Abstract
Background
Anesthesia given to immature rodents causes cognitive decline raising the possibility that the same might be true for millions of children undergoing surgical procedures under general anesthesia each year. We tested the hypothesis that anesthesia-induced cognitive decline in rats is treatable. We also tested if anesthesia-induced cognitive decline is aggravated by tissue injury.
Methods
7-day old rats underwent sevoflurane anesthesia (1 MAC, 4 hours) with or without tail clamping. At 4 weeks, rats were randomized to environmental enrichment or normal housing. At 8 weeks rats underwent neurocognitive testing, which consisted of fear conditioning, spatial reference memory and water maze-based memory consolidation tests, that interrogated working memory, short term memory and early long term memory.
Results
Sevoflurane-treated rats had a greater escape latency when the delay between memory acquisition and memory retrieval was increased from 1 minute to 1 hour, indicating that short term memory was impaired. Delayed environmental enrichment reversed the effects of sevoflurane on short term memory and generally improved many tested aspects of cognitive function, both in sevoflurane-treated and control animals. The performance of tail clamped rats did not differ from those rats receiving anesthesia alone.
Conclusion
Sevoflurane-induced cognitive decline in rats is treatable. Delayed environmental enrichment rescued the sevoflurane-induced impairment in short-term memory. Tissue injury did not worsen the anesthesia-induced memory impairment. These findings may have relevance to neonatal and pediatric anesthesia.
Introduction
Isoflurane, given as a single anesthetic agent, causes neurodegeneration in the infantile rodent brain (1–5) and long-term neurocognitive deficits later in life (4, 6–9) (but also see (5)). Sevoflurane is now more commonly used in pediatric anesthesia and its neurotoxicity profile appears more favorable than other popular anesthetic agents. Compared to isoflurane, sevoflurane caused less cytotoxicity and a smaller increase in intracellular calcium, both in vitro (10–12), as well as in infantile mice (13). Compared to propofol, sevoflurane caused less cytotoxicity and no long-term neurocognitive sequelae (14). Compared to desflurane, sevoflurane caused less cytotoxicity and did not impair working memory (15). However, there is also one report of sevoflurane causing both neurodegeneration and long-term deficits in social recognition memory and social interaction in mice (8), as well as a report of equal toxicity between sevoflurane and isofluarne (16). Thus, whether or not sevoflurane, given as the sole anesthetic during infancy, at clinically relevant concentrations, causes long-term neurocognitive dysfunction is not entirely clear.
Here, we tested the hypothesis that 1 minimum alveolar concentration (MAC) of sevoflurane, given to infantile rats for 4 hours, causes long-term neurocognitive dysfunction.
In humans, anesthesia during infancy is mostly administered for the purpose of tolerating a surgical procedure, which is rarely the case in animal studies of developmental anesthetic neurotoxicity. We routinely use tail clamping during anesthesia to titrate the anesthetic concentration to 1 MAC. In infantile rodents 1 MAC of isoflurane is not a stable anesthetic concentration, but instead, requires an initial anesthetic concentration that is 2–3 fold greater than the inspired anesthetic concentration required to achieve 1 MAC in adult rodents, followed by progressive reduction of the anesthetic concentration over a number of hours (15, 17). We therefore hypothesized, that the inspired MAC of sevoflurane in 7-day old rats also decreases with increasing duration of anesthesia.
MAC-determination in animals requires a painful stimulus, for example an alligator clamp to the tail or a surgical skin incision (17, 18). Therefore, MAC-determination in animals can serve as a model for anesthesia plus tissue injury. In adult rats anesthesia plus surgery causes hipppocampal inflammation and short-lived hippocampal dysfunction (19). It has been argued that the surgical procedure, and not the anesthetic, may be responsible for the long-term neurocognitive deficit following infantile anesthesia (20). If half of the animals undergoing a given anesthetic are subjected to tissue injury and half are not, it is possible to separate the effects of anesthesia from those of tissue injury. In doing so, we previously did not observe any additional effect of tail clamping on neurocognitive outcome of infantile anesthesia, using isoflurane (4) (unpublished observation). We therefore hypothesized, that rats undergoing sevoflurane anesthesia plus tail clamping have a similar long-term neurocognitive outcome as those rats undergoing sevoflurane anesthesia alone.
The mechanism of anesthesia-induced long-term neurocognitive dysfunction remains elusive. Although anesthesia has been shown to cause neurodegeneration (reviewed elsewhere (21)), alter synaptogenesis (22–25) and impair neurogenesis (7, 9, 26), evidence of a causal relationship between these anesthesia-induced cellular events and anesthesia-induced long-term neurocognitive dysfunction is lacking. Therefore, attempts to prevent anesthesia-induced neurocognitive dysfunction are currently not informed by a mechanistic understanding. Due to this current inability to specifically prevent neurocognitive dysfunction, it becomes important to determine, whether it is possible to treat the neurocognitive consequences of anesthesia-induced developmental neurotoxicity. Treatment, as opposed to prevention, by definition, requires some delay between the insult (anesthetic exposure) and the intervention. The present study tested the efficacy of such an approach, using a delayed, nonspecific, and powerful intervention: environmental enrichment. It is well-known that environmental enrichment fundamentally improves various aspects of brain function by harnessing a vast, often unrealized, brain plasticity (27–32). Hence, we tested the hypothesis, that delayed environmental enrichment is effective in treating sevoflurane induced long-term neurocognitive dysfunction.
Here, we show that sevoflurane causes long-term impairment in spatial short term memory, which is not exacerbated by tissue injury and is mitigated by delayed environmental enrichment.
Materials and Methods
Animals/Experimental Design
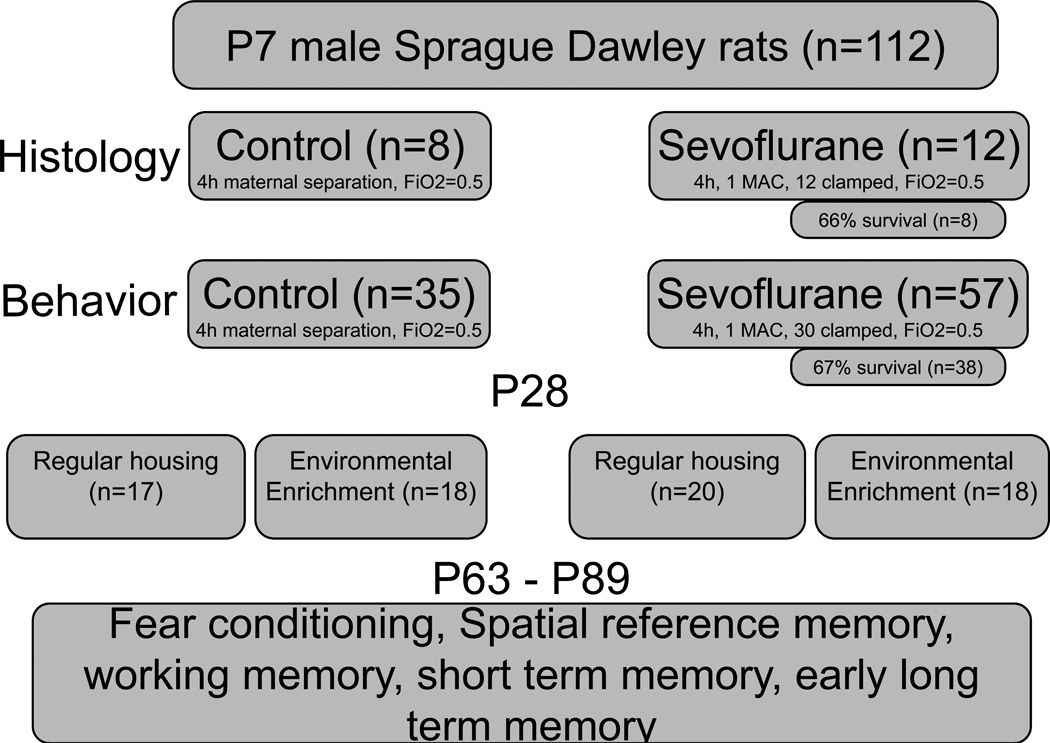
All experiments were conducted with approval of the Institutional Animal Care and Use Committee at the University of California, San Francisco. Sprague Dawley dams (n=12) with litters containing male pups (n=112) were purchased from Charles River Laboratories (Gilroy, CA). The study had a histologic arm designed to assess the acute effects of sevoflurane on brain cell death and a behavioral arm to assess the effect of sevoflurane on long-term neurocognitive outcome. Twenty male pups entered the histologic arm of the study whereas 92 rats were allocated to the behavioral arm of the study. Pups were cross-fostered and housed on a 12-hour light/dark cycle with ad libitum access to food and water. Pups (n=112) were randomly divided into one of three treatment groups: sevoflurane with tail clamping (n=42), sevoflurane without tail clamping (n=27), and sham anesthetized (n=43). At the end of 4 h survival in the histologic arm was 66% with 8 sevoflurane-treated animals and an equal number of sham anesthetized animals entering the analysis. Survival in the behavioral arm was 67% causing the group size of tail clamped rats in the behavioral arm to decreased to 21 and of non-clamped rats to 17. In the histologic arm, rats were killed 12 hours after anesthesia. In the behavioral arm, rats were further randomized after weaning from their mothers, at 28 days of age, to either environmental enrichment (n=36) or conventional housing (n=37). This resulted in the following experimental groups: Sham anesthesia, normal housing (n=17), sham anesthesia, environmental enrichment (n=18), sevoflurane, not clamped, normal housing (n=9), sevoflurane, not clamped, environmental enrichment (n=8), sevoflurane, tail-clamped, normal housing (n=11), sevoflurane, tail-clamped, environmental enrichment (n=10). Figure 1 summarizes the experimental design.
Figure 1.
Overview of experimental design. Four hypotheses were tested. 1) Inspired MAC of sevoflurane decreases with increasing duration of anesthesia. 2) Sevoflurane in infancy causes neurocognitive dysfunction later in life. 3) In a mixed model of tissue injury under anesthesia, the deficit is due to the anesthetic but not due to tissue injury. 4) Anesthesia-induced memory deficit is treatable, using delayed environmental enrichment.
FiO2 fraction of inspired oxygen, MAC minimum alveolar concentration, P postnatal day
Sevoflurane Anesthesia and Sham Anesthesia
Sevoflurane was simultaneously administered in O2/air (FiO2 50%) to up to 32 postnatal βday (P) 7 rats using a glove box. MAC of sevoflurane was determined by tail clamping of all animals in the histologic arm and half of the anesthetized animals in the behavioral arm every 15 minutes. The anesthetic concentration was adjusted so that 50% of the tail-clamped animals moved in response to the stimulus. A custom made, closed circuit, temperature controlling copper plate was set to automatically maintain the pericranial temperature of all rats at 36.5°C. Sham anesthesia was administered to up to 20 rats simultaneously. Rats were placed in the same anesthesia glove box used for sevoflurane treatment, but without exposure to the anesthetic; experimental conditions were identical otherwise, including maternal separation and fraction of inspired oxygen of 50%, with two notable exceptions: sham anesthetized rats did not undergo tail clamping, nor did they have temperature probes inserted into them. Instead, the temperature of the skin over their skull was measured with a laser infrared thermometer immediately upon entry into the glove box and maintained at this baseline level (around 33ºC). Five separate rats, that did not take part in any other portion of the experiment, were used to assess if sevoflurane causes a similar degree of hypercarbia as that previously observed by isoflurane (4). Blood was aspirated percutaneously from the left cardiac ventricle and analyzed in a Radiometer ABL 520 blood gas analyzer (Radiometer, Copenhagen, Denmark).
Histology
Tissue Processing and FluoroJade Staining
Sixteen hours after anesthesia or sham anesthesia rats were transcardially perfused with cold 4% paraformaldehyde in phosphate buffered saline. Brains were removed and postfixed overnight in the same solution, sucrose sunk and sliced to 40 µm thickness. FluoroJade staining of every 12th free floating slice was used to assess cell death as previously described (4, 9). Brain sections were mounted onto glass slides and allowed to air-dry. The slides were then washed in 100% ethanol for 3 minutes, then 70% ethanol for 1 minute, and then deionized water (dH2O) for 1 minute. They were incubated in 0.06% potassium permanganate for 20 minutes at 25°C, and again washed in dH2O for 1 minute. Next, the slides were incubated in a FluoroJade solution (4 ml of 0.01% FluoroJade (Millipore, Billerica, MA) [in dH2O] stock solution, 36 ml of 0.1% acetic acid in dH2O, 40 µl 4',6-diamidino-2-phenylindole (DAPI, 1:1000, Sigma, St. Louis, Missouri)) for 30 minutes at 25°C, and then washed 3 times in dH2O, for one minute per wash. After air-drying, the slides were washed with xylene for 2 minutes and then coverslipped using Depex® mounting solution (ElectronMicroscopySciences, Hatfield, PA).
Microscopy
FluoroJade stains were microscopically scanned and photographed at 4× followed by a tile scans at 20× of areas with appreciable cell death or of areas of special interest (23, 25), even if cell death was not obvious (e.g. medial prefrontal cortex, hippocampus, subiculum). The brain was mapped according to Paxinos’ atlas of the rat brain (33). All FJ+ cells were counted in 3 slices of the interrogated structure and the results were averaged. The thalamus was assessed as the entire structure. The hippocampal subregions analyzed were the cornu ammonis-1, cornu ammonis-2, cornu ammonis-3, pyramidal cell layer, and dentate gyrus. The subiculum was assessed as ventral and dorsal subiculum as well as the combined structure. Cortical areas included medial prefrontal, retrosplenial, visual, somatosensory, entorhinal, perirhinal and cingulate. All cortical layers were assessed in combination.
Behavior
Environmental Enrichment
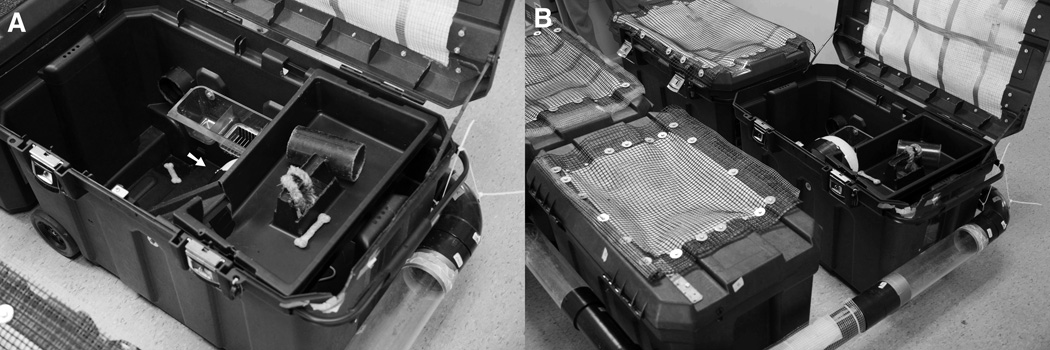
An enriched environment (Fig. 2) was constructed using four 50-gallon heavy-duty toolboxes (Pro Mobile job chest, The Stanley Works, New Britain, CT) purchased from a hardware store. Each chest was filled with at least 8 inch deep bedding, and contained a running wheel, a ramp to a tool tray suspended near the top of the box (“loft”), a rope and various toys (Fig. 2A). The four chests were connected by a series of tunnels so that rats had access to all chests and could interact with all other rats in the enriched environment (Fig. 2B). The tunnels were made of 4-inch diameter acrylonitrile butadiene styrene drain pipe alternating with 4-inch diameter clear acrylic pipes to help maintain the 12 h light/dark cycle. The pipes were connected to the enclosures with 4-inch diameter acrylonitrile butadiene styrene drain pipe fittings. Clear acrylic windows were installed at one end of each chest, and part of the lid was replaced with filter paper wrapped in plastic mesh, which was secured to the frame of the lid with nuts and bolts. The environmental enrichment was maintained from P28 until conclusion of behavioral testing on P 89.
Figure 2. Environmental Enrichment.
Four modified tool chests (A) were connected via a series of 4” ABS and clear acrylic pipes to provide environmental complexity, voluntary exercise (running wheel, arrow in 2A) and social interaction among 36 rats.
Fear Conditioning
On P63–P66, rats underwent fear conditioning. Four rats, counterbalanced for group assignment, were trained at a time. The chambers (length, 32 cm; width, 25 cm; height, 25 cm) were constructed of clear acrylic. The grid floor, used to deliver the shock, was composed of 19 stainless steel bars, each 4 mm in diameter and spaced 16 mm on center. These floors were connected to a shock delivery system (Med Associates, St. Albans, VT). The chambers were wiped with a pine-scented cleaner (5% Pine Scented Disinfectant; Midland, Inc., Sweetwater, TN) before and after each session. The room, in which training took place, was illuminated with overhead fluorescent bulbs, and a ventilation fan provided background noise (65 db). The appearance, odor, and texture of the chambers and room comprised the training context. After a 3-minute baseline exploratory period in the chambers, rats received three tone (2000 Hz, 90 db)–shock (1 mA, 2 seconds) pairings separated by 1 minute. Freezing, the absence of all movement except for respiration, is an innate defensive fear response in rodents and a reliable measure of learned fear. A minimum of three observers, blinded to group assignment, scored each animal’s freezing behavior every 8 seconds during the entire training period, and a percentage was calculated using the formula 100*f/n, where f is the number of freezing events per rat and n is the total number of observations per rat.
The next day, rats were tested for fear to the training context and to the tone. For the context test, each rat was placed in the chamber it had been trained in on the previous day for 8 minutes, but this time without the tone or shock. For the tone test, groups of rats were transported in separate plastic pots (height, 14 cm; diameter, 15.5 cm) to a distinct context in a different room, where the test chambers were triangular in shape, with an acrylic floor (length, 28 cm; width, 25 cm), and two acrylic sidewalls (length, 28 cm; width, 22 cm), connected to the floor at a 45-degree angle. The chambers were equipped with a speaker and were wiped down with acetic acid (1%; Fisher Scientific, St. Louis, MO) before and after each session. The room was lit by a single 30-Watt red bulb and appeared dark. Rats were given a 3-minute exploratory period before three 30-second tones (2000 Hz, 90 db) separated by 60 seconds. Rats were removed from the chamber after an additional 30 seconds. The order of the context and tone tests was counterbalanced such that half of each treatment group was first tested to context and then to tone and vice versa. A minimum of three observers, blinded to group assignment, scored each animal’s freezing behavior every 8 seconds during the context tests and the tone test.
Morris Water Maze Spatial Reference Memory
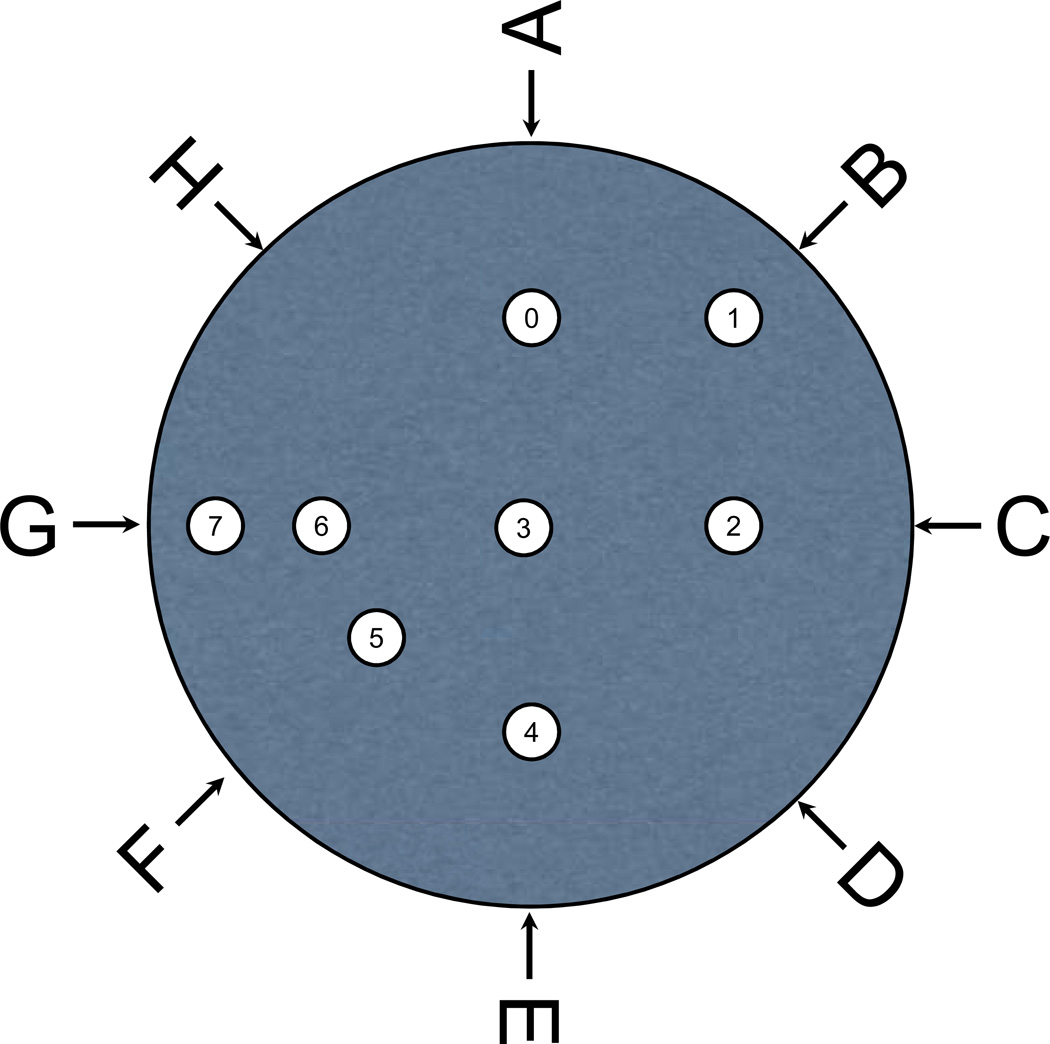
From P69 – P75 rats underwent spatial reference memory testing in the Morris water maze. A platform (diameter, 10.3 cm) was submerged in a circular pool (diameter, 180 cm; depth, 50 cm) filled with warm (24°C) opaque water. Two training sessions, spaced 7 hours apart, were administered each day. In each training session consisted of three trials, in each of which the rat was released from one of six pseudorandomly assigned release points (see Fig. 3) facing the wall of the tank. This resulted in one short, one medium, and one long swim per session, in random order. Animals were given 90 seconds to locate the hidden platform. If it did not locate the hidden platform in the allotted time, the rat was guided to the platform. In either case, the rat was removed from the platform after 15 seconds. To minimize any bias associated with platform location, equal numbers of rats in each group were assigned one of four platform locations for the duration of training (even numbers in Fig. 3). Training sessions were administered until the rats were able to locate the hidden platform in less than 15 seconds, averaged over a session (after five or more training sessions). Time to reach the platform (latency), path length, swimming speed, and time-integrated distance to the platform were analyzed using an EthoVision® video tracking system (Noldus Instruments, Wageningen, Holland) set to analyze ten samples per second.
Figure 3. Water maze setup.
Water maze tank with escape locations (numbers) and drop locations (letters). The numbers in the small circles represent escape locations for the spatial reference memory task (0,2,4 and 6) and the spatial working memory, short term memory and early long term memory tasks (1,3,5 and 7). The escape platform locations for the spatial reference memory task are the centers of the four quadrants. The escapes for the tasks assessing memory consolidation are half way between spatial working memory escape locations and the wall of the tank on the border of two quadrants (1) or within a quadrant (7), or in the center of the maze (3) or on a line perpendicular to the border between two quadrants that transects one of the first two escape locations (5).
Probe Trials
To assess memory retention for the hidden platform location, a probe trial, with the platform removed from the tank, was administered immediately after the last training session (P75), and three days later (P78). During the 60-second probe trial, the proportion of time spent in the target quadrant versus each of the other quadrants was determined both overall and separately for each of two 30-second intervals.
Cued Trials
On P78, after the probe trial, the appearance of the room was changed completely by relocating both the maze and adding a new set of remote spatial cues. The escape platform was moved and made visible by raising it 1 inch above the water surface and marking its edge with bright red tape, that could be seen clearly from the water surface but not by the tracking system. Rats were tested in three visible platform sessions (three trials per session); the platform was relocated between each session. The same dependent variables recorded and analyzed during the previous hidden platform trials were recorded and analyzed during visible platform trials. Cued testing concluded on P79.
Morris Water Maze Memory Consolidation
Memory consolidation describes the transition from unstable memories to stable memories (34). At least four different stages of memory consolidation are distinguished, three of which are assessed here: working memory (minutes), short term memory (minutes to hours), early long term memory (greater than 3 h) (35, 36). We did not assess remote long term memory, since interrogation of this process requires a delay between memory formation and recall of weeks to months (37, 38).
Working Memory
On P80 the room was again completely rearranged by repositioning the tank and adding a new set of remote spatial cues. The platform was submerged 1 inch below the water surface in one of four platform positions (odd numbers in Fig. 3), which were used, on average, once every fourth session. From P80 onwards, one session was administered per day, which consisted of a 60-second free swim (performance not scored), during which the rat was allowed to explore the maze, and three subsequent scored trials, with a 1-minute delay between the free swim and scored trials. If the rat found the platform during the free swim, it was allowed to remain on it for 15 seconds. The rat was guided to the platform and remained there for 15 seconds if it did not locate the platform during the free swim. After the free swim, three trials were administered, in which the rat was released from one of six pseudorandomly chosen locations facing the wall of the tank. The platform location was identical for all animals in a session, but the drop location was pseudorandomly varied to incorporate one short, one medium, and one long swim. Training sessions were administered until the rats were able to locate the hidden platform in less than 15 seconds, averaged over a session (after five or more training sessions). Time to reach the platform (latency), path length, swimming speed and time-integrated distance to the platform were recorded using an EthoVision® video tracking system (Noldus Instruments, Wageningen, Holland) set to analyze 10 samples per second.
Short Term Memory and Early Long Term Memory
When the rats achieved the predifined performance criterion (locating the hidden platform in less than 15 seconds, averaged over a session), on P87, indicating that working memory latencies no longer decrease, increasing delays between the free swim and the subsequent trials were implemented. On P88, the delay was extended to 1 hour delay, which tests short term memory (35) and on P89, the delay was extended to 4 hours, during which short term memory is no longer operant and early long term memory has been established (35). Time (latency) to reach the platform, time-integrated distance to platform, path length, and swim speed were again recorded with the tracking system, as described above. Performance on the first trial after the free swim on P87 (1-minute delay), P88 (1-hour delay), and P89 (4-hour delay) was used as a measure of working memory, short term memory and early long term memory, respectively.
Statistical Methods
Data were tested for normal distribution using Pearson and d’Agostino’s normality test. If the data of all groups being compared met parametric assumptions data were expressed as means and 95% confidence intervals and analyzed using parametric tests, specifically independent Student t-test to compare two groups (body weights of animals housed conventionally or in an enriched environment), one-way analysis of variance (ANOVA) with Bonferroni’s post-hoc test, when groups were compared at a single time point (for example fear conditioning data) and repeated measures two-way ANOVA with Bonferroni’s post-hoc test, whenever groups were compared over multiple time points (e.g. water maze data). Probe trial data were analyzed using a regular two-way ANOVA (no repeated measures). Whenever possible, the number of comparisons in the post-hoc tests was restricted to those necessary to test the hypotheses, i.e. normally housed control (Control NH) versus environmentally enriched control (Control EE), ControlNH versus normally housed sevoflurane (Sevo NH) and Sevo NH versus environmentally enriched sevoflurane (Sevo EE). If data from one or more groups did not fulfill parametric assumptions, data were expressed as medians and interquartile ranges and were analyzed using nonparametric tests (Mann Whitney U test or Kruskall Wallis test with Dunn’s Multiple Comparisons Test, when indicated). All comparisons were run as two-tailed tests. A P-value of 0.05 was considered statistically significant. For parametric multiple comparison post hoc tests the effect size is given as the difference in means and the 95% confidence interval (CI) of the difference in means. For nonparametric multiple comparison post hoc tests, the effect size is given as the rank sum difference.
Cell death data are presented as median fold increase from control and the respective ranges. Group comparisons were made in seven brain areas using the Kruskall Wallis test with Dunn’s correction for multiple comparisons.
Water maze data are given as latencies to the hidden platform, since swim speeds did not differ between groups during spatial reference memory testing, and since latency correlated very well with the other measures of performance in the water maze (path length, time-integrated distance to platform). Additionally, spatial reference memory data were expressed as the number of sessions (excluding the first three) required to reach a proficiency benchmark of finding the hidden platform in 30 seconds, averaged over one session (three trials). For probe trials, besides target quadrant dwell times and platform crossings, the percentage change in proportion of time spent in the target quadrant from the first to the second probe trial was calculated using the formula (percent duration of probe trial 2-percent duration of probe trial 1)/(percent duration of probe trial 1)*100. This parameter was calculated for each rat individually in an attempt to assess the degree of memory extinction between the first and second probe trial.
All analyses were performed using Prism for Macintosh 5.0 (GraphPad Software Inc., San Diego, CA).
Sample Size Calculation
We previously found that 4 hours of isoflurane at 1 MAC increases short term memory latencies at 1 hour delays from 40 to 52 sec compared to controls (4). Based on this effect size, and a standard deviation of the outcome variable in the anesthetic group of 12 seconds (4), we estimated that the minimum group size required to detect a similar difference with 80% power at a significance level of 0.05 is 17 animals per group. SAS version 9 (SAS Institute, Cary, NC) Proc POWER was used for sample size calculations.
Results
Missing data
There were no missing data.
MAC, anesthetic mortality, blood gases and body weight
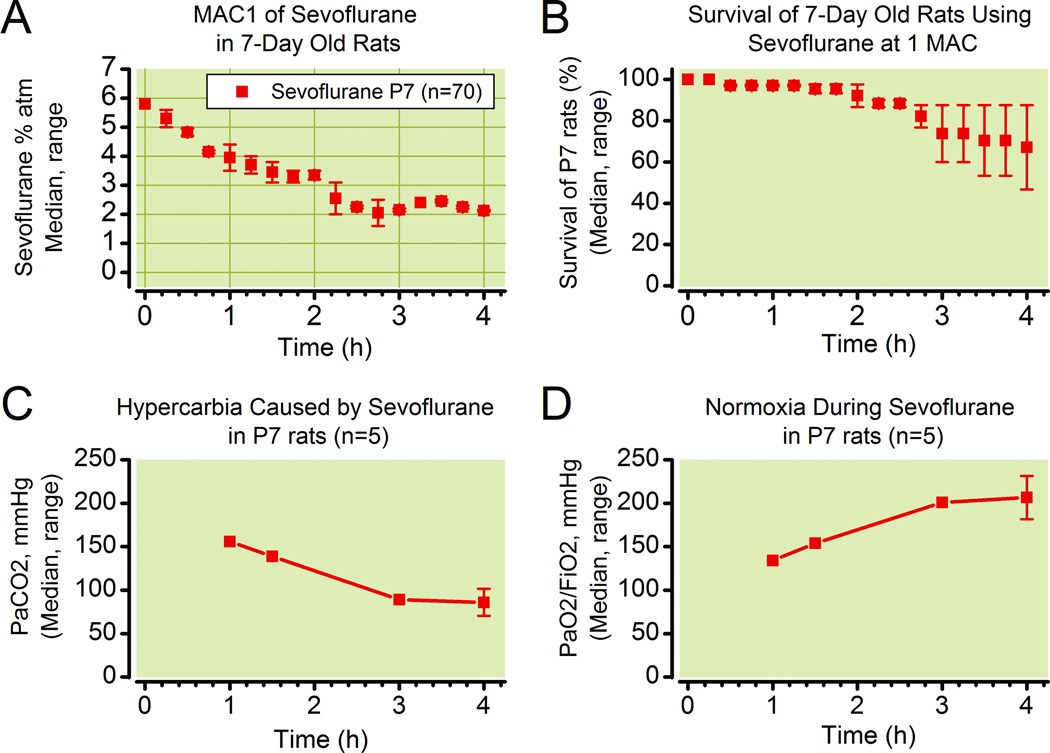
The inspired MAC of sevoflurane for P7 rats decreased continuously with increasing duration of anesthesia (Fig. 4A). Median MAC of sevoflurane was 5.3% atm (range 5.0 to 5.6) 15 minutes after induction of general anesthesia. This gradually decreased to a median of 2.1% atm (range 2.1 to 2.15) at the end of 4 hours of anesthesia. Median survival of P7 rats was 92% (range 87 to 98%) at the end of 2 hours of 1 MAC sevoflurane, and dropped rapidly in the third and fourth hours to a median of 73% (range 60 to 88%) and 67% (range 47 to 88%) respectively (Fig. 4B). Arterial blood gas analyses obtained by percutaneous puncture of the left ventricle from animals not participating in any other part of the experiment reveal moderate hypercarbia due to respiratory depression caused by sevoflurane at 1 MAC (Fig. 4C). Respiratory acidosis is a consquence with pH values between 6.9 (n=1) after 1 hour and a median of 7.13(range 7.07 to 7.19, n=2) after 4 hours (not shown). After three hours of anesthesia, base excess was negative in the three tested rats (-3.9 at 3 hours (n=1), −2.0 and −3.1 at 4 hours (n=2)) indicating that sevoflurane causes mild metabolic acidosis. Importantly, hyopxemia did not occur (Fig. 4D).
Figure 4.
Sevoflurane anesthesia in infancy - Minimum alveolar concentration (MAC) survival and arterial blood gas analyses.
Inspired MAC of sevoflurane, as determined by tail clamping, of P7 rats (n=30) during two separate 4 hour anesthetic exposures (A). Survival of P7 rats over four hours of sevoflurane at 1 MAC during three separate anesthetic exposures (B). Sevoflurane depressed respiration and caused hypercarbia (C) but not hypoxemia (D).
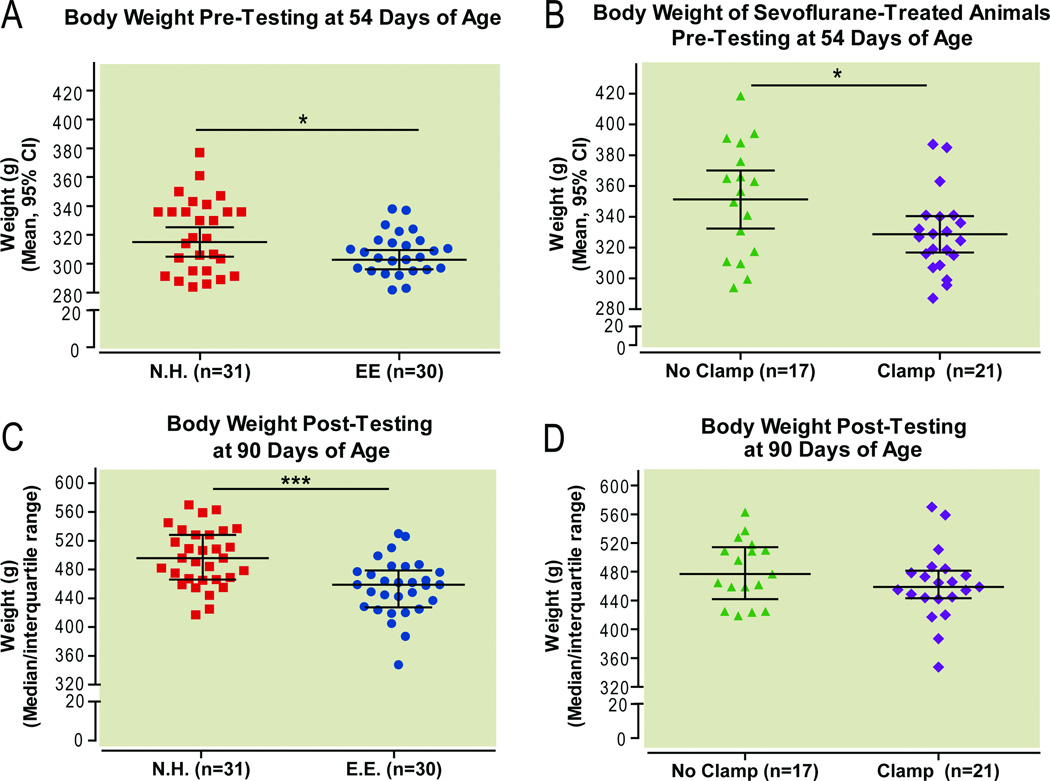
Before behavioral testing, animals housed in an enriched environment weighed significantly less than normally housed animals (Fig. 5A, P=0.044, unpaired t-test), and rats that had undergone tail clamping during anesthesia weighed less than those rats that were anesthetized without tail clamping (Fig. 5B, P=0.032, unpaired t-test). After behavioral testing, the effect of environmental enrichment on body weight persisted (Fig. 5C; P=0.0002, unpaired t-test), whereas the effect of tail clamping on body weight did not (Fig. 5D, P=0.20, unpaired t-test).
Figure 5.
Effect of environmental enrichment and tail clamping on body weight prior to behavioral testing (A,B) and after testing (C,D).
*P<0.05, ***P<0.01, unpaired t-test. EE environmental enrichment, NH normal housing, Sevo sevoflurane
Histology
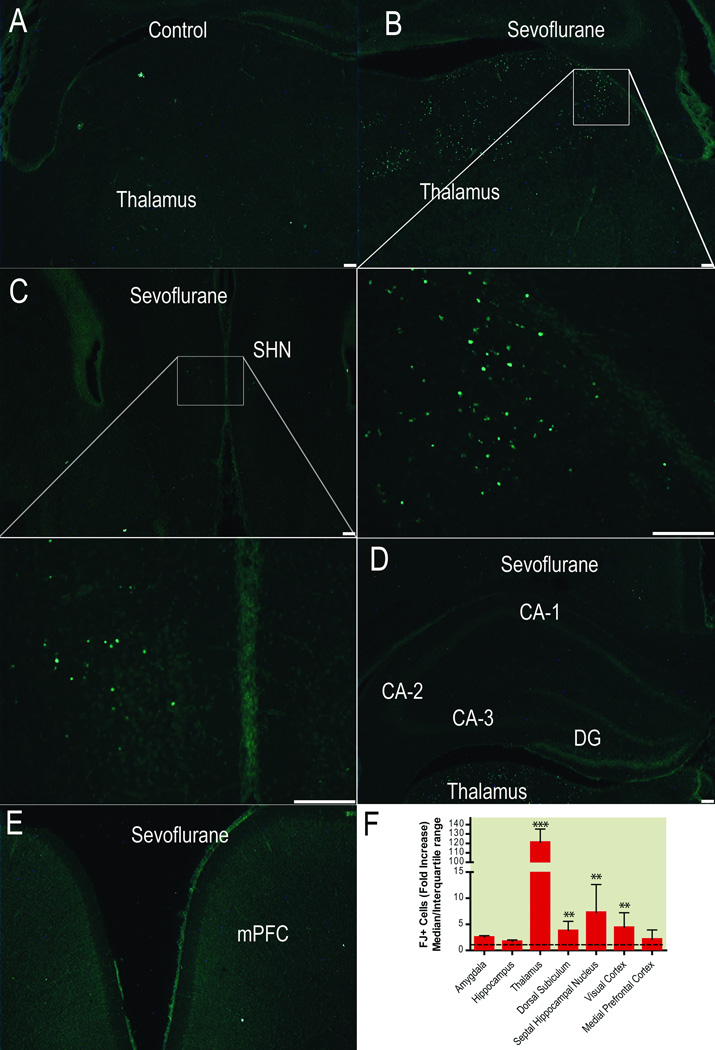
Sixteen hours after sham anesthesia, brains of sevoflurane-treated animals showed a greater amount of FJ+ staining than control animals (Fig. 6). Control animals had FJ+ cells in all examined structures, most prominently in the amygdala where a median of 6.7 (range 4 to 12) FJ+ cells per slice were found and least prominently in the thalamus where slices contained a median of 0.6 FJ+ cells (range 0 to 1.3) (Fig. 6A). Sevoflurane increased cell death (P<0.0001, Kruskall Wallis test) most dramatically in the thalamus (median 121 fold increase from control, range 54 to 140, rank sum difference [thalamus vs control]: 50.25, P<0.001, Dunn’s correction for multiple comparisons, Fig 6B,F). Other areas in which sevoflurane increased cell death were the septal hippocampal nucleus (median 7.3 fold increase from control, range 1.6 to15.1, rank sum difference [septal hippocampal nucelus vs control]: 33.38, P<0.01, Dunn’s correction for multiple comparisons, Fig. 6C,F), the dorsal subiculum (median 3.8 fold increase from control, range 2.5 to 15, rank sum difference [dorsal subiculum vs. control]: 29.38, P<0.01, Dunn’s correction for multiple comparisons, Fig. 6F), and the visual cortex (median 4.4 fold increase from control, range 2 to 13.1, rank sum difference [visual cortex vs control]: 29.79, P<0.01, Dunn’s correction for multiple comparisons, Fig. 6F). No other areas were affected by sevoflurane-induced cell death, including, notably, the hippocampus (median 1.73 fold increase from controls, range 0.67 to 2.1, rank sum difference [hippocampus vs. control]: 5.25, P>0.05, Dunn’s correction for multiple comparisons, Fig. 6D,F) and the medial prefrontal cortex (median 2.17 fold increase from controls, range 0.34 to 4, rank sum difference [medial prefrontal cortex vs. control]: 13.38, P>0.05, Dunn’s correction for multiple comparisons, Fig.6E,F).
Figure 6. Sevoflurane-induced brain cell death.
Cell death in the brain of 7-day old rats (n=8) treated with 4 hours of sevoflurane at 1 minimum alveolar concentration, as assessed by FluoroJade staining 16 hours after anesthesia. Photomicrograph (4× objective) of sporadic occurrence of FluoroJade+ cells in the thalamus of sham anesthetized animals (n=8) (A). Photomicrographs of FluoroJade+ cells in the thalamus (B) (4× with 20× inset) and the septal hippocampal nucleus (C) (4× with 20× inset) after sevoflurane anesthesia. In contrast, no significant increase in FJ stained cells can be found in the hippocampus (D) or the prefrontal cortex (E) of sevoflurane-treated animals. All regions with sevoflurane-induced cell death (F) as well as some regions not affected by sevoflurane-induced cell death, that are important in the behavioral outcome affected by sevoflurane (42, 50). Scale bar: 100µm.
** p<0.01, *** p<0.001. CA cornu ammonis, DG dentate gyrus, mPFC medial prefrontal cortex, SHN septal hippocampal nucleus.
Fear Conditioning
Training
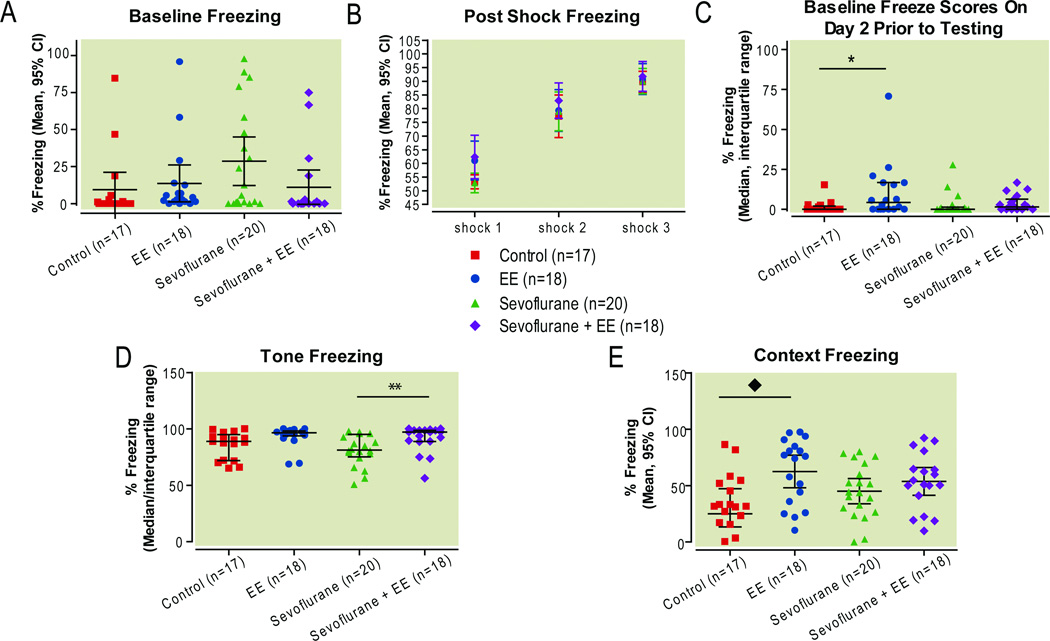
Baseline freezing in response to the fear conditioning environment, prior to tone/shock presentation, was similarly low, with an overall effect of treatment but no significant differences across groups after adjusting for multiple comparisons (Fig. 7A; P=0.034, Kruskall-Wallis test; rank sum difference [Control NH vs Control EE]: −14.56; [Control NH vs Sevo NH]: −15.98; [Sevo NH vs Sevo EE]: 13.59; all P>0.05 Dunn’s Multiple Comparisons Test). Administration of the tone/shock pairings resulted in progressive and robust post-shock freezing, representing immediate fear response conditioning to the tone/shock pairings. Post-shock freezing developed similarly cross all groups over the course of three shocks. (Treatment F(3,138)=1.9, P=0.14, Shock F(2,138)=169.9, P<0.001, Interaction (Treatment×Shock) F(6,138) =0.8, P=0.57, Fig. 7B).
Figure 7. Fear conditioning.
Freezing (absence of motion except respiration) was assessed prior to (A) and immediately after (B) three pairings of a conditioned stimulus (tone) and an unconditioned stimulus (shock). On the following day the association between the shock and the tone was tested in a completely different context. Freezing was assessed prior to (C) and after (D) re-exposure to the tone. Then, rats were returned to the original context, in the absence of tone or shock, and freezing was assessed once again (E).
*P<0.05, **P<0.01, Kruskall Wallis test with Dunn’s Multiple Comparisons Test (one or more groups did not pass normality test). ◆P< 0.05, one-way ANOVA, Bonferroni post hoc comparisons. EE enriched environment, NH normal housing, Sevo sevoflurane
Tone Test
There was no effect of sevoflurane on tone freezing 1 day after training, both prior to, and in response to the tone (rank sum difference [Control NH vs. Sevo NH]: −1.9 and 6.5 respectively, Fig. 7C,D). Control rats housed in an enriched environment had greater baseline freeze scores on day 2, prior to presentation of the first tone in the new context, compared with control rats that were normally housed (medians 4.1 vs. 0, range 0 to 70.8 vs. 0 to 15.3, respectively; P=0.028, Kruskall-Wallis test; rank sum difference [Control NH vs. Control EE]: −16.32, P<0.05, Dunn’s Multiple Comparisons Test, Fig. 7C). Furthermore, sevoflurane-treated rats, housed in an enriched environment, froze more in response to tone presentation, compared to normally housed, sevoflurane-treated rats (median 97.3 vs. 81.3, range 56.3 to 100 vs. 50.7 to 97.9, respectively; P=0.0029, Kruskall-Wallis test; rank sum difference [Sevo NH vs. Sevo EE]: 20.38, P<0.01, Dunn’s Multiple Comparisons Test, Fig. 7D). These results indicate that sevoflurane does not affect performance in the fear conditioning task, and that environmental enrichment improves fear conditioning performance.
Context Test
There was an overall effect of treatment in the context test (F(3,69)=3.3, p=0.025, one-way ANOVA). Sevoflurane treatment had no effect on freezing in response to the fear conditioning context, presented without the tone, 1 day after training (mean difference [Control NH vs. Sevo NH]: −8.13; 95%CI: −29 to 11.6; P>0.05 Bonferroni’s multiple comparison test, Fig. 7E). Control rats housed in an enriched environment had a higher percentage of freezing in the fear conditioning context than control rats that were normally housed (means 62.5 vs. 36.9, respectively; 95% CI of difference in means 4.5 to 46.7, P<0.05, Bonferroni’s multiple comparison test; Fig. 7E). These results, again, indicate that sevoflurane has no effect, and that environmental enrichment improved fear conditioning performance.
Spatial Reference Memory
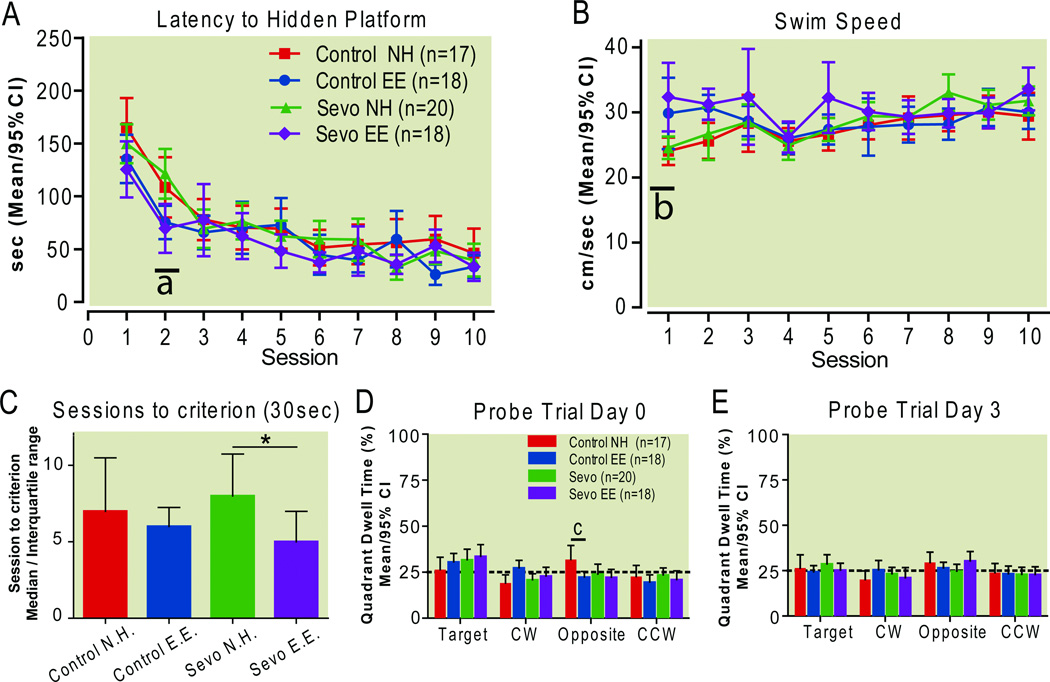
Repeated measures 2-way ANOVA showed an effect of treatment (F(3, 621)=3.15; P=0.03) on latency to hidden platform and an interaction of treatment-by-time (F(27,621)=1.64; P=0.02). Sevoflurane alone did not affect escape latencies but environmental enrichment decreased escape latencies in sevoflurane-treated animals during the second session of the task, at the steepest part of the learning curve (Difference in means[Sevo NH vs Sevo EE]: 51.8; 95%CI: 8.3 to 95.4; p<0.001, Bonferroni post hoc test, Fig. 8A).
Figure 8.
Spatial reference memory. Sevoflurane did not affect spatial reference memory. Environmental enrichment improved performance in sevoflurane-treated rats.
Escape latency to the hidden platform (A). At the second session, escape latency of sevoflurane-treated animals housed in an enriched environment is lower than latency of sevoflurane-treated rats housed conventionally. Swim speeds (B) and sessions to reach the platform in 30 seconds, averaged over three trials (C). Probe trial (platform removed) target quadrant dwell times on days 0 and 3 after completion of spatial reference memory testing (D–E). The dotted line indicates chance performance. *P<0.05, Kruskall Wallis test with Dunn’s Multiple Comparisons Test (data from one or more groups did not pass normality test). a P<0.001, b P<0.01, c P<0.05, Bonferroni post hoc comparisons.
CCW counterclockwise, CW clockwise EE enriched environment, NH normal housing, Sevo sevoflurane
Swim speeds did not differ between groups (treatment F(3,621)=1.91; P=0.14 repeated measures 2-way ANOVA, Fig. 8B), although there was a treatment-by-session interaction (F(27,621)=2.06; P=0.0014 repeated measures 2-way ANOVA). Bonferroni post hoc tests revealed that only during the first session sevoflurane-treated animals that were normally housed swam more slowly than their environmentally enriched counterparts (Difference in means 7.8 m/sec; 95% CI: 0.8 to 14.8; P<0.01).
Environmental enrichment in sevoflurane-treated animals decreased the number of training sessions needed to reach a 30-second benchmark latency to the hidden platform (P=0.049, Kruskall-Wallis test; rank sum difference [Sevo NH vs. Sevo EE]: 17.81, P<0.05, Dunn’s Multiple Comparisons Test, Fig. 8C).
Probe Trials
A probe trial was performed both immediately after the spatial reference memory sessions, and three days later. During each probe trial, the target platform was removed from the maze, and the percentage of time spent searching in the target quadrant, defined by the location of the removed platform, was measured. Two-way ANOVA showed that treatment did not affect target quadrant dwell times at either probe trial (1st trial F(3,276)=4.1×10−8; P=1.0; 2nd trial F(3,272)=6.6×10−6, P=1.0, 2-way ANOVA). There was, however, an interaction between quadrant and treatment during the first but not the second probe trial (1st trial F(9,276)=2.66; P= 0.0057; 2nd trial F(9,272)=1.14; P=0.33, 2-way ANOVA). Animals in all treatment groups spent similar amounts of time in each of the four quadrants, indicating near chance performance, with the exception of the normally housed control group, which, only when compared to controls housed in an enriched environment, showed a slight preference for the quadrant opposite of the target quadrant (Difference in means: 9.5, 95%CI: −20.0 to1.1; P<0.05, Bonferroni post hoc test, Fig. 8D,E). There was also no difference between groups in the percentage change in target quadrant dwell time from the first probe trial to the second probe trial (not shown). Taken together, these probe trial analyses indicate that animals in all treatment groups performed close to chance level, and that neither sevoflurane treatment, environmental enrichment, nor the amount of time between spatial reference memory training sessions and the probe trial had any impact on this performance.
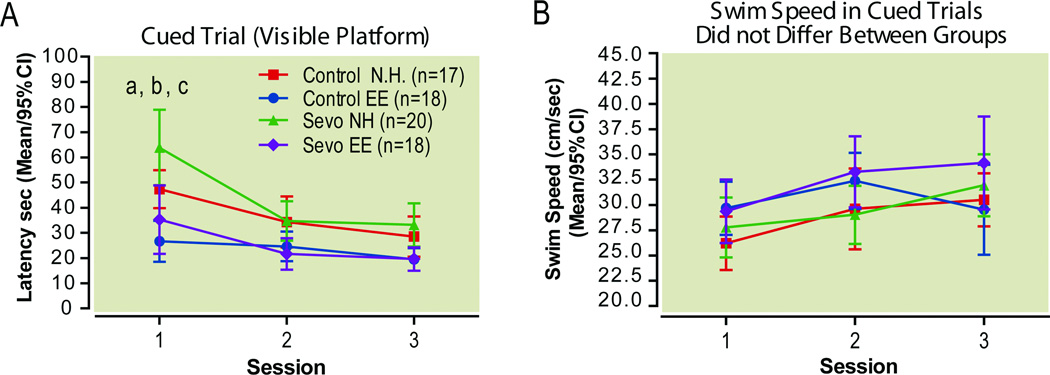
Cued Trials
There was a strong effect of treatment (F(3, 138)=14.99; P<0.0001, repeated measures 2-way ANOVA) and an insignificant treatment-by-session interaction (F(6,138)=2.15, P=0.051, repeated measures 2-way ANOVA). During the first session of the cued trials, normally housed sevoflurane-treated rats had longer escape latencies to the visible platform than normally housed controls (Difference in means [Sevo NH vs. Control NH]: 16.6, 95%CI: −1.8 to 34.9, P<0.05, Bonferrroni post hoc test, Fig. 9A). The groups housed in the enriched environment, whether control or sevoflurane-treated, had the lowest latencies at this session (Difference in means [Control NH vs. Control EE]: 20.7; 95%CI: 1.9 to 39.5, P<0.01; Bonferroni post hoc test, Difference in means [Sevo NH vs. Sevo EE]: 28.6; 95%CI: 10.6 to 46.7; P<0.001, Bonferroni post hoc test). During the second and third sessions of the cued trials, all groups had similar escape latencies, indicating that the greater escape latencies of sevoflurane-treated rats during the first session might be more appropriately interpreted as a problem related to learning a new task, rather than a sensiromotor deficit, which should have affected all sessions (39),or a problem relating to specific brain areas (40). Swim speeds did not differ between groups in this test (Treatment F(3, 138)=1.3, P=0.2781, Treatment-by-session interaction F(6,138)=1.7, P=0.13, repeated measures 2-way ANOVA, Fig. 9B)
Figure 9. Cued trials.
Sevoflurane-induced deficit on first session of cued trials is mitigated by delayed environmental enrichment. Escape Latencies to visible platform (A) and swim speeds (B).
a–c two-way ANOVA with Bonferroni post hoc comparisons. a Control NH vs. Sevo NH, P<0.05; b Control NH vs. Control EE, P<0.05; c Sevo NH vs. Sevo EE, P<0.001. EE enriched environment, NH normal housing, Sevo sevoflurane
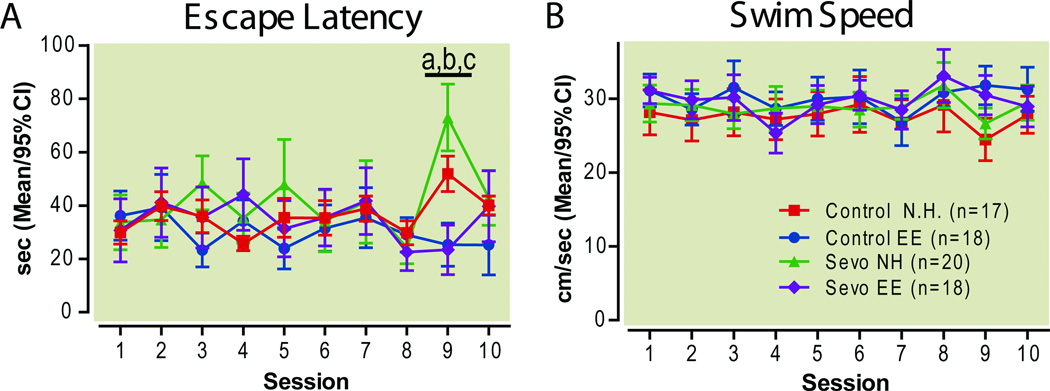
Spatial Working Memory, Short Term Memory and Early Long Term Memory
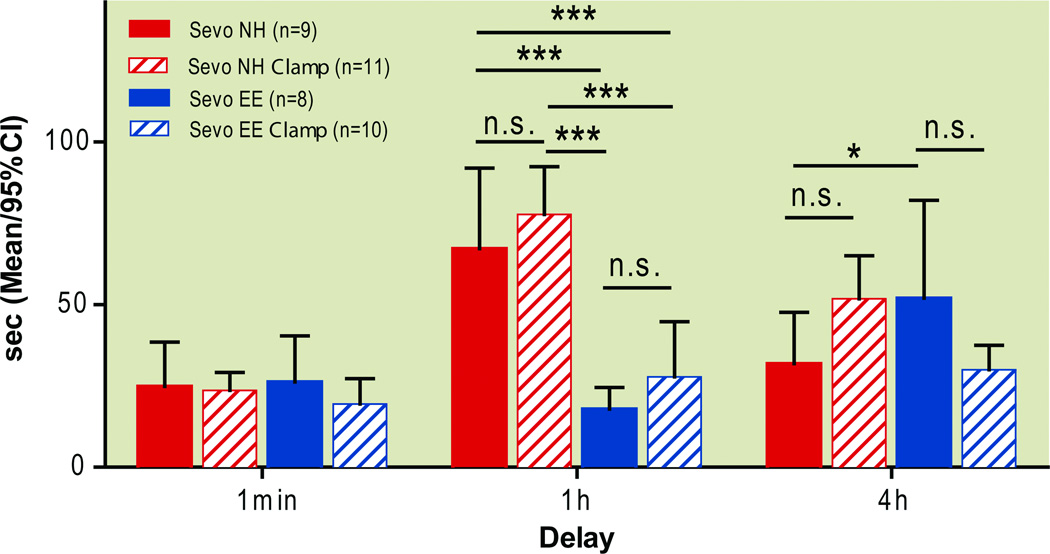
Next, the platform was moved to a different location each day. One unscored exploratory swim preceded three scored swims, the latencies of which were added. A repeated measures 2-way ANOVA revealed effects of treatment (F(3, 621)=4.76, P=0.0045), session (F(9,621)=3.26, P=0.0007) and a treatment-by-session interaction (F(27,621)=3.24, P<0.0001). Bonferroni post hoc tests showed that these effects were due entirely to the change in latencies at the 1 hour delay session (session 9), where normally housed, sevoflurane-treated rats had significantly longer escape latencies than normally housed controls, and where rats from an enriched environment had significantly lower escape latencies than their normally housed counterparts, regardless of exposure to sevoflurane (Differences in means [Control NH vs. Control EE]: 26.54; 95%CI: 1.3 to 51.9, P<0.01) [Control NH vs Sevo NH]: 21.13; 95%CI: −3.563 to 45.83, P<0.05, and [Sevo NH vs. Sevo EE]:49.63; 95%CI: 25.31 to 73.96, P<0.001, Fig. 10A). Sevoflurane-treatment had no effect when rats were housed in an enriched environment (Difference in means [Control EE vs. Sevo EE]: 1.956; 95%CI:-26.91 to 22.99, p>0.05, Bonferroni post hoc test). There was no significant difference in swim speeds between groups (Treatment F(3, 621)=1.18, P=0.32, repeated measures 2-way ANOVA, Fig. 10B) at sessions 1–8. Beginning at session 9, delays were introduced between an animal’s free swim (unscored exploratory swim to introduce the new platform location) and scored trials: a 1-hour delay at session 9, testing short term memory and a 4-hour delay at session 10, testing early long term memory. Repeated measures 2-way ANOVA of the escape latencies at the first swim of each delay session showed strong effects of treatment (F(3, 138)=12.85; P<0.0001), session (F(2, 138)=22.78, P<0.0001), as well as a treatment-by-session interaction (F(6,138)=8.7; P<0.0001). Bonferroni post hoc tests confirmed, again, that the differences between groups occurred at the 1-hour delay, where the normally housed, sevoflurane-treated group had significantly longer escape latencies than the normally housed control animals (Difference in means [Control NH vs. Sevo NH]: 14.32; 95%CI: −0.01 to 28.66, P<0.01, Bonferroni post hoc test). At the same time, both environmental enrichment groups performed equally well, with shorter latencies to the hidden platform than either of their normally housed counterparts (Difference in means [Control NH vs. Control EE]: 16.53; 95%CI:1.84 to 31.23, P<0.01; Difference in means [Sevo NH vs. Sevo EE]: 33.71; 95%CI: 19.59 to 47.83, P<0.001, Bonferroni post hoc tests, Fig. 11A). Once again, sevoflurane exposure had no effect on escape latencies when rats were housed in an enriched environment (Difference in means [Control EE vs. Sevo EE]: 2.853; 95%CI:-17.34 to 11.631, p>0.05, Bonferroni post hoc test) These results indicate that sevoflurane impaired performance in a spatial recognition memory task, when a 1-hour delay is introduced between memory encoding and memory retrieval, and that environmental enrichment negated this deficit and actually improved performance regardless of sevoflurane treatment. Interestingly, the Bonferroni post hoc tests showed no difference between normally housed sevoflurane and control groups at the 4-hour delay session, indicating that a 1-hour delay is critical for detecting sevoflurane-induced memory dysfunction.
Figure 10. Spatial memory consolidation.
Platform location was changed prior to each session. The criterion of having 80% of rats find the platform within an average of 10 seconds was not met until session 8, after which the delay between the initial introduction into the water maze and the subsequent scored trials was extended from 1 minute (working memory) to 1 hour at session 9 (short term memory) and 4 hours at session 10 (early long term memory). Latencies to the hidden platform (A) and swim speeds (B) averaged over 3 scored trials.
a P<0.05, Bonferroni post hoc test, Control NH vs. Sevo NH, b P<0.01, Bonferroni post hoc test, Control NH vs Control EE, c P<0.001, Bonferroni post hoc test, Sevo NH vs. Sevo EE. EE enriched environment, NH normal housing, Sevo sevoflurane
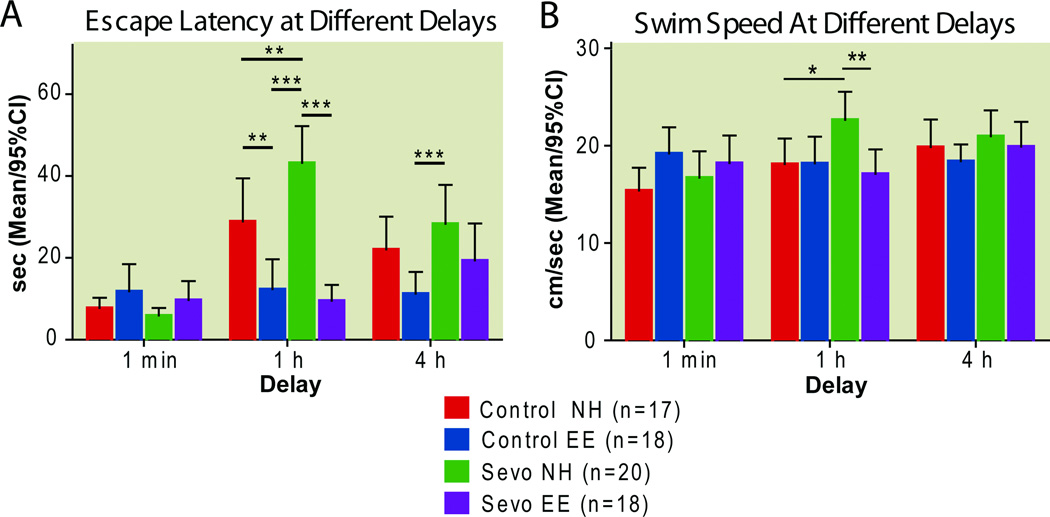
Figure 11. Spatial memory at different delays: Delayed environmental enrichment mitigates sevoflurane-induced short term memory deficit.
Sessions 8–10 of the spatial memory consolidation test introduced various delays between the rat’s initial introduction to the maze and the first of three subsequent “scored” trials. The overall change in latency from the first swim of session 8 (1-minute delay) to the first swim of session 9 (1-hour delay) is the measure of short term memory(A). Swim speeds during the first swim of each delay session (B).
* P<0.05, ** P<0.01, *** P<0.001,two-way ANOVA, Bonferroni post-tests. EE enriched environment, NH normal housing, Sevo sevoflurane
Repeated measures 2-way ANOVA revealed no effect of treatment on swim speeds during the first swims of sessions 8–10 (1min, 1 h and 4 h delays) (F(3,138)=1.34, P=0.27) but an effect of sessions (F(2,138)=5.16, P=0.0072) and a treatment-by-time interaction (F(6,138)=3.12; P=0.0067). Bonferrroni post hoc tests revealed that normally housed sevoflurane-treated animals increased their swim speeds significantly more than normally housed controls from session 8 to session 9 (Difference in means [Control NH vs. Sevo NH]: 4.566; 95%CI: −0.7368 to 9.869, P<0.05 Fig. 11B). Rats living in an enriched environment, by contrast, showed a decrease in swim speeds from session 8 to session 9 with environmentally enriched sevoflurane-treated rats swimming significantly more slowly than normally housed sevoflurane–treated animals (Difference in means [Sevo NH vs. Sevo EE]:5.551; 95%CI: 0.3281 to 10.77, P<0.01, Bonferroni post hoc test).
Taken together, these results suggest that despite faster swim speeds at the 1 h delay, normally housed sevoflurane-treated rats took longer to find the hidden platform. Conversely, despite swimming more slowly than their normally housed counterparts, sevoflurane-treated rats living in an enriched environment took less time to find the platform.
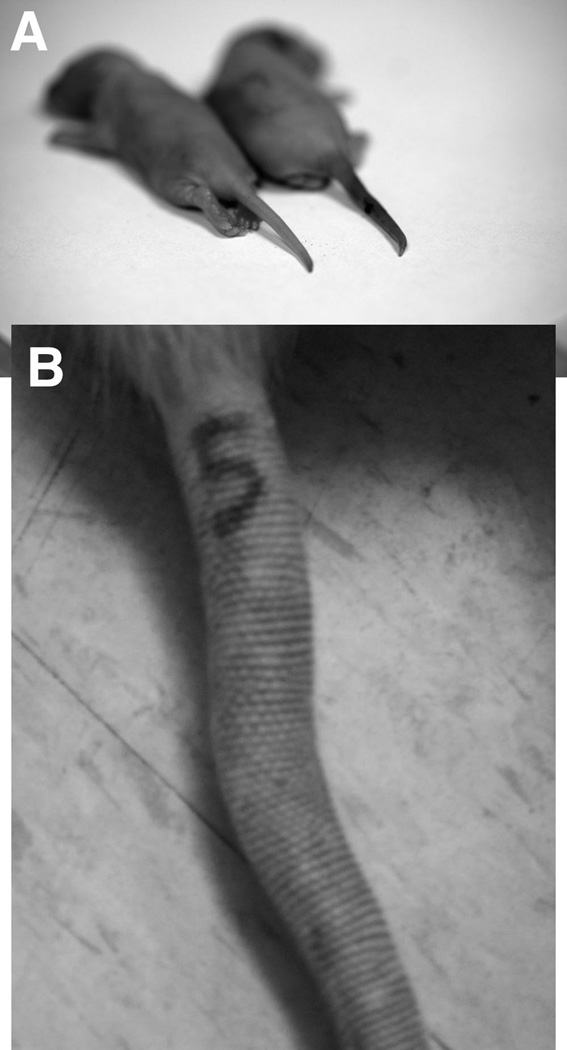
Effect of Tissue Injury
Tail clamping, which causes tissue injury (Fig. 12 A), hemorrhage (Fig. 12 A) and scarring (Fig. 12 B), had no effect on any of the behavioral outcomes. As an example, we show what happened when the data displayed in figure 11 are analyzed based on exposure to tail clamping during anesthesia. When the sevoflurane-treated groups (Sevo NH and Sevo EE) were broken down by tail clamping into four groups (Sevo NH clamped (n=11), Sevo NH not clamped (n=9), Sevo EE clamped (n=10) and Sevo EE not clamped (n=8)), the repeated measures 2-way ANOVA revealed an effect of tail clamping (F(3,36)=8.14, P=0.0003), an effect of session (F(2,36)=15.1, P<0.0001) and an interaction between tail clamping and session (F(6,36)=7.52, P<0.0001). Despite the small group sizes, Bonferroni post hoc tests confirmed the strong effect of environmental enrichment in improving performance of sevoflurane-treated non-clamped animals (Differences in mean latencies [Sevo NH not clamped vs. Sevo EE not clamped]: 49.33; 95%CI:19.09 to 79.58, P<0.001 and [Sevo NH clamped vs. Sevo EE clamped]: 49.98; 95% CI: 22.78 to 77.18, P<0.001). However, at no time point was there any difference between clamped and not clamped animals for a given housing condition (Differences in means [Sevo NH clamped vs. Sevo NH not clamped]: 10.51 at the 1 h delay (95%CI: −17.46 to 38.49) and 19.95 at the 4 h delay (95%CI: −8.028 to 47.92), both P>0.05. Differences in means [Sevo EE clamp vs. Sevo EE not clamped]: 9.87 at the 1 h delay (95%CI: −19.66 to 39.39) and −21.98 (95% CI: −51.50 to 7.546) at the 4h delay, both P>0.05, Fig 13). This indicates that performance is affected by the housing condition and not by tail clamping.
Figure 12. Tissue injury caused by tail clamping.
Photograph of tails of 7-day old rats that underwent tail clamping (A, right rat) and no tail clamping (A, left rat) during 4 hours of sevoflurane anesthesia. Photograph of representative example of a clamped tail 3 months after anesthesia. There is a fixed deformity caused by scarring of the tail (B).
Figure 13. Tail clamping does not affect spatial memory 3 months after tissue injury under anesthesia.
Escape latencies were similar between sevoflurane-treated groups that were tail-clamped and not tail-clamped during anesthesia at P7. The effect of delayed environmental enrichment on short term memory escape latency 3 months after anesthesia (see figure 10) persists both in the tail clamped and the not tail clamped groups. * P<0.05, *** P<0.001, two-way ANOVA, Bonferroni post-tests. EE enriched environment, NH normal housing, Sevo sevoflurane
Discussion
The main findings of this study are:
1) Sevoflurane, given to infantile rats, caused acute, mostly thalamic, cell death and impaired short term memory 3 months after the anesthetic. 2) Delayed environmental enrichment mitigated the effects of sevoflurane on memory. 3) Tissue injury did not further impair neurocognitive function.
Effects of Sevoflurane on Memory
Prior studies are controversial, as to whether sevoflurane in infancy does (8) or does not (13–15) cause long-term neurocognitive sequelae. The present study suggests that sevoflurane does cause long term neurocognitive sequelae. In our previous experiments (4) isoflurane impaired spatial memory when the delay between memory acquisition and retrieval was extended from 1 minute to 1 hour but not to 4 hours. We now show an identical pattern using sevoflurane. Intuitively, one would expect that performance worsens, as the delay between memory encoding and retrieval increases. However, the various stages of memory consolidation (34) may be selectively vulnerable to the effects of infantile anesthesia. The experimental paradigm used here tested working memory (1min delay), short term memory (1 hour delay) and, early long term memory (4 hour delay, 24 hour delays, 3 day probe trial) (36, 41). We did not test if anesthesia affects the later stages of memory consolidation, i.e. remote long-term memory, since the delay between memory acquisition and retrieval would have had to be extended to at least 14 – 25 days (37, 38).
If acute sevoflurane-induced brain cell death were responsible for the behavioral outcome, one possibility that must be considered is that the sevoflurane-induced loss of episodic memory at the 1-hour delay is thalamic (as opposed to hippocampal) in nature (42). The thalamus was the brain region most severely affected by sevoflurane-induced neurodegeneration. This is consistent with a prior study in mice, although the cell death observed by us was virtually exclusive to the thalamus with only minor cell death in a few other areas. It is possible that the different dosing regimens, and the different animal species may have contributed to the discrepant results. Previously 3% sevoflurane for 6 hours caused extensive neurodegeneration (8). Since MAC of volatile agents decreases with increasing duration of anesthesia in infantile rodents (4, 9, 15, 17, 43) 3% sevoflurane for 6 hours (8) is less than 1 MAC for the first 2 hours of exposure and more than 1 MAC for the remainder of the anesthetic (43), which is a greater anesthetic dose than the 1 MAC for 4 hours used in the current study. Importantly, 4 hours of isoflurane at 1 MAC causes substantial thalamic and cortical cell death (4), suggesting that the common denominator of the two volatile agents is thalamic cell death. We wish to point out that neither this study nor any prior investigation provides sufficient evidence for a causal relationship between anesthesia-induced thalamic or other cell death and anesthesia-induced cognitive dysfunction.
Cortical cell death in this study was minor compared to other studies (1, 4, 8, 9). Of the cortical structures the visual cortex was the most severely affected by sevoflurane-induced cell death (median 4.4 fold increase from control). This raises the remote possibility, that the deficits caused by sevoflurane might be related to visual disturbances, especially since the cued trial performance of sevoflurane-treated rats (platform was visible) was affected. This explanation remains unlikely, since blind rats can perform the spatial recognition memory task of the Morris Water maze better than cognitively impaired seeing rats (39) and since the cued trial performance of blind rats involves all sessions (39) instead of only the first, as occurred here. It is more likely that sevoflurane treated rats had difficulties learning the rules of a new task.
Rats in this study were moderately hypercarbic during anesthesia, raising the possibility that hypercarbia may have contributed to anesthesia-induced neurodegeneration as has previously been shown for isoflurane (4). However, we previously also showed that hypercarbia alone improves long term cognitive function (4). Therefore, hypercarbia should, if anything, have mitigated the effect of sevoflurane on cognitive function. The current findings might most appropriately be interpreted as sevoflurane-induced impairment of short term memory despite moderate hypercarbia.
Effect of Environmental Enrichment on Neurocognitive Function
Since the mechanism by which anesthesia in infancy impairs brain function in rodents is not yet known, preventive strategies that target an anesthesia-specific mechanism, are currently not available. Therefore, we decided to test if anesthesia-induced brain dysfunction can be treated instead. Indeed, environmental enrichment reversed the anesthetic effect on memory and rendered the performance of anesthetized rats indistinguishable from their environmentally enriched controls, and far superior to conventionally housed rats, regardless of sevoflurane treatment. It is remarkable that this intervention was effective, even when instituted with substantial delay (3 weeks after the anesthetic). The environmental enrichment paradigm combined three major stimuli: physical exercise (voluntary wheel running), social interaction and environmental complexity. Notably, our paradigm lacked novelty, i.e. frequent rearrangement of features within the environment, including food sources, which has been considered an important component of environmental enrichment (28, 29) but is obviously not required for its efficacy in treating anesthesia-induced cognitive decline. The effect of environmental enrichment and the components, that make up an enriched environment, on cellular and molecular events in the brain have been reviewed in detail elsewhere (27–29). In the current study, rats that were randomized to environmental enrichment had unlimited access to running wheels for at least 4 weeks before neurocognitive testing. An average odometer-reading of 4.5 miles/day per running wheel, as well as decreased body weights of enriched rats, suggest that greater voluntary physical activity in the enrichment groups may have contributed to the efficacy of environmental enrichment. It is not known if exercise alone is sufficient to rescue the behavioral phenotype following anesthesia in infancy. Since we already live in a vastly enriched environment, compared to lab rats, it would be difficult to assess the effects of further environmental enrichment on postanesthesia neurocognitive outcome in humans. However, it appears quite feasible to study the effect of exercise on neurocognitive function following anesthesia.
Effect of Tissue Injury on Neurocognitive Function
It has been suggested, that if an episode of anesthesia during early infancy impaired cognition in humans, it might be due to, or at least inseparable from, an effect of surgical tissue injury and the associated inflammatory response (20, 44, 45). This appears logical, because most anesthetics during infancy are administered for surgical procedures. The contributions of anesthesia and tissue injury to cognitive outcome would be difficult to separate in clinical studies. Therefore, we sought to dissociate the effect of tissue injury from those of the anesthetic using an animal model. Tissue injury was inflicted by tail clamping, which causes hematoma formation, hemorrhage and, ultimately, scarring of the tail (Fig. 12). We routinely use tail clamping to titrate the concentration of a volatile anesthetic to 1 MAC in a cohort of immature rats. Adding an equally sized cohort of rats, that receive the same anesthetic, without undergoing tail clamping, enables dissociation of the effects of tissue injury from anesthesia. Despite not being caused by a surgical blade, we suspect that the tissue injury caused by tail clamping as least as traumatic and inflammatory as surgical tissue injury because MAC in response to a surgical blade is lower than MAC in response to tail clamping (46). Tail clamping did not affect cognitive outcome in this study, nor in one prior study using isoflurane (4) (unpublished observation). This is at odds with another study showing that abdominal surgery in adult rats causes neuroinflammation and impairs neurocognitive function, albeit in a very short lived manner (19). It is possible, that the different developmental stages might explain the discrepant results.
It has been suggested that increased neuronal activity, such as during acute or chronic painful stimuli, cause mild cell death, decrease activity, impair attention and decrease spatial learning later in life (47). These ill effects were mitigated by analgesic doses of ketamine, presumably by reducing neuronal excitation (47). Why is it then, that in this study the balance of painful stimulus on the one hand, and anesthetic on the other did not mitigate the cognitive deficit of the anesthetic alone? Further, how is it possible that a general anesthetic alone, which presumably causes even greater neuronal depression than analgesic doses, kills brain cells and impairs cognition? One possible explanation is that sevoflurane does not cause neuronal depression but rather neuronal excitation: GABAergic agents (such as sevoflurane) cause neuronal excitation in immature neurons (48, 49) and inhibit neuronal activity only later, after maturation of the NKCC1 chloride transporter (49). If the cognitive outcome of sevoflurane in this study is in fact mediated by neuronal excitation, we would have to hypothesize that the painful stimulus during anesthesia (tail clamping) either did not further increase the state of neuronal excitation or excited neurons in brain areas, which do not contribute to the observed cognitive outcome. An alternative explanation is that anesthesia-induced neuronal excitation or inhibition does not mediate anesthesia-induced cognitive outcome.
Limitations
Shortcomings of the present study include the absence of data to suggest how sevoflurane impairs cognition in infantile rats and how environmental enrichment mitigates these effects.
Multiple comparisons increase the chance of type1 error inflation. While multiple comparisons within a family of comparisons were controlled for, we made no attempt to control for the multiple families of comparisons that were necessary to test our hypotheses. We have carefully reported the effect sizes of all planned and relevant unplanned comparisons, to enable the readers to make their own interpretation of the data and to draw their own conclusions.
The current study design does not allow conjectures regarding the effect of environmental impoverishment, had it been introduced at 4 weeks on the background of environmental enrichment. Further, the study design does not support comparative conclusions regarding the relative effect sizes of the environmental condition versus anesthetic exposure. The presented data suggest that the effect of anesthesia on cognition was dwarfed by the impact of environment, which might be interpreted as a reassuring finding. In other words, if it turns out that anesthesia impairs cognition in human infants, as it does in animals, one would hope that in humans it is also true that cognitive outcome can be substantially improved by environmental factors, so that the effect of anesthesia can be negated. This may be so, but would need to be tested in a clinical trial. It is, of course, impossible to draw conclusions from this or other animal studies, on how sevoflurane might affect long-term cognitive outcome in human infants or how environmental enrichment, or certain aspects thereof, affects cognition after anesthesia. If it turned out that anesthesia does not impair cognition in humans, the discrepancy with the animal literature, which, for the most part, has anesthesia in infancy impair recognition memory tasks later in life, might be explained by differences in environmental factors between humans and lab animals.
Either way, it is important not to change clinical practice, based on the current findings or other findings, generated by research not specifically designed to address clinical practices.
Conclusion
We have proven the principle that the behavioral consequences of anesthesia-induced developmental neurotoxicity are treatable in rats. Sevoflurane impaired short term memory 3 months after anesthesia, and delayed environmental enrichment rescued memory function. The long-term cognitive deficit caused by anesthesia is not exacerbated by tissue injury. Environmental enrichment, including voluntary exercise, might be a potential therapeutic strategy, in case long-term cognitive impairment caused by anesthesia in infancy occurs in humans. The cause of the anesthesia-induced neurocognitive dysfunction, its exact nature and the mechanism by which environmental enrichment mitigates the effects of anesthesia remain to be determined.
Acknowledgments
This work was supported by grants from the Anesthesia Patient Safety Foundation Indianapolis, Indiana.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Elaine W. Lee, B.A. is a member of the 2009 Medical Student Anesthesia Research Fellowship Program of the Foundation of Anesthesia Education and Research, Rochester, Minnesota.
Meetings at which presented:
American Society of Anesthesiologists 2009 Annual Meeting, Medical Student Anesthesia Research Fellowship Symposium, October 20, 2009, New Orleans, Louisiana
Smart Tots Session of the International Anesthesia Research Society
Annual Meeting, May 23, 2011, Vancouver, Canada
Bibliography
- 1.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma D, Williamson P, Januszewski A, Nogaro MC, Hossain M, Ong LP, Shu Y, Franks NP, Maze M. Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology. 2007;106:746–753. doi: 10.1097/01.anes.0000264762.48920.80. [DOI] [PubMed] [Google Scholar]
- 3.Johnson SA, Young C, Olney JW. Isoflurane-induced neuroapoptosis in the developing brain of nonhypoglycemic mice. J Neurosurg Anesthesiol. 2008;20:21–28. doi: 10.1097/ANA.0b013e3181271850. [DOI] [PubMed] [Google Scholar]
- 4.Stratmann G, May LD, Sall JW, Alvi RS, Bell JS, Ormerod BK, Rau V, Hilton JF, Dai R, Lee MT, Visrodia KH, Ku B, Zusmer EJ, Guggenheim J, Firouzian A. Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats. Anesthesiology. 2009;110:849–861. doi: 10.1097/ALN.0b013e31819c7140. [DOI] [PubMed] [Google Scholar]
- 5.Loepke AW, Istaphanous GK, McAuliffe JJ3, Miles L, Hughes EA, McCann JC, Harlow KE, Kurth CD, Williams MT, Vorhees CV, Danzer SC. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg. 2009;108:90–104. doi: 10.1213/ane.0b013e31818cdb29. [DOI] [PubMed] [Google Scholar]
- 6.Sanders RD, Xu J, Shu Y, Januszewski A, Halder S, Fidalgo A, Sun P, Hossain M, Ma D, Maze M. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110:1077–1085. doi: 10.1097/ALN.0b013e31819daedd. [DOI] [PubMed] [Google Scholar]
- 7.Zhu XH, Yan HC, Zhang J, Qu HD, Qiu XS, Chen L, Li SJ, Cao X, Bean JC, Chen LH, Qin XH, Liu JH, Bai XC, Mei L, Gao TM. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci. 2010;30:12653–12663. doi: 10.1523/JNEUROSCI.6414-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 9.Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–848. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 10.Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, Eckenhoff RG. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res. 2005;1037:139–147. doi: 10.1016/j.brainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology. 2008;109:243–250. doi: 10.1097/ALN.0b013e31817f5c47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang QJ, Li KZ, Yao SL, Li ZH, Liu SS. Different effects of isoflurane and sevoflurane on cytotoxicity. Chin Med J (Engl) 2008;121:341–346. [PubMed] [Google Scholar]
- 13.Liang G, Ward C, Peng J, Zhao Y, Huang B, Wei H. Isoflurane causes greater neurodegeneration than an equivalent exposure of sevoflurane in the developing brain of neonatal mice. Anesthesiology. 2010;112:1325–1334. doi: 10.1097/ALN.0b013e3181d94da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bercker S, Bert B, Bittigau P, Felderhoff-Muser U, Buhrer C, Ikonomidou C, Weise M, Kaisers UX, Kerner T. Neurodegeneration in newborn rats following propofol and sevoflurane anesthesia. Neurotox Res. 2009;16:140–147. doi: 10.1007/s12640-009-9063-8. [DOI] [PubMed] [Google Scholar]
- 15.Kodama M, Satoh Y, Otsubo Y, Araki Y, Yonamine R, Masui K, Kazama T. Neonatal desflurane exposure induces more robust neuroapoptosis than do isoflurane and sevoflurane and impairs working memory. Anesthesiology. 2011;115:979–991. doi: 10.1097/ALN.0b013e318234228b. [DOI] [PubMed] [Google Scholar]
- 16.Istaphanous GK, Howard J, Nan X, Hughes EA, McCann JC, McAuliffe JJ, Danzer SC, Loepke AW. Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology. 2011;114:578–587. doi: 10.1097/ALN.0b013e3182084a70. [DOI] [PubMed] [Google Scholar]
- 17.Stratmann G, Sall JW, Eger EI, Laster MJ, Bell JS, May LD, Eilers H, Krause M, Heusen F, Gonzalez HE. Increasing the duration of isoflurane anesthesia decreases the minimum alveolar anesthetic concentration in 7-day-old but not in 60-day-old rats. Anesth Analg. 2009;109:801–806. doi: 10.1213/ane.0b013e3181aff364. [DOI] [PubMed] [Google Scholar]
- 18.Eger EI2, Saidman LJ, Brandstater B. Minimum alveolar anesthetic concentration: A standard of anesthetic potency. Anesthesiology. 1965;26:756–763. doi: 10.1097/00000542-196511000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: A possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106:436–443. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Sun LS, Li G, Dimaggio C, Byrne M, Rauh V, Brooks-Gunn J, Kakavouli A, Wood A, Coinvestigators of the Pediatric Anesthesia Neurodevelopment Assessment (PANDA) Research Network Anesthesia and neurodevelopment in children: Time for an answer? Anesthesiology. 2008;109:757–761. doi: 10.1097/ALN.0b013e31818a37fd. [DOI] [PubMed] [Google Scholar]
- 21.Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681–1707. doi: 10.1213/ane.0b013e318167ad77. [DOI] [PubMed] [Google Scholar]
- 22.De Roo M, Klauser P, Briner A, Nikonenko I, Mendez P, Dayer A, Kiss JZ, Muller D, Vutskits L. Anesthetics rapidly promote synaptogenesis during a critical period of brain development. PLoS One. 2009;4:e7043. doi: 10.1371/journal.pone.0007043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briner A, De Roo M, Dayer A, Muller D, Kiss JZ, Vutskits L. Bilateral whisker trimming during early postnatal life impairs dendritic spine development in the mouse somatosensory barrel cortex. J Comp Neurol. 2010;518:1711–1723. doi: 10.1002/cne.22297. [DOI] [PubMed] [Google Scholar]
- 24.Head BP, Patel HH, Niesman IR, Drummond JC, Roth DM, Patel PM. Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology. 2009;110:813–825. doi: 10.1097/ALN.0b013e31819b602b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lunardi N, Ori C, Erisir A, Jevtovic-Todorovic V. General anesthesia causes longlasting disturbances in the ultrastructural properties of developing synapses in young rats. Neurotox Res. 2010;17:179–188. doi: 10.1007/s12640-009-9088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefovska VG, Uckermann O, Czuczwar M, Smitka M, Czuczwar P, Kis J, Kaindl AM, Turski L, Turski WA, Ikonomidou C. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann Neurol. 2008;64:434–445. doi: 10.1002/ana.21463. [DOI] [PubMed] [Google Scholar]
- 27.Baroncelli L, Braschi C, Spolidoro M, Begenisic T, Sale A, Maffei L. Nurturing brain plasticity: Impact of environmental enrichment. Cell Death Differ. 2010;17:1092–1103. doi: 10.1038/cdd.2009.193. [DOI] [PubMed] [Google Scholar]
- 28.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 29.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 30.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 31.Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: Sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney: Academic Press; 2005. [DOI] [PubMed] [Google Scholar]
- 34.McGaugh JL. Memory--A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 35.Izquierdo I, Izquierdo LA, Barros DM, Mello e Souza T, de Souza MM, Quevedo J, Rodrigues C, Sant'Anna MK, Madruga M, Medina JH. Differential involvement of cortical receptor mechanisms in working, short-term and long-term memory. Behav Pharmacol. 1998;9:421–427. doi: 10.1097/00008877-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Izquierdo I, Barros DM, e Souza TM, de Souza MM, Izquierdo LA, Medina JH. Mechanisms for memory types differ. Nature. 1998;393:635–636. doi: 10.1038/31371. [DOI] [PubMed] [Google Scholar]
- 37.Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- 38.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 39.Lindner MD, Plone MA, Schallert T, Emerich DF. Blind rats are not profoundly impaired in the reference memory Morris water maze and cannot be clearly discriminated from rats with cognitive deficits in the cued platform task. Brain Res Cogn Brain Res. 1997;5:329–333. doi: 10.1016/s0926-6410(97)00006-2. [DOI] [PubMed] [Google Scholar]
- 40.Packard MG, Teather LA. Amygdala modulation of multiple memory systems: Hippocampus and caudate-putamen. Neurobiol Learn Mem. 1998;69:163–203. doi: 10.1006/nlme.1997.3815. [DOI] [PubMed] [Google Scholar]
- 41.Izquierdo I, Medina JH. Memory formation: The sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- 42.Aggleton JP, O'Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT. Hippocampal-anterior thalamic pathways for memory: Uncovering a network of direct and indirect actions. Eur J Neurosci. 2010;31:2292–2307. doi: 10.1111/j.1460-9568.2010.07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stratmann G, Alvi RS. Can minimum alveolar concentrations in immature rodents be a single number? Anesthesiology. 2011;115:1132–1133. doi: 10.1097/ALN.0b013e3182303c66. [DOI] [PubMed] [Google Scholar]
- 44.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalkman CJ, Peelen L, Moons KG, Veenhuizen M, Bruens M, Sinnema G, de Jong TP. Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology. 2009;110:805–812. doi: 10.1097/ALN.0b013e31819c7124. [DOI] [PubMed] [Google Scholar]
- 46.Merkel G, Eger EI2. A comparative study of halothane and halopropane anesthesia including method for determining equipotency. Anesthesiology. 1963;24:346–357. doi: 10.1097/00000542-196305000-00016. [DOI] [PubMed] [Google Scholar]
- 47.Anand KJ, Garg S, Rovnaghi CR, Narsinghani U, Bhutta AT, Hall RW. Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr Res. 2007;62:283–290. doi: 10.1203/PDR.0b013e3180986d2f. [DOI] [PubMed] [Google Scholar]
- 48.Edwards DA, Shah HP, Cao W, Gravenstein N, Seubert CN, Martynyuk AE. Bumetanide alleviates epileptogenic and neurotoxic effects of sevoflurane in neonatal rat brain. Anesthesiology. 2010;112:567–575. doi: 10.1097/ALN.0b013e3181cf9138. [DOI] [PubMed] [Google Scholar]
- 49.Ben-Ari Y. Excitatory actions of gaba during development: The nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 50.Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–489. [PubMed] [Google Scholar]