Summary
In the large-scale analysis of GWAS-identified risk variants for other cancers on endometrial cancer risk, a SNP near TET2 gene, rs7679673, previously associated with prostate and breast cancer risk demonstrated a robust association with endometrial cancer (P = 7.37 × 10− 5).
Abstract
Genome-wide association studies (GWAS) have identified a large number of cancer-associated single nucleotide polymorphisms (SNPs), several of which have been associated with multiple cancer sites suggesting pleiotropic effects and shared biological mechanisms across some cancers. We hypothesized that SNPs associated with other cancers may be additionally associated with endometrial cancer. We examined 213 SNPs previously associated with 14 other cancers for their associations with endometrial cancer in 3758 endometrial cancer cases and 5966 controls of European ancestry from two consortia: Population Architecture Using Genomics and Epidemiology and the Epidemiology of Endometrial Cancer Consortium. Study-specific logistic regression estimates adjusted for age, body mass index and the most significant principal components of genetic ancestry were combined using fixed-effect meta-analysis to evaluate the association between each SNP and endometrial cancer risk. A Bonferroni-corrected P value of 2.35×10−4 was used to determine statistical significance of the associations. SNP rs7679673, ~6.3kb upstream of TET2 and previously reported to be associated with prostate cancer risk, was associated with endometrial cancer risk in the direction opposite to that for prostate cancer [meta-analysis odds ratio = 0.87 (per copy of the C allele), 95% confidence interval = 0.81, 0.93; P = 7.37×10−5] with no evidence of heterogeneity across studies (P heterogeneity = 0.66). This pleiotropic analysis is the first to suggest TET2 as a susceptibility locus for endometrial cancer.
Introduction
Endometrial cancer is the most common gynecologic cancer, with >52 600 new cases expected in the USA in 2014 (1). Although survival is favorable, with a survival rate similar to that of breast cancer, >8500 women are estimated to die of this disease in 2014 (1). Three genome-wide association studies (GWAS) of endometrial cancer (2–4) have been conducted to date with only one identifying a novel genome-wide significant risk variant for endometrial cancer, namely, rs4430796 at the 17q12 (HNF1B) locus (3).
GWAS have successfully identified a large number of susceptibility loci for various cancers. Among the many risk loci identified, several have been associated with multiple cancer sites supporting the existence of carcinogenic pleiotropy (5). For example, HNF1B at 17q12 has been identified as a susceptibility locus for endometrial, prostate and ovarian cancers (3,6–8). Loci in the 8q24 region (9) and a locus on chromosome 5 (5p15.33) that includes the telomerase reverse transcriptase (TERT) gene (10) have been associated with multiple cancer sites. Despite these striking examples, pleiotropic effects have not been systematically explored in endometrial cancer. Evidence of carcinogenic pleiotropy can improve our understanding of disease etiology by identifying shared molecular components underlying disease risk and by validating the pathogenicity of variants at a locus (11).
In this study, we examined variants identified by GWAS for 14 other cancers for their association with endometrial cancer in a large-scale analysis of cases and controls from nine studies in two consortia: the National Human Genome Research Institute (NHGRI) Population Architecture Using Genomics and Epidemiology (PAGE) (12) and the Epidemiology of Endometrial Cancer Consortium (E2C2) (13).
Materials and methods
Study population
Two consortia contributed data to this meta-analysis study: PAGE (6,12) and E2C2 (13,14). Due to a limited number of cases of non-European descent in the contributing studies, the current analysis was restricted to women of European descent and included 3758 primary incident invasive endometrial cancer cases and 5966 controls who were free of endometrial cancer and did not have a history of hysterectomy (Table I). Contributing PAGE studies included: Multiethnic Cohort (MEC) (15); Women’s Health Initiative (WHI) (16) and Epidemiologic Architecture for Genes Linked to Environment (EAGLE), which accesses the Vanderbilt University biorepository (BioVU) (17). Participating E2C2 studies included: Connecticut Endometrial Cancer Study (CECS) (18); Fred Hutchinson Cancer Research Center case-control study (FHCRC) (19); Polish Endometrial Cancer Case-Control Study (PECS); MEC; California Teachers’ Study (CTS) (20); Nurses’ Health Study (NHS) (21) and the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) (22,23). All studies are from the USA, except for the Polish study. While MEC participates in both PAGE and E2C2, only MEC data as part of E2C2 were used. Details of these studies have been published elsewhere (6,14). Study design characteristics for each study (case–control definitions and matching factors) are summarized in the Supplementary Material, available at Carcinogenesis Online. Institutional review board approval was obtained for all studies.
Table I.
Study population characteristics by study
| Study name | Study design | Location | Study period | Number of cases/controls | Mean agea (years) | Mean BMI (kg/m2) | ||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |||||
| Connecticut Endometrial Cancer Study (CECS) | Case–control | Connecticut, USA | 2004–2009 | 477/567 | 60.6 | 61.9 | 32.5 | 26.6 |
| California Teachers Study (CTS) | Cohort | California, USA | 1995–2004 | 295/285 | 65.3 | 66.7 | 27.0 | 25.2 |
| Epidemiologic Architecture for Genes Linked to Environment (Eagle-BioVU) | Case–control | Tennesse, USA | 2007 – | 20/156 | 88.1 | 89.0 | 25.8 | 25.5 |
| Fred Hutchinson Cancer Center (FHCRC) | Case–control | Washington, USA | 1994–2005 | 697/693 | 59.7 | 59.2 | 30.2 | 25.6 |
| Multiethnic Cohort (MEC) | Cohort | Hawaii/California, USA | 1993–2008 | 100/199 | 65.4 | 64.5 | 28.8 | 25.5 |
| Nurses’ Health Study (NHS) | Cohort | 11 states, USA | 1976–2008 | 396/348 | 62.4 | 63.0 | 28.6 | 26.3 |
| Polish Endometrial Cancer Case-Control Study (PECS) | Case–control | Poland | 2001–2003 | 459/558 | 60.9 | 55.6 | 28.6 | 26.4 |
| Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) | Cohort | USA | 1993–2008 | 446/123 | 67.9 | 62.7 | 29.3 | 26.9 |
| Women’s Health Initiative (WHI) | Cohort | USA | 1991–2009 | 868/3037 | 63.9 | 65.2 | 28.9 | 27.3 |
BMI, body mass index.
aAge at diagnosis for cases and at reference date for controls.
SNP selection and genotyping
Single nucleotide polymorphisms (SNPs) previously associated with 14 cancers other than endometrial cancer were identified by PAGE researchers from the NHGRI GWAS catalog (P < 5.0 × 10− 5) as of January 2010 (24,25) and review of more recent cancer GWAS and fine mapping literature. These SNPs were selected irrespective of racial/ethnic composition of the initial published GWAS. Out of >300 SNPs identified, 213 SNPs have been genotyped and passed quality control in at least two studies across PAGE and E2C2 (Supplementary Table 1, available at Carcinogenesis Online). In PAGE, these SNPs were genotyped using a custom panel for each study (26). In E2C2, genotype data were abstracted from previously generated stage 1 GWAS data (2).
To control for potential bias due to population stratification, each PAGE study genotyped 128 ancestry informative markers that capture the major continental genetic diversity (European, East Asian, Amerindian, African, South Asian, Mexican and Puerto Rican) (27). Principal components of genetic ancestry were estimated from these markers by EIGENSTRAT (28) and included in regression models as an estimate of genetic ancestry. In E2C2, principal components of genetic ancestry were derived from the GWAS data using EIGENSTRAT (28). We did not exclude individuals based on principal component-based ancestry in the PAGE. In the E2C2 studies, we excluded 146 participants due to non-European principal component-based ancestry (2).
Standard quality assurance and quality control measures were utilized to ensure genotyping quality. In PAGE, samples and SNPs were excluded based on call rates (<90%), concordance of blinded replicates (≤98%) and departure from Hardy–Weinberg equilibrium (P < 0.001). Each PAGE laboratory also genotyped 360 HapMap samples to serve as cross-laboratory and cross-platform quality control samples (12). In E2C2, samples were excluded based on call rates (<90%), unexpected duplicates, heterozygosity, departure from Hardy–Weinberg equilibrium (P < 0.0001), minor allele frequencies (<1%) and outlying the CEU HapMap2 cluster in principal component analysis (as only participants of European descent were included) (2). The majority of the 213 SNPs of interest were available across most studies (89% of the SNPs were genotyped in at least six studies; Supplementary Table 1, available at Carcinogenesis Online).
Statistical analyses
For each study, the association between each SNP and endometrial cancer was estimated using unconditional logistic regression. The modeled allele was the ‘risk’ allele for each SNP as defined as the allele associated with an increased risk of cancer in prior publications. For SNPs associated with multiple cancer sites, the first reported GWAS was used in assigning the risk allele. SNPs were coded additively with 0, 1, 2 referring to the number of risk alleles. Models were adjusted for age (years), body mass index [kg/m2 obtained from self-report at time of diagnosis for cases and interview for controls, or for most cohort (nested case–control) studies at baseline assessment] and the most relevant principal components of genetic ancestry to account for population substructure for each study. Log odds regression estimates were combined across studies using inverse-variance weighted, fixed-effect meta-analysis as implemented in METAL (29). Heterogeneity P values were estimated based on Cochran’s Q statistics. A Bonferroni-corrected P = 2.35 × 10− 4 (nominal alpha/number of SNPs tested = 0.05/213) was used to determine the statistical significance of the association for each SNP with endometrial cancer.
Results
The study population characteristics are shown in Table I. The number of endometrial cases ranged from 20 in the BioVU to 868 in the WHI. The average age varied across studies, but the majority of women were older than 55 years. On average, cases had higher body mass index than controls.
A total of 213 risk variants for 14 cancers other than endometrial cancer were tested in 3758 cases and 5966 controls from a total of nine studies in the two consortia (Figure 1; Supplementary Table 1, available at Carcinogenesis Online). Fourteen variants were nominally associated with endometrial cancer at P < 0.05 (Table II). These 14 variants were previously associated with breast cancer (5 SNPs), prostate cancer (3 SNPs), pancreatic cancer (2 SNPs), testicular cancer (1 SNP), colorectal cancer (1 SNP), lung cancer (1 SNP) and esophageal cancer (1 SNP). Three of the breast cancer-associated variants in FGFR2 are in linkage disequilibrium (r 2 ≥ 0.92 in HapMap CEU) and thus may not represent independent results.
Fig. 1.
Manhattan plot of the meta-analysis association between risk variants of 14 other cancers and endometrial cancer. The solid line is the Bonferroni-corrected significance threshold (0.05/213 = 2.35×10− 4). Each association result is color coded according to the cancer for which the SNP was originally reported and positioned on the x-axis according to its genomic position.
Table II.
Meta-analysis association results between the top 14 SNPs (P < 0.05) and endometrial cancer
| SNP | Region | Position (bp) | Locus | Risk Allelea | Other Allele | Number of studiesb | OR per allelec (95% CI) | P value | P heterogeneity | Initial GWAS (reference) |
|---|---|---|---|---|---|---|---|---|---|---|
| rs10936599 | 3q26.2 | 169492101 | MYNN | C | T | 7 | 1.10 (1.00, 1.20) | 0.042 | 0.52 | Colorectal cancer (34) |
| rs7679673 | 4q24 | 106061534 | TET2 | C | A | 8 | 0.87 (0.81, 0.93) | 7.37×10−5 | 0.66 | Prostate cancer (30) |
| rs889312 | 5q11.2 | 56031884 | MAP3K1 | C | A | 9 | 0.93 (0.87, 1.00) | 0.048 | 0.02 | Breast cancer (35) |
| rs4624820 | 5q31.3 | 141681788 | SPRY4 | G | A | 8 | 1.06 (1.00, 1.14) | 0.049 | 0.12 | Testicular cancer (36) |
| rs763780 | 6p12.2 | 52101739 | IL17F | G | A | 6 | 0.76 (0.62, 0.94) | 0.012 | 0.73 | Pancreatic cancer (37) |
| rs9502893 | 6p25.3 | 1340189 | FOXQ1 | G | A | 7 | 1.10 (1.01, 1.19) | 0.023 | 0.32 | Pancreatic cancer (38) |
| rs2981582 | 10q26.13 | 123352317 | FGFR2 | A | G | 8 | 0.90 (0.84, 0.97) | 0.005 | 0.07 | Breast cancer (35) |
| rs1219648 | 10q26.13 | 123346190 | FGFR2 | G | A | 8 | 0.92 (0.87, 0.99) | 0.024 | 0.02 | Breast cancer (39) |
| rs2981579 | 10q26.13 | 123337335 | FGFR2 | T | C | 8 | 0.93 (0.87, 0.99) | 0.032 | 0.02 | Breast cancer (40) |
| rs7127900 | 11p15.5 | 2233574 | IGF2, IGF2AS, INS, TH | A | G | 8 | 0.91 (0.84, 0.98) | 0.021 | 0.60 | Prostate cancer (30) |
| rs9600079 | 13q22.1 | 73728139 | None | T | G | 7 | 0.91 (0.84, 0.99) | 0.027 | 0.08 | Prostate cancer (41) |
| rs1978503 | 18q21.2 | 53664282 | None | A | G | 9 | 0.88 (0.81, 0.95) | 0.002 | 0.55 | Breast cancer (42) |
| rs16951095 | 18p11.31 | 7042911 | LAMA1 | C | T | 6 | 0.78 (0.94, 0.66) | 0.011 | 0.08 | Lung cancer (43) |
| rs738722 | 22q12.1 | 29130012 | CHEK2 | T | C | 7 | 1.10 (1.01, 1.20) | 0.037 | 0.71 | Esophageal cancer (44) |
CI, confidence interval; OR, odds ratio.
aAllele associated with increased risk as indicated in the first published GWAS paper.
bNumber of studies with association result.
cAdjusted for age, body mass index, top principal components for each study and calculated using fixed effects model.
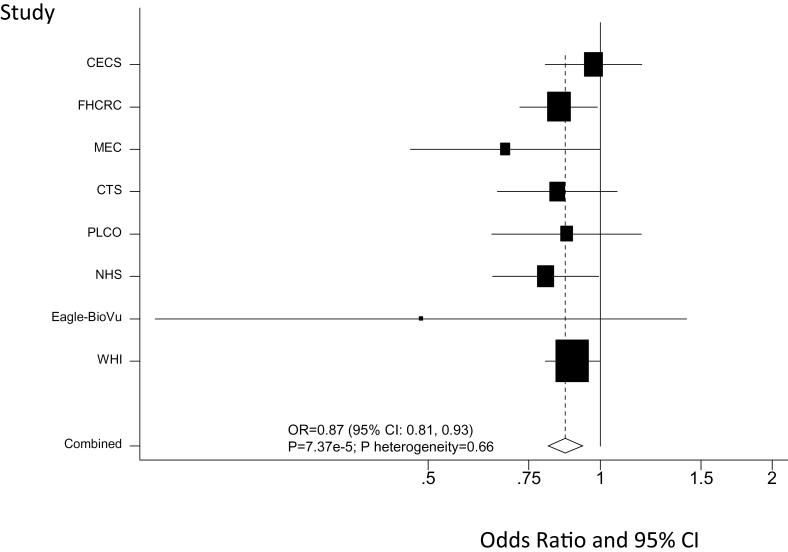
One SNP (rs7679673) in the 4q24 region (~6kb upstream of TET2) previously reported to be associated with prostate cancer risk (30) demonstrated a statistically significant association with endometrial cancer risk in the direction opposite to that for prostate cancer [overall meta-analysis odds ratio = 0.87 (per copy of the C allele), 95% confidence interval = 0.81, 0.93; P = 7.37 × 10− 5]. This SNP surpassed our conservative Bonferroni-corrected criterion of significance (P < 2.35 × 10− 4) and showed a consistency in the results across studies (P heterogeneity = 0.66) (Figure 2). A nearby breast cancer risk variant, rs9790517 (31), located ~23 kb from rs7679673 (r 2 = 0.42 in CEU samples), was not statistically significantly associated with endometrial cancer risk (per copy of the T allele: odds ratio = 1.05; 95% confidence interval: 0.95, 1.17).
Fig. 2.
Forest plot of the association between rs7679673 near the TET2 gene region 4q24 and endometrial cancer risk. Study-specific and meta-analysis associations are plotted, modeling the C risk allele for prostate cancer.
Discussion
We conducted a large meta-analysis among women of European ancestry to investigate pleiotropic effects of GWAS-identified risk variants for other cancers on endometrial cancer risk. To our knowledge, this is the first systematic analysis for pleiotropic associations in endometrial cancer. We found that a SNP at chromosome 4q24, rs7679673, previously associated with prostate cancer risk demonstrated a robust association with endometrial cancer risk, using a conservative criterion for statistical significance. This SNP resides ~6.3kb from the transcription start site of TET2.
The TET2 gene encodes a methylcytosine dioxygenase involved in myelopoiesis. This gene has been characterized as a tumor suppressor gene involved in pathogenesis of acute myeloid leukemia, myelodysplastic syndrome and myeloproliferative neoplasms (32). The TET2 region has previously been identified as a risk locus for both prostate and breast cancer (30,31). The risk SNPs in the TET2 region, rs7679673 and rs9790517 (r 2 = 0.42), were genotyped in our study, but only rs7679673 was statistically significantly associated with endometrial cancer risk. The C allele of rs7679673 was associated with a 13% decreased risk of endometrial cancer, which is in the opposite direction as seen for prostate cancer (30). Currently, there is no direct information about the function of rs7679673. The T allele of an intronic SNP rs9790517 has been associated with a 5% increased risk of breast cancer (31). We noted the same direction and magnitude of effect with the T allele of rs9790517 although the results were not statistically significant. Despite the opposite direction of association in endometrial and prostate cancer, our results suggest a shared pathway between these two cancers and possibly breast cancer. The different directions of association between cancer sites may be due to linkage disequilibrium with two different functional SNPs that have different effects in the different tissues, or context-specific differences in regulation of nearby genes, just as transcription factors can serve as both oncogenes and tumor suppressors (33). The underlying biological mechanism for which TET2 may influence carcinogenesis remains to be elucidated.
The major strength of our study is the large number of subjects from well-designed endometrial cancer studies. The limitation of this study is that our analysis was based on individual SNPs from each/most loci and thus we did not have broader coverage of the area. As more recent GWAS have identified many new cancer risk loci, these SNPs remain to be examined for their pleiotropic effects with endometrial cancer. The statistical power to detect an association for the 213 SNPs varied; nonetheless, 89% of the SNPs were genotyped in more than two-third of the studies. We identified 14 variants were nominally associated with endometrial cancer at P < 0.05 which was more than the ~11 associations expected by chance (213 SNPs × 0.05 = 10.7). The small numbers of non-European ancestry women in the available studies precluded the possibility of exploring generalizability across race/ethnicity. Finally, because the large majority of cases in this study were type 1 tumors (i.e. endometrioid adenocarcinomas), our results apply mainly to these tumors.
In summary, our cross-cancer pleiotropy analysis suggested a possible role of TET2 in endometrial cancer susceptibility. Further replication of our results and research into the biological mechanisms by which inherited differences in pleiotropic cancer risk loci influence endometrial cancer will expand our understanding of the key contributors to endometrial cancer development.
Supplementary material
Supplementary Table 1 can be found at http://carcin.oxfordjournals.org/
Funding
PAGE: The PAGE program is funded by the National Human Genome Research Institute (NHGRI) , supported by U01HG004803 (CALiCo), U01HG004798 (EAGLE), U01HG004802 (MEC), U01HG004790 (WHI) and U01HG0s04801 (Coordinating Center), and their respective NHGRI ARRA supplements. The complete list of PAGE members can be found at http://www.pagestudy.org. EAGLE-BioVU: The ‘Epidemiologic Architecture for Genes Linked to Environment (EAGLE)’ is funded through the NHGRI PAGE program (U01HG004798 and its NHGRI ARRA supplement). The dataset(s) used for the analyses described were obtained from Vanderbilt University Medical Center’s BioVU which is supported by institutional funding and by the Vanderbilt CTSA grant UL1 TR000445 from NCATS/NIH. The Vanderbilt University Center for Human Genetics Research, Computational Genomics Core provided computational and/or analytical support for this work. MEC: The MEC characterization of epidemiological architecture is funded through the NHGRI PAGE program (U01HG004802 and its NHGRI ARRA supplement). The MEC study is funded through the National Cancer Institute (R37CA54281, R01 CA082838). WHI: Funding support for the ‘Epidemiology of putative genetic variants: The Women’s Health Initiative’ study is provided through the NHGRI PAGE program (U01HG004790). The WHI program is funded by the National Heart, Lung and Blood Institute; NIH and U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 32115, 32118–32119, 32122, 42107–26, 42129–32 and 44221. CTS: This research was supported by grants R01 CA082838, R01 CA91019 and R01 CA77398 from the National Cancer Institute (NCI). The collection of cancer incidence data was supported by the California Department of Public Health and the NCI Surveillance, Epidemiology and End Results Program (SEER) as part of the statewide cancer reporting program. FHCRC: This work was supported by R01 CA082838, R35 CA39779, R01 CA75977, R03 CA80636, N01 HD 2 3166, K05 CA 92002, R01 CA 105212, R01 CA87538 and funds from the Fred Hutchinson Cancer Research Center. NHS: This work was supported by the National Institutes of Health (R01 CA134958, R01CA082838). The NHS was supported by the National Institutes of Health (P01 CA87969, R01 CA49449). We would like to thank the participants and staff of the Nurses’ Health Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. CECS: NIH R01 CA098346 and R01 CA082838. The cooperation of 28 Connecticut hospitals in the Connecticut Endometrial Cancer Study, including Charlotte Hungerford Hospital, Bridgeport Hospital, Danbury Hospital, Hartford Hospital, Middlesex Hospital, New Britain General Hospital, Bradley Memorial Hospital, Yale/New Haven Hospital, St. Francis Hospital and Medical Center, St. Mary’s Hospital, Hospital of St. Raphael, St. Vincent’s Medical Center, Stamford Hospital, William W. Backus Hospital, Windham Hospital, Eastern Connecticut Health Network, Griffin Hospital, Bristol Hospital, Johnson Memorial Hospital, Day Kimball Hospital, Greenwich Hospital, Lawrence and Memorial Hospital, Milford Hospital, New Milford Hospital, Norwalk Hospital, MidState Medical Center, John Dempsey Hospital and Waterbury Hospital, in allowing patient access, is gratefully acknowledged. PECS: This study is supported by the Intramural Research Program of the NCI. PLCO: This study is supported by the Extramural and the Intramural Research Programs of the NCI.
Supplementary Material
Acknowledgements
The studies would like to thank all participants, staff, physicians and investigators for making this project possible. We thank Ms P.Wan at University of Southern California for her assistance in the analysis. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- E2C2
Epidemiology of Endometrial Cancer Consortium
- GWAS
genome-wide association studies
- MEC
Multiethnic Cohort
- PAGE
Population Architecture Using Genomics and Epidemiology
- SNP
single nucleotide polymorphism.
References
- 1.American Cancer Society. (2014). Cancer Facts & Figures 2014. American Cancer Society, Atlanta, GA [Google Scholar]
- 2. De Vivo I., et al. Australian National Endometrial Cancer Study Group. (2014). Genome-wide association study of endometrial cancer in E2C2. Hum. Genet., 133, 211–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spurdle A.B., et al. Australian National Endometrial Cancer Study Group; National Study of Endometrial Cancer Genetics Group. (2011). Genome-wide association study identifies a common variant associated with risk of endometrial cancer. Nat. Genet., 43, 451–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Long J., et al. (2012). Genome-wide association study identifies a possible susceptibility locus for endometrial cancer. Cancer Epidemiol. Biomarkers Prev., 21, 980–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sakoda L.C., et al. (2013). Turning of COGS moves forward findings for hormonally mediated cancers. Nat. Genet., 45, 345–348 [DOI] [PubMed] [Google Scholar]
- 6. Setiawan V.W., et al. (2012). HNF1B and endometrial cancer risk: results from the PAGE study. PLoS One, 7, e30390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elliott K.S., et al. Australian Melanoma Family Study Investigators; PanScan Consortium. (2010). Evaluation of association of HNF1B variants with diverse cancers: collaborative analysis of data from 19 genome-wide association studies. PLoS One, 5, e10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Permuth-Wey J., et al. Australian Cancer Study; Australian Ovarian Cancer Study; Consortium of Investigators of Modifiers of BRCA1/2. (2013). Identification and molecular characterization of a new ovarian cancer susceptibility locus at 17q21.31. Nat. Commun., 4, 1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghoussaini M., et al. UK Genetic Prostate Cancer Study Collaborators/British Association of Urological Surgeons’ Section of Oncology; UK ProtecT Study Collaborators. (2008). Multiple loci with different cancer specificities within the 8q24 gene desert. J. Natl Cancer Inst., 100, 962–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mocellin S., et al. (2012). Telomerase reverse transcriptase locus polymorphisms and cancer risk: a field synopsis and meta-analysis. J. Natl Cancer Inst., 104, 840–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sivakumaran S., et al. (2011). Abundant pleiotropy in human complex diseases and traits. Am. J. Hum. Genet., 89, 607–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matise T.C., et al. PAGE Study. (2011). The Next PAGE in understanding complex traits: design for the analysis of Population Architecture Using Genetics and Epidemiology (PAGE) Study. Am. J. Epidemiol., 174, 849–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olson S.H., et al. (2009). Maximizing resources to study an uncommon cancer: E2C2–Epidemiology of Endometrial Cancer Consortium. Cancer Causes Control, 20, 491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Setiawan V.W., et al. (2009). Two estrogen-related variants in CYP19A1 and endometrial cancer risk: a pooled analysis in the Epidemiology of Endometrial Cancer Consortium. Cancer Epidemiol. Biomarkers Prev., 18, 242–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kolonel L.N., et al. (2000). A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am. J. Epidemiol., 151, 346–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Women’s Health Initiative Study Group. (1998). Design of the Women’s Health Initiative clinical trial and observational study. Control Clin. Trials, 19, 61–109 [DOI] [PubMed] [Google Scholar]
- 17. Bush W.S., et al. (2013). Enabling high-throughput genotype-phenotype associations in the epidemiologic architecture for genes linked to environment (eagle) project as part of the population architecture using genomics and epidemiology (page) study. Pac. Symp. Biocomput., 373–384 [PMC free article] [PubMed] [Google Scholar]
- 18. Lu L., et al. (2011). Long-term overweight and weight gain in early adulthood in association with risk of endometrial cancer. Int. J. Cancer, 129, 1237–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doherty J.A., et al. (2011). Polymorphisms in nucleotide excision repair genes and endometrial cancer risk. Cancer Epidemiol. Biomarkers Prev., 20, 1873–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee E., et al. (2010). Genetic variation in the progesterone receptor gene and risk of endometrial cancer: a haplotype-based approach. Carcinogenesis, 31, 1392–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Vivo I., et al. (2002). A functional polymorphism in the promoter of the progesterone receptor gene associated with endometrial cancer risk. Proc. Natl Acad. Sci. USA, 99, 12263–12268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prorok P.C., et al. Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Project Team. (2000). Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control. Clin. Trials, 21 (suppl. 6), 273S–309S [DOI] [PubMed] [Google Scholar]
- 23. Gohagan J.K., et al. Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Project Team. (2000). The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control. Clin. Trials, 21 (suppl. 6), 251S–272S [DOI] [PubMed] [Google Scholar]
- 24. Hindorff L.A., et al. (2009). Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc. Natl Acad. Sci. USA, 106, 9362–9367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Welter D., et al. (2014). The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res., 42, D1001–D1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng I., et al. (2014). Pleiotropic effects of genetic risk variants for other cancers on colorectal cancer risk: PAGE, GECCO and CCFR consortia. Gut, 63, 800–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kosoy R., et al. (2009). Ancestry informative marker sets for determining continental origin and admixture proportions in common populations in America. Hum. Mutat., 30, 69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Price A.L., et al. (2006). Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet., 38, 904–909 [DOI] [PubMed] [Google Scholar]
- 29. Willer C.J., et al. (2010). METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics, 26, 2190–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eeles R.A., et al. UK Genetic Prostate Cancer Study Collaborators/British Association of Urological Surgeons’ Section of Oncology; UK ProtecT Study Collaborators; PRACTICAL Consortium. (2009). Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat. Genet., 41, 1116–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Michailidou K., et al. Breast and Ovarian Cancer Susceptibility Collaboration; Hereditary Breast and Ovarian Cancer Research Group Netherlands (HEBON); kConFab Investigators; Australian Ovarian Cancer Study Group; GENICA (Gene Environment Interaction and Breast Cancer in Germany) Network. (2013). Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat. Genet., 45, 353–61, 361e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Delhommeau F., et al. (2009). Mutation in TET2 in myeloid cancers. N. Engl. J. Med., 360, 2289–2301 [DOI] [PubMed] [Google Scholar]
- 33. Rowland B.D., et al. (2005). The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat. Cell Biol., 7, 1074–1082 [DOI] [PubMed] [Google Scholar]
- 34. Houlston R.S., et al. COGENT Consortium; CORGI Consortium; COIN Collaborative Group; COINB Collaborative Group. (2010). Meta-analysis of three genome-wide association studies identifies susceptibility loci for colorectal cancer at 1q41, 3q26.2, 12q13.13 and 20q13.33. Nat. Genet., 42, 973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Easton D.F., et al. SEARCH collaborators; kConFab; AOCS Management Group. (2007). Genome-wide association study identifies novel breast cancer susceptibility loci. Nature, 447, 1087–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rapley E.A., et al. UK Testicular Cancer Collaboration. (2009). A genome-wide association study of testicular germ cell tumor. Nat. Genet., 41, 807–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Innocenti F., et al. (2012). A genome-wide association study of overall survival in pancreatic cancer patients treated with gemcitabine in CALGB 80303. Clin. Cancer Res., 18, 577–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Low S.K., et al. (2010). Genome-wide association study of pancreatic cancer in Japanese population. PLoS One, 5, e11824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hunter D.J., et al. (2007). A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat. Genet., 39, 870–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thomas G., et al. (2009). A multistage genome-wide association study in breast cancer identifies two new risk alleles at 1p11.2 and 14q24.1 (RAD51L1). Nat. Genet., 41, 579–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takata R., et al. (2010). Genome-wide association study identifies five new susceptibility loci for prostate cancer in the Japanese population. Nat. Genet., 42, 751–754 [DOI] [PubMed] [Google Scholar]
- 42. Murabito J.M., et al. (2007). A genome-wide association study of breast and prostate cancer in the NHLBI’s Framingham Heart Study. BMC Med. Genet., 8 (suppl. 1), S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yoon K.A., et al. (2010). A genome-wide association study reveals susceptibility variants for non-small cell lung cancer in the Korean population. Hum. Mol. Genet., 19, 4948–4954 [DOI] [PubMed] [Google Scholar]
- 44. Abnet C.C., et al. (2010). A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat. Genet., 42, 764–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.