Abstract
G-protein mutations are one of the most common mutations occurring in uveal melanoma activating the protein kinase C (PKC)/mitogen-activated protein kinase (MAPK) and phosphoinositide 3-Kinase (PI3K)/AKT pathways. In this study, we described the effect of dual pathway inhibition in uveal melanoma harboring GNAQ and GNA11 mutations via PKC inhibition with AEB071 (Sotrastaurin) and PI3k/AKT inhibition with BYL719, a selective PI3Kα inhibitor. Growth inhibition was observed in GNAQ/GNA11 mutant cells with AEB071 versus no activity in WT cells. In the GNAQ-mutant cells, AEB071 decreased phosphorylation of MARCKS, a substrate of PKC, along with ERK1/2 and ribosomal S6, but persistent AKT activation was present. BYL719 had minimal anti-proliferative activity in all uveal melanoma cell lines, and inhibited phosphorylation of AKT in most cell lines. In the GNA11 mutant cell line, similar effects were observed with ERK1/2 inhibition, mostly inhibited by BYL719. With the combination treatment, both GNAQ and GNA11 mutant cell lines showed synergistic inhibition of cell proliferation and apoptotic cell death. In vivo studies correlated with in vitro findings showing reduced xenograft tumor growth with the combination therapy in a GNAQ mutant model. These findings suggest a new therapy treatment option for G-protein mutant uveal melanoma with a focus on specific targeting of multiple downstream pathways as part of combination therapy.
Keywords: Pi3Kα, PKC, BYL719, AEB071
INTRODUCTION
Uveal melanoma is one of the most common intraocular malignancies of the adult eye, affecting six individual per million per year. Most cases of uveal melanoma metastasize to the liver and have a poor survival rate after initial diagnosis. Few treatment options for this fatal disease exist(1). Chemotherapy is ineffective. There has been recent interest in targeting the mitogen-activated protein kinase (MAPK) pathway (2). MAPK activation is found to be present among 80% of uveal melanoma although it rarely occurs through mutations such as BRAF (3, 4). Activation of MAPK in uveal melanoma can be attributed to mutations in GNA11 or GNAQ, an early event of malignant transformation (5, 6). Collectively these genetic alterations are present in 85% of diagnosed cases (5, 7). These genes encode members of the q class of G-protein alpha-subunits involved in mediating signals between G-protein-coupled receptors (GPCRs) and downstream effectors. Activation is through stimulation of phospholipase C-β (PLCβ), which cleaves phosphatidylinositol (4,5)-bisphosphate(PIP2) to inositol triphosphate (IP3) and diacyl glycerol (DAG), which activates protein kinase C (PKC) further activating the MAPK pathway(2, 8).
To date, most experimental therapies used to treat GNAQ and GNA11 mutant uveal melanoma have focused on the targeting of the MEK/ERK pathway. However, these mutations have been shown to activate other signaling pathways upstream of MEK including protein kinase C (PKC) family members which are involved in cell proliferation and apoptosis. The PKC family is a group of serine/threonine kinases composed of different isoforms which are divided into (1) the classical (or conventional) PKC isoforms α,βI,βII,γ, (2) the novel PKC isoforms δ,ε,η,μ and (3) the atypical PKC isoforms λ and ζ each having different roles and functions in cancer (9). Myristoylated alanine-rich C-kinase substrate (MARCKS) is a major PKC substrate expressed in many different cell types. MARCKS, when phosphorylated by PKC, has such roles as calcium/calmodulin binding and actin-membrane interactions (10–12).
PI3K/AKT signaling pathways are also activated in the setting of G-protein mutations in uveal melanoma (2). There are also signaling interactions involved with GNAQ and some PI3K isoforms (13). The PI3K/AKT pathway has been implicated in uveal melanoma, as well as in many other cancers, to drive cell survival and cell migration (14, 15). PI3K mediates phosphorylation of PIP2 to PIP3 [phosphatidylinositol (3,4,5)-tris-phosphate], which activates AKT to promote tumor growth and proliferation. The PI3Ks are classified as Class 1A and IB. Class1A consist of heterodimers with an inhibitory adaptor unit (p85) as well as a catalytic subunit (p110). There are three known isoforms of the class 1A p110 subunits, which include p110α, p110β and p110δ. Class 1B consists of p110γ and a regulatory subunit, p101 (16). p110α (PI3Kα) has been linked to activation of AKT in cell lines. It has been shown that siRNA knockdown of p110α in C2C12 myoblasts reduced phosphorylation of AKT stimulated by IGF-1 while silencing of p110β did not affect AKT (17). While AKT phosphorylation has been shown to be increased in uveal melanoma (18, 19), it remains to be determined whether a pan-PI3k or AKT inhibitor can be clinically developed in this or other diseases because of concerns for systemic toxicity. Rather, an inhibitor of PI3Kα, by providing isoform specificity for the pathway, may avoid toxicity and off target effects thus far associated with this class of drugs (20). This will be particularly relevant in uveal melanoma where activation of multiple downstream pathways including PI3K/AKT, MEK and PKC drive tumor growth. Combination therapies will ultimately be absolutely essential to maximize effective growth inhibition.
Toward this end, we elected to evaluate dual target inhibition of PI3kα and PKC in GNAQ and GNA11 mutant uveal melanoma cells. It has been previously reported that PKC inhibitors have antiproliferative activity in vitro against uveal melanoma cells carrying GNAQ mutations (21, 22). AEB071 (sotrastaurin) is a potent inhibitor of many forms of PKC shown to have anti-proliferative activity in other cell types as well (23). It is currently being evaluated in a phase I clinical trial for patients with metastatic uveal melanoma (NCT01430416). BYL719 is a next generation PI3Kα specific inhibitor which has been tested in a broad cancer cell line panel (24) and currently under clinical evaluation to asses efficacy in PI3kα driven tumors (25). We now show that the combination of the PKC inhibitor AEB071 with BYL719, a selective PI3kα inhibitor, results in an enhanced anti-tumor effect due to inhibition of both PKC and PI3K/AKT signaling in G-protein mutant uveal melanoma cells in vitro as well as in vivo. These findings would support the clinical development of this drug combination such that both of these pathways are inhibited in patients with uveal melanoma.
MATERIALS AND METHODS
Cell Culture
All cell lines were maintained in RPMI1640 supplemented with heat inactivated 10% Fetal Bovine serum supplemented with 100 units/ml penicillin and 100 ug/ml streptomycin and maintained at 37°C and 5% CO2. 92.1 cells were provided by Dr. William Harbour (Washington University, St. Louis, MO). Omm1.3 and Mel270 have been provided by Dr. Bruce Ksander (Harvard Medical School, Boston, MA). Omm1 was kindly provided by Dr. Boris Bastian (University California of San Francisco, San Francisco, CA). Mel290 was from David Folberg (University of Illinois, Chicago, IL). C918 were obtained from Dr. David H. Abramson (Memorial Sloan Kettering Cancer Center, New York, NY) originally from David Folberg. As previously reported, uveal melanoma cell lines have been sequenced for the presence of activating mutations in codons 209 (exon 5) and 183 (exon 4) of GNAQ and GNA11. Two cell lines had Q209L mutation (92.1, Mel202), whereas Omm1.3 and Mel270 had Q209P mutation (26). Omm1 (GNA11) cell line had a Q209L mutation. A karyotype test was also performed for each cell line in 2012, confirming the authenticity of the cell lines as reported previously (27).
Chemicals
AEB071 (Sotrastaurin) and BYL719 were supplied by Novartis (Switzerland) and dissolved in DMSO at 10mM concentration and stored aliquoted at −20 C.
Cell Viability Assays and Combination Index Analysis
Cells were plated in a 96-well plate and treated with AEB071, BYL719 or DMSO at indicated concentrations for a period of 5 days. Viability was assessed using Cell Counting Kit from Dojindo Molecular Technologies (Rockville, MD, USA) as per manufacturer’s instructions. The Combination Index values were calculated using the CompuSyn software (Combosyn; Paramus, NJ, USA) as developed by Chou TC e.t a.l in 2010 (28). Briefly explained, the plots generated by the CompuSyn software demonstrate the Y-axis combination index values, where CI <1, =1, and >1 indicate synergism, additive effect, and antagonism, respectively. The X-Axis represents the fractional activity, which reflects the fraction of cells inhibited by the treatments relative to vehicle control. For combination index studies, the concentrations tested included AEB071 (0, 125, 250, 500, 1000 nM) and BYL719 (0, 250, 500, 1000, 2000 nM).
Gene Silencing
Experiments with small interfering RNAs (siRNA) were performed as previously described (29). Briefly, the cells were transfected using Liptofectamine RNAiMax (Invitrogen) according to manufacturer’s instructions. Next day, cells were treated with drug for an additional 24 hrs. Cells were then harvested and lysates were collected for Western Blot analysis. Small interfering RNA for human PI3Kα was purchased from Thermo Scientific, On Target plus SMARTpool (L-003018-00), and control siRNA (sc-37007) was from Santa Cruz Biotechnology.
Western Blots
Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer supplemented with protease inhibitor cocktail tablet (Roche Diagnostics) and 1 mmol/L Na3VO4. Equal amounts of protein were loaded and separated on a 4–12% PAGE gel (Invitrogen). Proteins were transferred to polyvinylidenedifluoride (PVDF) membranes, which were blocked in 5% nonfat dried milk. Membranes were then incubated with primary and secondary antibody and developed by ECL. Antibodies used to probe were pAKT (Ser473), Pan AKT, pS6 (S240/244), S6 ribosomal protein, pMARCKS (S152/156), MARCKS, alpha-Tubulin, ERK1/2, Cleaved PARP (Cell Signaling) and pERK1/2 (Y204) (Santa Cruz Biotechnology). For time course evaluation, media from plated cells was aspirated and freshly prepared drug in media was added to the plates. Cells were then harvested and lysed in RIPA buffer at 2, 6 and 24 hrs.
Flow Cytometry
Cell cycle analysis was performed as previously described (30, 31). Briefly, cell cycle analysis was performed after 72 hours of treatment. Cells were ethanol fixed, stained with propidium iodide and MPM-2 antibody, and analyzed by flow cytometry.
Xenograft Studies
6–8 week nu/nu SCID female mice bearing subcutaneously injected 92.1 tumors (7 mice/group) of 100mm3 diameter were treated with vehicle, AEB071 (80mg/kg/d) TID and or BYL719 orally (50mg/kg/d) QD as single agents and in combination, 5 days/week for 2 weeks. After 2 weeks, two animals from each group were sacrificed and tumors were collected to analyze for Western blot. For Omm1 xenogratfs, 6–8 weeks athymic female mice bearing subcutaneously injected Omm1 tumors (7 mice/group) of 100 mm3 diameter were treated with vehicle, AEB071 (80mg/kg/d) TID and or BYL719 orally (50mg/kg/d) QD as single agents and in combination, 5 days/week for 3 weeks. Tumors were homogenized with grinding resins kits from GE Healthcare as per manufacturer’s instructions. Tumors were collected to analyze for H&E, and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining as previously described (31). Tumors were measured every 2 to 3 days with calipers, and tumor volumes were calculated by the formula . Toxicity was monitored by weight loss. Experiments were carried out under institutional guidelines addressing the proper and humane use of animals. The Memorial Sloan- Kettering Cancer Center Animal Care and Use Committee and Research Animal Resource center approved this study. The study is also in accordance of the Principles of Laboratory Animal Care (NIH Publication No. 85-23, released 1985).
Statistical analysis
All in vitro experiments were carried out at least 2–3 times. Standard error was calculated as the standard deviation divided by the square root of number of samples. Statistical analysis for in vivo studies was determined by the two-sided t test. We chose P= 0.05 as statistically significant in individual comparisons.
RESULTS
AEB071 inhibits cell proliferation in GNAQ/GNA11-mutant Uveal Melanoma cell lines with inhibition of the PKC/ERK1/2 pathway
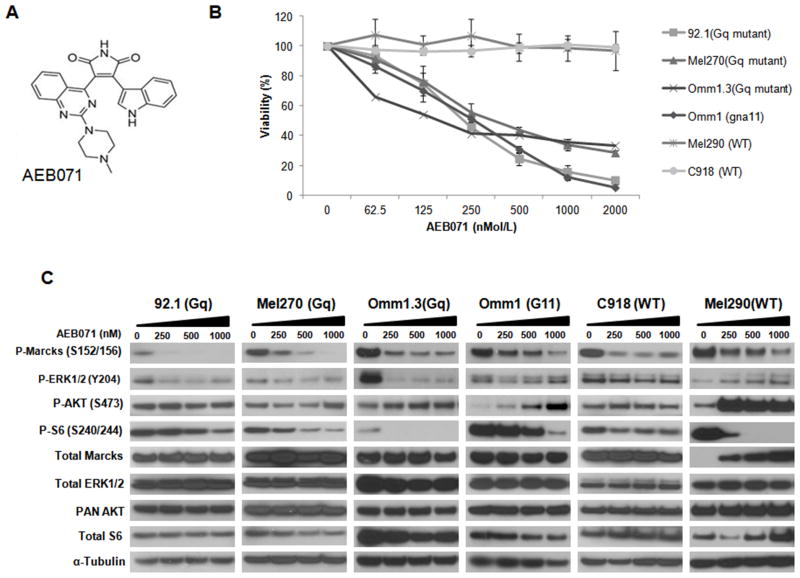
We evaluated the cell growth effect of the PKC inhibitor, AEB071 (structure Figure 1A), utilizing six uveal melanoma cell lines with different genotypes. The cell lines included GNAQ mutant cell lines 92.1, Mel270, Omm1.3 and the GNA11 mutant cell line Omm1. We also included wild type (WT) cell lines C918 and Mel290, without GNAQ/GNA11 mutations. We examined the single agent anti-proliferative effect on all cell lines utilizing increasing concentrations 0–2 μM of AEB071. We observed a dose dependent inhibition of proliferation with GI50 values ranging from 250–500nM for the GNAQ and GNA11 mutant cell lines (Figure 1B), while the cell lines with no mutations (WT) were not inhibited by the drug up to the highest concentration of 2 μM. We next examined target inhibition of PKC signaling with increasing concentrations of the drug from 0–1000nM (Figure 1C). AEB071 inhibited p-MARCKS, a PKC substrate, and pS6 in all the cell lines, independently of the mutational status. We also found an inhibition of ERK phosphorylation only in the GNAQ mutant cells. There was a slight inhibition of pERK at lower doses also in the GNA11 mutant cells, but not in the WT cells at any concentrations. This is consistent with previous reports indicating that AEB071 inhibits ERK1/2 phosphorylation in GNAQ mutant cell lines (22). Phosphorylation of AKT at Ser473 was minimally affected in the GNAQ mutant cells, while it increased in the GNA11-mutant and WT cells. In Mel290 (WT), the activation of AKT in response to AEB071 was particularly evident, indicating a feedback mechanism, possibly dependent on EGFR, which has been reported to be overexpressed in this cell line (32).
Figure 1. AEB071 reduces cell viability in G-protein mutant cell lines with minimal impact on the AKT pathway.
A. Structure of AEB071. B. AEB071 as single agent selectively inhibits cell proliferation of GNAQ/GNA11-mutant cells. Cell lines were treated with 0, 62.5, 125, 250, 500, 1000, 2000 nM of AEB071 for 5 days. Results represent the mean of three independent experiments. C. AEB071 inhibits PKC and mTOR pathways but not AKT. Western Blot of MARCKS, ERK, ribosomal S6 and AKT phosphorylation following drug treatments for 24 hrs. α-Tubulin was used as a loading control.
Silencing of PI3kα enhances the anti-proliferative effects of the PKC inhibitor in GNAQ mutant uveal melanoma cell lines
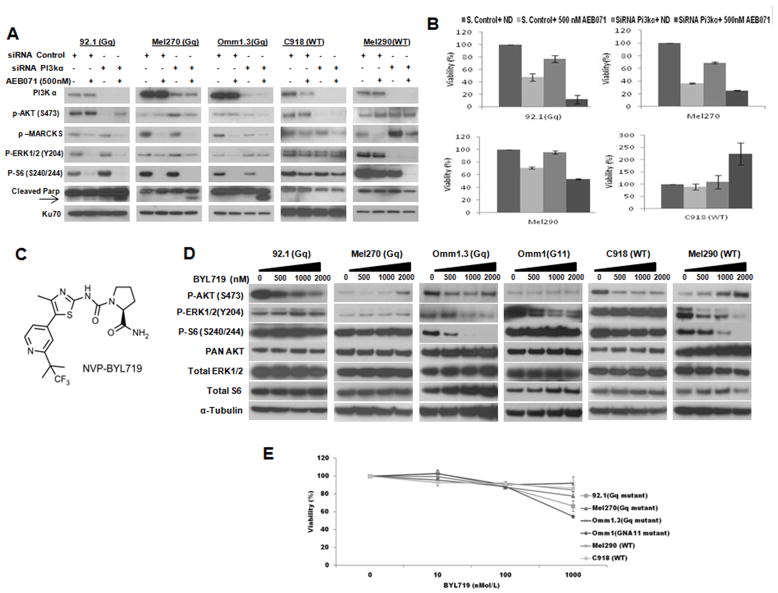
To explore whether selective PI3kα inhibition contributes to the PKC inhibitory effects in uveal melanoma, we performed gene silencing of p110α with or without the presence of AEB071 (Figure 2A). Depletion of p110α inhibited AKT phosphorylation in the GNAQ mutant (92.1, Omm1.3) and WT (C918) cells. There was no AKT inhibition by p110α siRNA in Mel270 and this was still maintained at basal levels in the presence of AEB071, and in Mel290. However, treatment with AEB071 in the presence of p110α siRNA suppression induced PARP cleavage only in the mutant cells, under which condition p-MARCKS, p-ERK, p-AKT and p-S6 were inhibited (Figure 2A). This corresponded to a significant decrease in cell viability in the GNAQ mutant cells (Figure 2B). In contrast, the WT cell lines showed no PARP cleavage, and the C918 cells showed an increase in cell viability. This enhancement of cell growth in the WT cell line with PI3kα suppression and AEB071 may be attributed to the absence of ERK1/2 decrease as seen with the GNAQ mutant cells (Figure 2A).
Figure 2. Selective inhibition of PI3kα enhances AEB071 antiproliferative effect in GNAQ mutant cells.
A, PI3kα siRNA inhibits AKT phosphorylation in uveal melanoma cell lines. siRNA knockdown of p110α isoform was performed with or without AEB071 in 92.1, Mel270, Omm1.3 (GNAQ mutant), and Mel290, C918 (WT) cell lines. A non-specific siRNA was used as control. Western blots of PI3Kα, AKT, MARCKS, ERK, S6 and cleaved PARP were then performed. The nuclear protein Ku70 was used as a loading control. B, PI3Kα and PKC inhibition reduces cell proliferation in GNAQ mutant uveal melanoma. Cell viability was assessed after transfection, with or without AEB071 for 5 days. C, Structure of BYL719. D, BYL719 inhibits AKT (ser473) phosphorylation, and/or p-ERK in some cell lines. Cells were treated with indicated concentrations of BYL719 for 24 hrs, and analyzed for pAKT, pERK, pS6 and the respective total proteins. α-Tubulin was used as a loading control. E, BYL719 as single agent has minimal to no effect on cell viability in uveal melanoma after 5 days of exposure. Concentrations tested include 0, 10, 100,1000 nM. Results represent the mean of three independent experiments.
We next examined the effect of single agent BYL719 (structure in Figure 2C) on the same cell lines, utilizing concentrations ranging from 0–2μM (Figure 2D). We observed inhibition of phosphorylation of AKT (Ser473) up to 1 μM in most cell lines (Figure 2D), even though there was reactivation at higher doses in Omm1.3, Mel270 and Mel290. Some of the cell lines, such as Omm1.3, Omm1, and Mel290 showed inhibition of p-ERK1/2 by BYL719. There is recent evidence supporting the inhibition of ERK1/2 phosphorylation by the selective PI3kα inhibitor BYL719 (33). There was also significant inhibition of S6 phosphorylation in the Omm1.3 and Mel290 cell lines. However, PI3Kα/pAKT inhibition in itself was clearly not sufficient to have an anti-proliferative effect in any cell line (Figure 2E). The exception to this was Omm1, the GNA11 cell line, in which cell growth inhibition was observed at 1000nM, a concentration at which there was inhibition of p-ERK.
The combination AEB0711/BYL719 induces a synergistic decrease in cell viability in GNAQ/GNA11 mutant uveal melanoma cell lines
In order to examine the effects of the AEB071/BYL719 combination on cell viability, we performed combination index analysis utilizing the Chou–Talay method to determine whether the effects are synergistic (28). The combination of AEB071 and BYL719 at different doses exhibited a combination index (CI) < 1, indicative of synergy and a fractional activity of more than 50% ((Fa)>0.5) in all GNAQ/GNA11 mutant cell lines (Supplementary Figure 1). CI values for WT cell lines C918 and Mel290 ranged from > 1 to 30 with a (Fa) < 0.2, showing that the combination was in fact antagonistic in cells that are wild-type for G proteins.
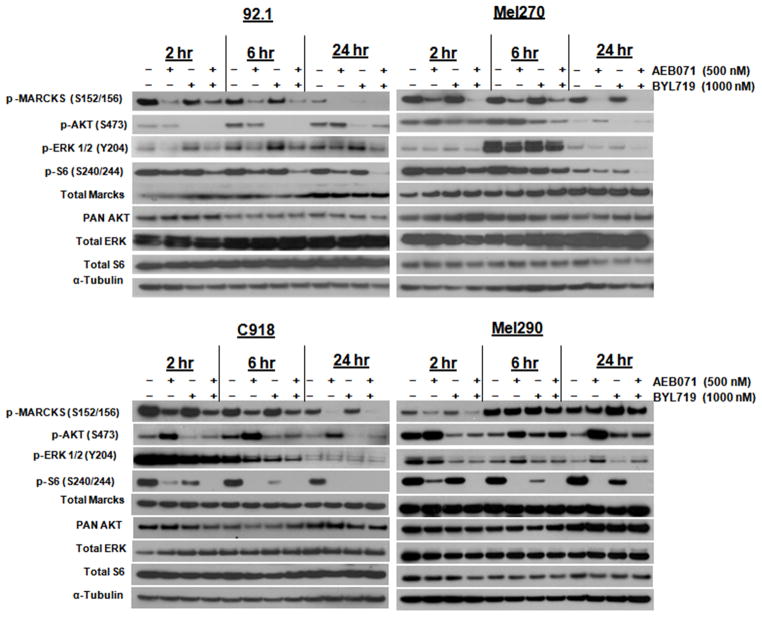
We next investigated the occurrences of molecular events with the combination treatment (Figure 3). We treated two GNAQ (92.1, Mel270), and two WT (C918, Mel290) cell lines with concentrations of 500nM of AEB071 and 1μM of BYL719 respectively and examined the effect on downstream signaling at 2, 6, and 24 hours. In contrast to single agent AEB071 or BYL719, the combination therapy resulted in inhibition of p-MARCKS and p-AKT over time and all downstream signaling pathways such as p-ERK and p-S6 in the GNAQ-mutant cell lines. Single agent BYL719 caused a slight activation of p-ERK that was detectable as early as 2 hours after drug exposure, especially in the 92.1 cells, but this activation was suppressed with the combination treatment. This would in fact suggest that the reactivation of p-ERK in this setting may be mediated through PKC. The reactivation of p-ERK at 6 hours, especially in the Mel270 cell line, even in untreated controls, may reflect a DMSO effect. In the Mel290 WT cell line, there was activation of AKT to both AEB071 and BYL719 along with reactivation of p-MARCKS at later time points suggesting that this cell line may have a dependency on other survival pathways such as EGFR (32). For the WT C918 cell line, p-MARCKS, p-AKT and p-S6 are inhibited over time but minimal p-ERK inhibition occurs. We also tested the GNA11 mutant cell line, Omm1, with the combination therapy (Supplementary Figure 2A). In these cells AEB071 inhibited p-MARCKS and p-S6 but induced activation of p-AKT over time, which was inhibited by BYL719 in the combination therapy. Interestingly, BYL719 also inhibited pERK in this cell line. Thus, these results indicate that inhibition of PKC and PI3kα together achieves a synergistic effect in GNAQ and GNA11 cells with inhibition of downstream survival pathways that is not achievable with single agent therapy alone.
Figure 3. The combination AEB071/BYL719 synergistically reduces tumor cell viability in GNAQ mutant cells due to cooperative inhibition of PKC/ERK and PI3kα/AKT pathways.
Cells were treated with indicated AEB071/BYL719 concentrations for 2, 6 and 24 hrs and Western blots were then performed. Suppression of PKC/ERK and PI3Kα/AKT pathways was observed with the combination treatment in the GNAQ mutant cells, as evidenced by inhibition of MARCKS, ERK, AKT (Ser473) and S6 phosphorylation. α-Tubulin was used as a loading control.
AEB071/BYL719 combination induces apoptosis
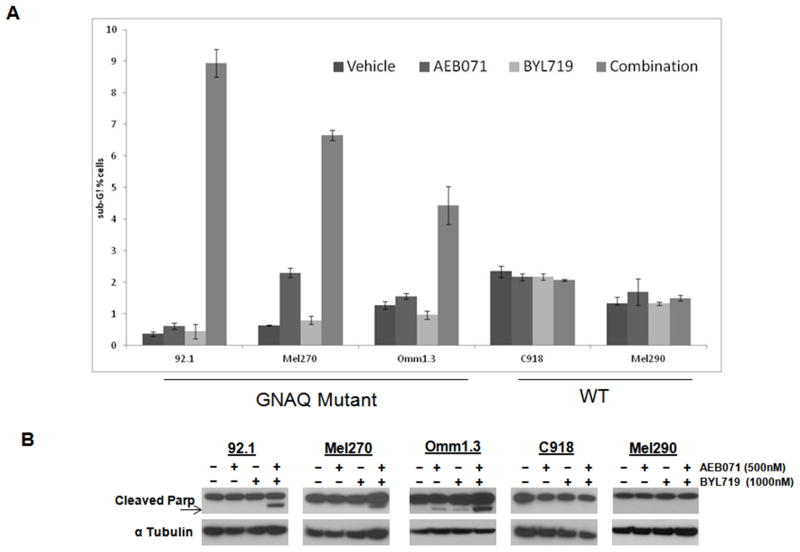
We next elected to determine whether this inhibition of cell growth was due to growth arrest or induction of apoptosis. We assessed cell cycle effects of the drug combination with bi-parameter flow cytometry analysis for DNA content (detected with propidium iodide) and MPM-2, a mitotic marker, as previously described (31). Apoptosis was assessed by the detection of a sub-G1 peak and of PARP cleavage. The flow cytometry data indicated essentially minimal growth arrest (data not shown) with AEB071, but there was evidence of a sub-G1 peak with the combination therapy only in the GNAQ mutant cell lines (Figure 4A). Furthermore, the combination treatment induced PARP cleavage, an early apoptotic marker, in GNAQ mutant cells, while there was no evidence of PARP cleavage in the WT cell lines (Figure 4B).
Figure 4. The combination AEB071/BYL719 induces apoptosis in GNAQ mutant cells.
A. Cells were treated with AEB071/BYL719 for 72 hrs and labeled with MPM-2, stained with propidium iodide, and analyzed by flow cytometry. SubG1 fractions were quantified using Flow jo. Results represent the mean of three independent experiments. B. Uveal melanoma cell lines were treated with drug for 24 hr. Western Blots were performed for cleaved PARP (Poly (ADP-ribose) polymerase). α-Tubulin was used as a loading control.
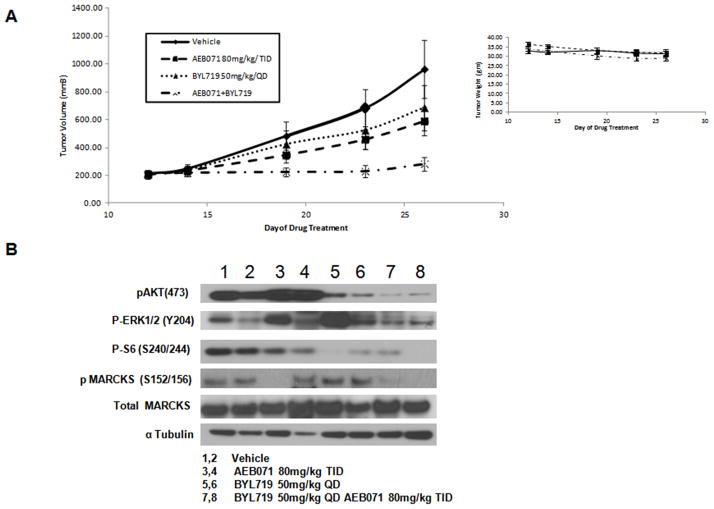
AEB071/BYL719 combination inhibits tumor growth in vivo in a GNAQ mutant xenograft model
In view of the synergistic effects of the combination therapy observed in vitro, we elected to determine whether the combination therapy would be superior to single agent therapy in a GNAQ mutant xenograft mouse model. As shown in Figure 5A, both single agents had a modest effect on inhibiting tumor growth at their respective single agent MTDs. The combination therapy resulted in a significantly enhanced reduction in tumor volume when compared to either AEB071 or BYL719 alone (p = 0.049 vs. BYL719 and p= 0.022 vs. AEB071 at day 26). There was even a greater effect when compared to vehicle control (p = 0.016). Examination of the tumor lysates following drug therapy (day # 26) confirmed the in vitro data indicating that the combination therapy resulted in concomitant inhibition of PKC and PI3K signaling with inhibition pMARCKS, pAKT, pERK and pS6 that was not observed with either single agent alone. Toxicity was measured along with tumor volume by weight loss, which was less than 14.7% for all treatments (Figure 5A, right panel). Examination of apoptosis by TUNEL indicates the presence of apoptotic cells only with the combination therapy (Supplemental Figure 3).
Figure 5. AEB071/BYL719 combination inhibits in vivo tumor growth in a GNAQ mutant xenograft model.
A, AEB071/BYL719 inhibited tumor growth in a xenograft model with the GNAQ mutant 92.1 cell line. 6-8 week nu/nu SCID female mice were subcutaneously injected with 92.1 cells. Drug treatments began after tumors reached 100 mm3. Mice with tumors were treated three times daily with AEB071 (80mg/kg/d) or once daily with BYL719 (50 mg/kg/d) or in combination for 5 days each week for a total of 2 weeks. Tumors were measured with calipers every 2 to 3 days. Tumor volume was compared between groups of mice at various time points. P-value when compared to either AEB071 or BYL719 alone p = 0.049 vs. BYL719 and p= 0.022 vs. AEB071 at day 26. Toxicity was measured by weight loss (right panel). B, Two animals from each cohort were sacrificed and tumors were collected on Day 26. Tumors were lysed with RIPA buffer and Western blots were performed for pAKT, pERK, pS6, pMARCKS and αtubulin.
The Omm1(GNA11) mutant xenograft mouse model was also performed with both single agents having an effect on inhibiting tumor growth at their respective single agent MTDs, which appeared greater than that observed in the GNAQ xenograft. Furthermore, the combination therapy did not result in a significant reduction in tumor volume when compared to either AEB071 or BYL719 alone, though a trend favored the combination therapy (Supplementary Figure 2B). For unclear reasons the animals bearing the GNA11 xenografts experienced a 23.9% weight loss with combination therapy (Supplementary Figure 2B, right panel).
DISCUSSION
PKC, MAPK and PI3K/AKT pathways are highly activated in uveal melanoma (3, 4, 18, 34). MEK/ERK signaling has been considered as a major target in uveal melanoma and clinical trials are underway targeting this pathway (35). Alternatively, dual targeted therapy is now being considered as a treatment option as further studies elucidate the importance of targeting activated signal pathways. Previous studies have focused on combining inhibitors of MEK with mTOR and PI3K/AKT pathways (26, 36, 37). Targeted therapy directed against PI3K isoforms has usually been effective in cancers harboring mutations of the isoform but PI3K mutations have not been reported in uveal melanoma. Clinically, the benefit of targeting a specific isoform of PI3K has led to more complete target inhibition at lower doses resulting in less adverse effects (38). The blocking of AKT activation in other cell types through PI3kα has been widely studied (17, 39). The role of PI3Kα isoform in its active state allows for cell survival and migration leading to tumor formation and metastases (40). We postulated that preventing this activation with a selective PI3Kα inhibitor would provide a means to inhibit AKT in these cells as part of a combinatorial approach. The G-protein oncogenic mutations have been shown to activate PKC. In fact, GNAQ mutations rely mostly on PKC and MAPK pathways for survival (26, 41). This present study demonstrates that inhibiting PKC and ERK1/2 signaling with AEB071 together with the PI3kα inhibitor, BYL179, results in a synergistic anti-tumor effect in uveal melanoma harboring GNAQ and GNA11 mutations in vitro. Previous reports have shown that PKC inhibition has a negative regulatory effect on PI3K/AKT by stimulating Akt/PKB phosphorylation at Ser 473 (42). This may explain the minimal single agent effect of AEB071 on inhibiting pAKT in the GNAQ and GNA11 mutant cell lines. Interestingly, our current results also show inhibition of ribosomal S6 phosphorylation with PKC inhibition and combination treatment. S6 overexpression has been reported as a prognostic marker for uveal melanoma and mTor signaling may similarly provide a growth advantage to these cells (19). This could explain why inhibition of this pathway also enhances the anti-tumor effect we have observed in these cells. In the setting of GNAQ/GNA11 mutations, the inhibition of MARCKS, ERK, AKT and S6 phosphorylation results in apoptosis by the combination treatment. Similar pathway targeting in pancreatic cells have shown an apoptotic outcome by sulforaphane treatment due to synergistic inhibition of PI3K/AKT and MEK/ERK (43).
The effects of this combination treatment demonstrate the importance of targeting parallel survival and growth pathways that are downstream of GNAQ and GNA11 in uveal melanoma. However, simply inhibiting several of these pathways may not be sufficient to inhibit tumor growth inhibition in vivo. For example, we previously reported that blockade of Torc1 and Torc2 with AZD8055 in combination with the MEK inhibitor selumetinib (AZD6244) in GNAQ mutant cells is insufficient to suppress tumor growth in vivo (36). In contrast, we have shown that inhibition of p-AKT with MK2206, an allosteric inhibitor of AKT, in combination with selumetinib results in enhanced anti-tumor effects (26). This would suggest that the development of combination therapy for the treatment of GNAQ uveal melanoma will depend largely on the selective targeting of pathways that are critical for tumor growth. Based on our MK2206 and selumetinib data, inhibition of p-ERK in combination with inhibition of p-AKT appears to be the minimal requirement necessary to promote an anti-tumor effect with combination therapy. As AEB071 results in inhibition of PKC as well as p-ERK, this dual pathway inhibition, when combined with inhibition of PI3kα/AKT by BYL719, appears to provide a means to broaden pathway inhibition above and beyond MEK and AKT alone for cell growth inhibition of GNAQ/GNA11 mutant cells.
All together our findings indicate that the targeting of PKC/ERK, along with a selective PI3kα isoform inhibitor, cooperates in inhibiting growth in GNAQ mutant uveal melanoma cells. In the GNA11 cell line this same effect is ultimately achieved though it is BYL719, the PI3kα inhibitor, rather than AEB071, the PKC inhibitor, that contributes to the inhibition of p-ERK. We also show a translatable GNAQ xenograft model of uveal melanoma demonstrating effective PKC/ERK and PI3K/AKT pathway inhibition, which results in enhanced in vivo tumor growth inhibition with the combination therapy. The combination effects in vivo on the GNA11 xenograft were less definitive despite synergy in vitro. There was unexpected weight loss with the combination therapy which may have been due to the difference in mouse species (nude mice rather than SCID mice) needed to establish the GNA11 xenograft.
The combination of AEB071 and MEK162, the MEK inhibitor, has recently been reported in uveal melanoma xenografts (41). Our studies indicate that both AEB071 and BYL719 can inhibit p-ERK as single agents or as part of combination therapy. However, only BYL719 can inhibit p-AKT which appears critical to the synergistic effect. In conclusion, our results suggest an effective new therapy for the treatment of uveal melanoma, and targeting these pathways with AEB071 and BYL719 could have therapeutic implications for the future treatment of patients with this disease. Based on these results a phase Ib/II clinical trial of this drug combination in patients with metastatic uveal melanoma is now planned.
Supplementary Material
Acknowledgments
Financial Support: E. Musi, G. Ambrosini and G.K. Schwartz received grant support from Cycle for Survival (Philanthropy). E.de Stanchina received grant support from NIH grant # P30 CA 008748.
We thank Vinagolu K. Rajasekhar for assisting with our in vivo studies.
Footnotes
Conflicts of Interest: None of the authors have conflicts of interest except Dr. Gary Schwartz who has a consulting agreement with Novartis.
References
- 1.Augsburger JJ, Correa ZM, Shaikh AH. Effectiveness of treatments for metastatic uveal melanoma. American journal of ophthalmology. 2009;148:119–27. doi: 10.1016/j.ajo.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 2.Patel M, Smyth E, Chapman PB, Wolchok JD, Schwartz GK, Abramson DH, et al. Therapeutic Implications of the Emerging Molecular Biology of Uveal Melanoma. Clinical Cancer Research. 2011;17:2087–100. doi: 10.1158/1078-0432.CCR-10-3169. [DOI] [PubMed] [Google Scholar]
- 3.Weber A, Hengge UR, Urbanik D, Markwart A, Mirmohammadsaegh A, Reichel MB, et al. Absence of mutations of the BRAF gene and constitutive activation of extracellular-regulated kinase in malignant melanomas of the uvea. Laboratory investigation; a journal of technical methods and pathology. 2003;83:1771–6. doi: 10.1097/01.lab.0000101732.89463.29. [DOI] [PubMed] [Google Scholar]
- 4.Zuidervaart W, van Nieuwpoort F, Stark M, Dijkman R, Packer L, Borgstein AM, et al. Activation of the MAPK pathway is a common event in uveal melanomas although it rarely occurs through mutation of BRAF or RAS. British journal of cancer. 2005;92:2032–8. doi: 10.1038/sj.bjc.6602598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O’Brien JM, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onken MD, Worley LA, Long MD, Duan S, Council ML, Bowcock AM, et al. Oncogenic mutations in GNAQ occur early in uveal melanoma. Investigative ophthalmology & visual science. 2008;49:5230–4. doi: 10.1167/iovs.08-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, et al. Mutations in GNA11 in uveal melanoma. The New England journal of medicine. 2010;363:2191–9. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. Journal of Cellular Physiology. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- 9.Martiny-Baron G. Classical PKC isoforms in cancer. Pharmacological research. 2007;55:477. doi: 10.1016/j.phrs.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Thelen M, Rosen A, Nairn AC, Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991;351:320–2. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- 11.Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992;356:618–22. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- 12.Graff JM, Young TN, Johnson JD, Blackshear PJ. Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. The Journal of biological chemistry. 1989;264:21818–23. [PubMed] [Google Scholar]
- 13.Ballou LM, Chattopadhyay M, Li Y, Scarlata S, Lin RZ. Galphaq binds to p110alpha/p85alpha phosphoinositide 3-kinase and displaces Ras. The Biochemical journal. 2006;394:557–62. doi: 10.1042/BJ20051493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye M, Hu D, Tu L, Zhou X, Lu F, Wen B, et al. Involvement of PI3K/Akt signaling pathway in hepatocyte growth factor-induced migration of uveal melanoma cells. Investigative ophthalmology & visual science. 2008;49:497–504. doi: 10.1167/iovs.07-0975. [DOI] [PubMed] [Google Scholar]
- 15.Stephens L, Williams R, Hawkins P. Phosphoinositide 3-kinases as drug targets in cancer. Current opinion in pharmacology. 2005;5:357–65. doi: 10.1016/j.coph.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT Pathway for Cancer Drug Discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 17.Matheny RW, Jr, Adamo ML. PI3K p110 alpha and p110 beta have differential effects on Akt activation and protection against oxidative stress-induced apoptosis in myoblasts. Cell death and differentiation. 2010;17:677–88. doi: 10.1038/cdd.2009.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saraiva VS, Caissie AL, Segal L, Edelstein C, Burnier MN., Jr Immunohistochemical expression of phospho-Akt in uveal melanoma. Melanoma research. 2005;15:245–50. doi: 10.1097/00008390-200508000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Populo H, Soares P, Rocha AS, Silva P, Lopes JM. Evaluation of the mTOR pathway in ocular (uvea and conjunctiva) melanoma. Melanoma research. 2010;20:107–17. doi: 10.1097/CMR.0b013e32832ccd09. [DOI] [PubMed] [Google Scholar]
- 20.Brana I, Siu LL. Clinical development of phosphatidylinositol 3-kinase inhibitors for cancer treatment. BMC medicine. 2012;10:161. doi: 10.1186/1741-7015-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X, Zhu M, Fletcher JA, Giobbie-Hurder A, Hodi FS. The protein kinase C inhibitor enzastaurin exhibits antitumor activity against uveal melanoma. PloS one. 2012;7:e29622. doi: 10.1371/journal.pone.0029622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu X, Li J, Zhu M, Fletcher JA, Hodi FS. Protein Kinase C Inhibitor AEB071 Targets Ocular Melanoma Harboring GNAQ Mutations via Effects on the PKC/Erk1/2 and PKC/NF-κB Pathways. Molecular Cancer Therapeutics. 2012;11:1905–14. doi: 10.1158/1535-7163.MCT-12-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naylor TL, Tang H, Ratsch BA, Enns A, Loo A, Chen L, et al. Protein kinase C inhibitor sotrastaurin selectively inhibits the growth of CD79 mutant diffuse large B-cell lymphomas. Cancer research. 2011;71:2643–53. doi: 10.1158/0008-5472.CAN-10-2525. [DOI] [PubMed] [Google Scholar]
- 24.Huang AFC, Wilson C, Reddy A, Liu M, Lehar J, et al. Single agent activity of PIK3CA inhibitor BYL719 in a broad cancer cell line panel. Cancer Res; Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research; 2012 Mar 31–Apr 4; Chicago, IL. Philadelphia (PA): AACR; 2012. p. Abstract nr 3749. [Google Scholar]
- 25.Furet P, Guagnano V, Fairhurst RA, Imbach-Weese P, Bruce I, Knapp M, et al. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorganic & medicinal chemistry letters. 2013;23:3741–8. doi: 10.1016/j.bmcl.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Ambrosini G, Musi E, Ho AL, de Stanchina E, Schwartz GK. Inhibition of Mutant GNAQ Signaling in Uveal Melanoma Induces AMPK-Dependent Autophagic Cell Death. Mol Cancer Ther. 2013 doi: 10.1158/1535-7163.MCT-12-1020. [DOI] [PubMed] [Google Scholar]
- 27.Griewank KG, Yu X, Khalili J, Sozen MM, Stempke-Hale K, Bernatchez C, et al. Genetic and molecular characterization of uveal melanoma cell lines. Pigment Cell & Melanoma Research. 2012;25:182–7. doi: 10.1111/j.1755-148X.2012.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer research. 2010;70:440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 29.Ambrosini G, Cheema HS, Seelman S, Teed A, Sambol EB, Singer S, et al. Sorafenib inhibits growth and mitogen-activated protein kinase signaling in malignant peripheral nerve sheath cells. Mol Cancer Ther. 2008;7:890–6. doi: 10.1158/1535-7163.MCT-07-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motwani M, Delohery TM, Schwartz GK. Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 1999;5:1876–83. [PubMed] [Google Scholar]
- 31.Nair JS, de Stanchina E, Schwartz GK. The Topoisomerase I Poison CPT-11 Enhances the Effect of the Aurora B Kinase Inhibitor AZD1152 both In vitro and In vivo. Clinical Cancer Research. 2009;15:2022–30. doi: 10.1158/1078-0432.CCR-08-1826. [DOI] [PubMed] [Google Scholar]
- 32.Amaro A, Mirisola V, Angelini G, Musso A, Tosetti F, Esposito AI, et al. Evidence of epidermal growth factor receptor expression in uveal melanoma: inhibition of epidermal growth factor-mediated signalling by Gefitinib and Cetuximab triggered antibody-dependent cellular cytotoxicity. European journal of cancer (Oxford, England : 1990) 2013;49:3353–65. doi: 10.1016/j.ejca.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Brady SW, Zhang J, Seok D, Wang H, Yu D. Enhanced PI3K p110alpha signaling confers acquired lapatinib resistance which can be effectively reversed by a p110alpha-selective PI3K inhibitor. Mol Cancer Ther. 2013 doi: 10.1158/1535-7163.MCT-13-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babchia N, Calipel A, Mouriaux F, Faussat AM, Mascarelli F. The PI3K/Akt and mTOR/P70S6K signaling pathways in human uveal melanoma cells: interaction with B-Raf/ERK. Investigative ophthalmology & visual science. 2010;51:421–9. doi: 10.1167/iovs.09-3974. [DOI] [PubMed] [Google Scholar]
- 35.Carvajal RDA, Wolchok JD, Chapman PB, Dickson MA, D’Angelo SP, et al. Pharmacodynamic activity of selumetinib to predict radiographic response in advanced uveal melanoma. J Clin Oncol. 2012;30:15s. (suppl; abstr 8598) [Google Scholar]
- 36.Ho AL, Musi E, Ambrosini G, Nair JS, Deraje Vasudeva S, de Stanchina E, et al. Impact of combined mTOR and MEK inhibition in uveal melanoma is driven by tumor genotype. PloS one. 2012;7:e40439. doi: 10.1371/journal.pone.0040439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khalili JS, Yu X, Wang J, Hayes BC, Davies MA, Lizee G, et al. Combination Small Molecule MEK and PI3K Inhibition Enhances Uveal Melanoma Cell Death in a Mutant GNAQ- and GNA11-Dependent Manner. Clinical Cancer Research. 2012;18:4345–55. doi: 10.1158/1078-0432.CCR-11-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Courtney KD, Corcoran RB, Engelman JA. The PI3K Pathway As Drug Target in Human Cancer. Journal of Clinical Oncology. 2010;28:1075–83. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jamieson S, Flanagan JU, Kolekar S, Buchanan C, Kendall JD, Lee WJ, et al. A drug targeting only p110alpha can block phosphoinositide 3-kinase signalling and tumour growth in certain cell types. The Biochemical journal. 2011;438:53–62. doi: 10.1042/BJ20110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase—Moving towards therapy. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2008;1784:159–85. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Wu Q, Tan L, Porter D, Jager MJ, Emery C, et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene. 2013 doi: 10.1038/onc.2013.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen HC, Huang WC, Ali A, Woodgett JR, Lin WW. Negative regulation of phosphatidylinositol 3-kinase and Akt signalling pathway by PKC. Cellular signalling. 2003;15:37–45. doi: 10.1016/s0898-6568(02)00047-5. [DOI] [PubMed] [Google Scholar]
- 43.Roy S, Srivastava R, Shankar S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. Journal of Molecular Signaling. 2010;5:10. doi: 10.1186/1750-2187-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.