- 7. Pratt M.M., et al. (2011). Polycyclic aromatic hydrocarbon (PAH) exposure and DNA adduct semi-quantitation in archived human tissues. Int. J. Environ. Res. Public Health, 8, 2675–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tretyakova N., et al. (2012). Quantitation of DNA adducts by stable isotope dilution mass spectrometry. Chem. Res. Toxicol., 25, 2007–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yun B.H., et al. (2013). Human formalin-fixed paraffin-embedded tissues: an untapped specimen for biomonitoring of carcinogen DNA adducts by mass spectrometry. Anal. Chem., 85, 4251–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grollman A.P. (2013). Aristolochic acid nephropathy: Harbinger of a global iatrogenic disease. Environ. Mol. Mutagen., 54, 1–7 [DOI] [PubMed] [Google Scholar]
- 11. Grollman A.P., et al. (2007). Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc. Natl Acad. Sci. USA, 104, 12129–12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nortier J.L., et al. (2000). Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N. Engl. J. Med., 342, 1686–1692 [DOI] [PubMed] [Google Scholar]
- 13. Pfau W., et al. (1990). Aristolochic acid binds covalently to the exocyclic amino group of purine nucleotides in DNA. Carcinogenesis, 11, 313–319 [DOI] [PubMed] [Google Scholar]
- 14. Moriya M., et al. (2011). TP53 mutational signature for aristolochic acid: an environmental carcinogen. Int. J. Cancer, 129, 1532–1536 [DOI] [PubMed] [Google Scholar]
- 15. Chen C.H., et al. (2012). Aristolochic acid-associated urothelial cancer in Taiwan. Proc. Natl Acad. Sci. USA, 109, 8241–8246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yun B.H., et al. (2012). Biomonitoring of aristolactam-DNA adducts in human tissues using ultra-performance liquid chromatography/ion-trap mass spectrometry. Chem. Res. Toxicol., 25, 1119–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gilbert M.T., et al. (2007). The isolation of nucleic acids from fixed, paraffin-embedded tissues-which methods are useful when? PLoS ONE, 2, e537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jelakovic B., et al. (2012). Aristolactam-DNA adducts are a biomarker of environmental exposure to aristolochic acid. Kidney Int., 81, 559–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dong H., et al. (2006). Quantitative determination of aristolochic acid-derived DNA adducts in rats using 32P-postlabeling/polyacrylamide gel electrophoresis analysis. Drug Metab. Dispos., 34, 1122–1127 [DOI] [PubMed] [Google Scholar]
- 20. Turashvili G., et al. (2012). Nucleic acid quantity and quality from paraffin blocks: defining optimal fixation, processing and DNA/RNA extraction techniques. Exp. Mol. Pathol., 92, 33–43 [DOI] [PubMed] [Google Scholar]
- 21. Dubeau L., et al. (1986). Southern blot analysis of DNA extracted from formalin-fixed pathology specimens. Cancer Res., 46, 2964–2969 [PubMed] [Google Scholar]
- 22. Maniatis T, et al. (1982). Molecular Cloning - A Laboratory Manual. Cold Spring Harbor Laboratory Press, Plainview, NY [Google Scholar]
- 23. Zimmermann J., et al. (2008). DNA damage in preserved specimens and tissue samples: a molecular assessment. Front. Zool., 5, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sidorenko V.S., et al. (2012). Lack of recognition by global-genome nucleotide excision repair accounts for the high mutagenicity and persistence of aristolactam-DNA adducts. Nucleic Acids Res., 40, 2494–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.