Abstract
Background & Aims
Liver transplantation has become the standard of care treatment for hepatocellular carcinoma (HCC) that falls within size and numeric criteria in cirrhotic patients. Cirrhotomimetic (CMM) hepatocellular carcinoma is an uncommon growth pattern that infiltrates cirrhotic parenchyma, can become extensive in size, and can evade detection via radiologic studies. Liver transplant outcomes for this type of HCC is not well reported but generally considered to be poor. We wished to better describe this variant of HCC in explanted livers, derive a classification system for this tumor type, and assess the outcomes of liver transplantation for this tumor variant.
Methods
Upon retrospective analysis of all patients transplanted at a single center for HCC in 1996–2009 (358 patients) a series of 26 patients exhibiting CMM growth pattern were identified. We developed a classification system for this tumor growth pattern variant and determined patient and tumor-specific outcomes.
Results
We derived a classification schema of CMM HCC based upon tumor extent and cellular histopathology with clear cell pathology being associated with favorable outcome. We note a 100% 3-year and 58.3% 5-year recurrence free survival after transplant in those with tumor confined to one lobe who have clear cell pathology versus 16.2% 3- and 5-year recurrence free survival in those who do not meet these criteria.
Conclusion
Cirrhotomimetic HCC features are noted in 7% of patients transplanted for HCC in our center with favorable outcomes inpatients with clear cell histology and growth involving less than 50% of the liver.
Keywords: Hepatocellular, carcinoma, infiltrative, transplant, liver, survival, classification
Introduction
Hepatocellular carcinoma (HCC) is among the common cancers worldwide and is the most common primary malignant neoplasm in patients with cirrhosis 1. Due to the high incidence of this cancer in those with cirrhosis, periodic surveillance for HCC by cross-sectional imaging (CSI) or ultrasonography is recommended. Typically, HCC develops as discrete tumor nodules which progress in size if not treated. Multicentric HCC is common in cirrhotic patients but generally follows the pattern of discrete nodular growth 2, 3. HCC has become one of the major indications for orthotopic liver transplantation (OLT) with good graft and patient survival in those with ideal tumor characteristics based on number and size of tumors and without vascular involvement or metastasis 4–6. A standardized MELD score exception system has been adopted to allow expeditious transplantation in cirrhotic patients with HCC who fall within ideal criteria of a single lesion of up ≤5cm and not more than three lesions of under 3 cm in size 5, 7, 8. Tumor-specific outcomes for transplantation in HCC is classically attributed to factors including tumor burden and vascular invasion, and patients whose tumors exceed the quantitative restrictions of Milan and/or UCSF criteria are generally excluded from transplantation.
Cirrhotomimetic (CMM) HCC is a rare variant characterized by small cirrhosis-like tumor nodules that interdigitate within cirrhotic liver parenchyma. At the sensitivity of currently available radiographic imaging modalities, the nodules of CMM HCC are indistinguishable from regenerative cirrhotic nodules and are thus often undetected prior to transplantation. CMM HCCs, by definition, are discovered during pathologic assessment of the explanted specimen, either upon gross examination, or incidentally on histologic sections taken adjacent to macroscopically identified tumor nodules. CMM HCCs appear synonymous with the “single nodule with extranodular growth” and “confluent multinodular” HCCs as described by Shimada et al, as well as the “diffuse cirrhosis-like” HCCs described by Jakate et al. CMM involvement as described by Jakate et al may range from CMM growth adjacent to a dominant mass, to a diffuse distribution occupying up to 50% of the explanted liver volume. Regardless of their extent, CMM HCCs all share the feature of unanticipated tumor burden. There is little published data regarding outcomes following diagnosis of CMM HCC. Small case series have described outcomes of OLT in those with CMM HCC and depict tumors generally outside of the Milan criteria with high degrees of local invasion 9, 10. Existing studies, though limited not only in number of cases, but also in follow-up interval, have suggested unexpectedly favorable survival in some patients despite the amount of tumor present.
We report our institution’s experience with CMM HCCs, consisting of 26 patients transplanted over the period of 1996–2009 whose explanted livers exhibited CMM tumor growth. We describe the clinical and pathologic features of patients with CMM HCCs, assess survival following transplantation, and characterize the pathologic features associated with survival.
Materials and Methods
Patient Selection
This a retrospective analysis of patients with CMM HCC transplanted at the University of Pennsylvania between 1996 and 2009. The diagnosis of HCC was based on explant evaluation. This data analysis was approved by the local IRB and did not require consent for de-identified data analysis as deemed by the IRB. The study protocol conforms to the ethical guidelines of the1975 Declaration of Helsinki as reflected in a priori approval by the institutional human research committee.
Pathologic Analysis
Patients demonstrating hepatocellular carcinoma with a CMM growth pattern were identified from patients who underwent liver transplantation at our institution over a thirteen year period (1996–2009). At the time of whole liver explantation, each liver was serially sectioned in the fresh state along the long axis at 3–5 mm intervals. Lesions were documented and entirely submitted for histologic examination, including 1 cm of peripheral liver parenchyma surrounding each nodule. Following fixation in 10% formalin, the sections were routinely processed, paraffin-embedded, and stained with hematoxylin and eosin. On retrospective review, the initial diagnoses were confirmed by review of the histologic sections, gross description, and when available, specimen photographs. Pathologic features including tumor size, number of nodules, extent of cirrhotomimetic growth pattern, architectural pattern, histologic grade, cytologic features, and lymphovascular invasion were assessed. Each case was independently reviewed by two pathologists (EFC and EEF), and evaluated for the previously mentioned pathologic features. Following derivation of definitions for cirrhotomimetic extent, tumors were evaluated by a third pathologist (HP) who was blinded to initial results.
Radiographic Analysis
Hepatocellular carcinoma was identified pre-transplantation in the majority of patients included in this study by cross-sectional imaging 11. Hepatic masses were identified radiographically as hepatocellular carcinoma if the patient had a CT scan demonstrating vascular blush or an MRI with contrast enhancement and post-perfusion contrast washout corresponding to the suspicious area. HCC was also identified in patients who had a non-enhancing hepatic mass and an alpha-fetoprotein (AFP) level greater than 200 ng/ml. Biopsy of the lesion was not required to diagnose HCC. Patients were surveyed with additional imaging every three months while on the wait list to maintain MELD exception status, and exception point candidacy was determined using the largest dimension of the lesion(s).
Statistical Analysis
Statistical analyses were performed with SPSS statistical software (Version 19.0 for Windows; Chicago, IL). Descriptive analyses included Student’s t-test or one-way ANOVA where appropriate for continuous variables, and Fisher’s exact test or the Chi square test statistic were used to compare categorical variables. Univariate survival analysis was performed using the Kaplan-Meier method, and survival curves were compared with the log-rank test. Multivariate survival analysis included Cox Proportional Hazards regression analysis. Test results were deemed statistically significant when p≤.05.
Results
Case Selection and Pre-transplant Imaging
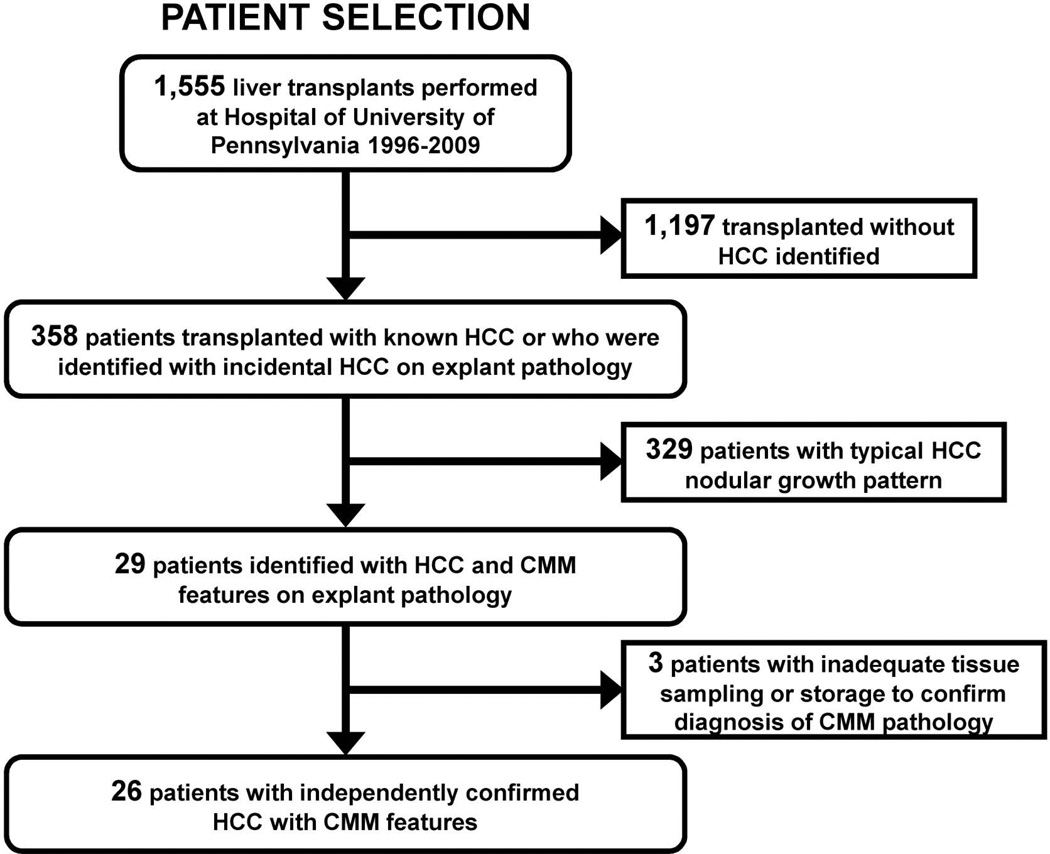
Of the 1555 total liver transplants performed from 1996 to 2009 at our center, 26 were identified with CMM growth pattern that could be confirmed by sample re-review (1.6%) (Figure 1). Nineteen of these 26 cases (73%), had HCC identified pre-transplant on screening CSI, while 7 patients were not known to have tumor pre-transplant. These 19 patients with known HCC and CMM growth pattern represent 5.3% of the 358 total patients transplanted with preoperative identification of HCC from 1996–2009. Overall patient demographic data is shown in Table 1.
Figure 1. Selection algorithm for inclusion/exclusion into study group.
Table 1.
| Recipient and Donor Characteristics | Total | Clear and Confined | Other | p |
|---|---|---|---|---|
| n | 26 | 10 | 16 | |
| Recipient data | ||||
| Age in years (mean +/− SD) | 57.1 +/− 7.5 | 55.2 +/− 5.3 | 57.6 +/− 4.9 | 0.22 |
| Male Gender, %n | 76.9% (20) | 50% (5) | 93.8% (15) | 0.02 |
| Race, %n | ||||
| White | 73.1% (19) | 80.0% (8) | 68.8% (11) | 0.53 |
| Black | 15.4% (4) | 10.0% (1) | 18.8% (3) | 1.00 |
| Hispanic | 3.8% (1) | 0.0% (0) | 6.2% (1) | 1.00 |
| Other | 7.7% (2) | 10% (1) | 6.2% (1) | 1.00 |
| Etiology of Disease, %n | ||||
| Hep C | 65.4% (17) | 50.0% (5) | 75.0% (12) | 0.23 |
| Hep C + Other | 15.4% (4) | 20.0% (2) | 12.5% (2) | 0.63 |
| Hep B | 0.0% (0) | 0.0% (0) | 0.0% (0) | n/a |
| EtOH | 7.7% (2) | 10.0% (1) | 6.2% (1) | 1.00 |
| PSC/PBC/Autoimmune Hepatitis | 3.8% (1) | 10.0% (1) | 0.0% (0) | 0.39 |
| NASH | 0.0% (0) | 0.0% (0) | 0.0% (0) | n/a |
| Cryptogenic/Other | 7.7% (2) | 10.0% (1) | 6.2% (1) | 1.00 |
| Listed MELD at TX (mean +/− SD) | 23.6 +/− 6.3 | 23.0 +/− 3.7 | 22.0 +/− 4.8 | 0.77 |
| Biological MELD at TX (mean +/− SD) | 18.1 +/− 8.2 | 13.4 +/− 3.4 | 15.3 +/− 5.4 | 1.00 |
| Median Peak Alpha-Fetoprotein (range) | 51 (2-2298) | 15 (2-936) | 71 (4-2298) | 0.09 |
| Donor data | ||||
| Age in years (mean +/− SD) | 37.4 +/− 17.7 | 42.2 +/− 15.4 | 33.5 +/− 18.0 | 0.60 |
| Donor Type, %n | ||||
| Deceased | 88.5% (23) | 80.0% (8) | 93.8% (15) | 0.29 |
| Living | 3.8% (1) | 10.0% (1) | 0.0% (0) | 0.39 |
| CDC High Risk | 7.7% (2) | 10.0% (1) | 6.2% (1) | 1.00 |
| CIT in mins (mean +/− SD) | 319.7 +/− 143.2 | 244.0 +/− 101.0 | 384.7 +/− 154.6 | <0.01 |
| WIT in mins (mean +/− SD) | 54.9 +/− 10.2 | 54.2 +/− 6.6 | 52.5 +/− 7.7 | 0.31 |
All patients listed for OLT at our center undergo serial contrast MRI (preferred) or CT scan imaging for HCC monitoring or screening: every three months for those with known HCC and at least every 12 months for those undergoing HCC screening. Those with tumor identified preoperatively had “known tumor” (n = 19; 73%) as in Table 2 and tumor numbers identified by imaging are noted in the top section of this table. These patients were listed with MELD exception points for HCC after the adoption of this policy when applicable. Those without known pre-transplant tumor had similar radiologic surveillance as those with identified tumor prior to transplant. It should be noted that 25% of the patients with known tumor were outside of Milan criteria – in part due to being transplanted prior to the adoption of the MELD system.
Table 2.
| Tumor Characteristics | Total | Clear and Confined | Other | p |
|---|---|---|---|---|
| n | 26 | 10 | 16 | |
| Data Based on Pre-Transplant Imaging Studies | ||||
| Known Tumor | 73.1% (19) | 60.0% (6) | 81.2% (13) | 0.36 |
| # of Dominant Tumor Nodules | ||||
| 1 | 42.1% (8) | 16.7% (1) | 53.8% (7) | 0.18 |
| 2 | 36.8% (7) | 50.0% (3) | 30.8% (4) | 0.62 |
| 3+ | 21.1% (4) | 33.3% (2) | 15.4% (2) | 0.56 |
| Largest Nodule Diameter in cm (mean +/− SD) | 2.87 +/− 1.28 | 2.28 +/− 0.38 | 2.78 +/− 1.23 | 0.80 |
| Total Tumor Diameter in cm (mean +/− SD) | 3.79 +/− 1.74 | 3.76 +/− 0.77 | 3.31 +/− 1.35 | 0.15 |
| Bilobar in Patients, % of patients w/ Known | 29.4% (5) | 50% (3) | 15.4% (2) | 0.26 |
| Treatment in Patients, % of patients w/ Known | ||||
| TACE | 35.3% (6) | 16.7% (1) | 38.5% (5) | 0.60 |
| RFA | 5.0% (1) | 0.0% (0) | 7.7% (1) | 1.00 |
| Resection | 0.0% (0) | 0.0% (0) | 0.0% (0) | n/a |
| None | 64.7% (11) | 83.3% (5) | 53.8% (7) | 0.33 |
| Outside Milan, % of patients w/ Known | 35.3% (6) | 33.3% (2) | 30.8% (4) | 1.00 |
| Outside UCSF, % of patients w/ Known | 23.5% (4) | 33.3% (2) | 15.4% (2) | 0.55 |
| Data Based on Pathology Examination of Explant | ||||
| # of Dominant Tumor Nodules | ||||
| 1 | 34.6% (9) | 30.0% (3) | 37.5% (6) | 1.00 |
| 2 | 23.1% (6) | 30.0% (3) | 18.8% (3) | 0.64 |
| 3+ | 42.3% (11) | 40.0% (4) | 43.8% (7) | 1.00 |
| Largest Nodule Diameter in cm (mean +/− SD) | 3.02 +/− 1.44 | 3.04 +/− 0.76 | 3.57 +/− 1.61 | 0.34 |
| Cumulative Tumor Nodule Diameter in cm (mean +/− SD) |
4.91 +/− 2.96 | 6.52 +/− 0.15 | 5.23 +/− 2.87 | 0.68 |
| Incidental Tumor(Y), % | 26.9% (7) | 40.0% (4) | 18.8% (3) | 0.37 |
| Bilobar (Y), % | 88.5% (23) | 90.0% (9) | 87.5% (14) | 0.85 |
| Differentiation, % | ||||
| Poor | 3.8% (1) | 10.0% (1) | 0.0% (0) | 0.41 |
| Moderate to Poor | 15.4% (4) | 10.0% (1) | 18.8% (3) | 1.00 |
| Moderate | 46.2% (12) | 50.0% (5) | 43.8% (7) | 1.00 |
| Moderate to Well | 11.5% (3) | 10.0% (1) | 7.7% (2) | 1.00 |
| Well | 3.8% (1) | 0.0% (0) | 6.2% (1) | 1.00 |
| Macroscopic Vascular Invasion | 0.0% (0) | 0.0% (0) | 0.0% (0) | n/a |
| Microscopic Vascular Invasion | 88.5% (23) | 90.0% (9) | 87.5% (14) | 0.85 |
| Positive Surgical Margin | 0.0%(0) | 0.0% (0) | 0.0% (0) | n/a |
| Positive Lymph Nodes | 0.0% (0) | 0.0% (0) | 0.0% (0) | n/a |
Pathologic features
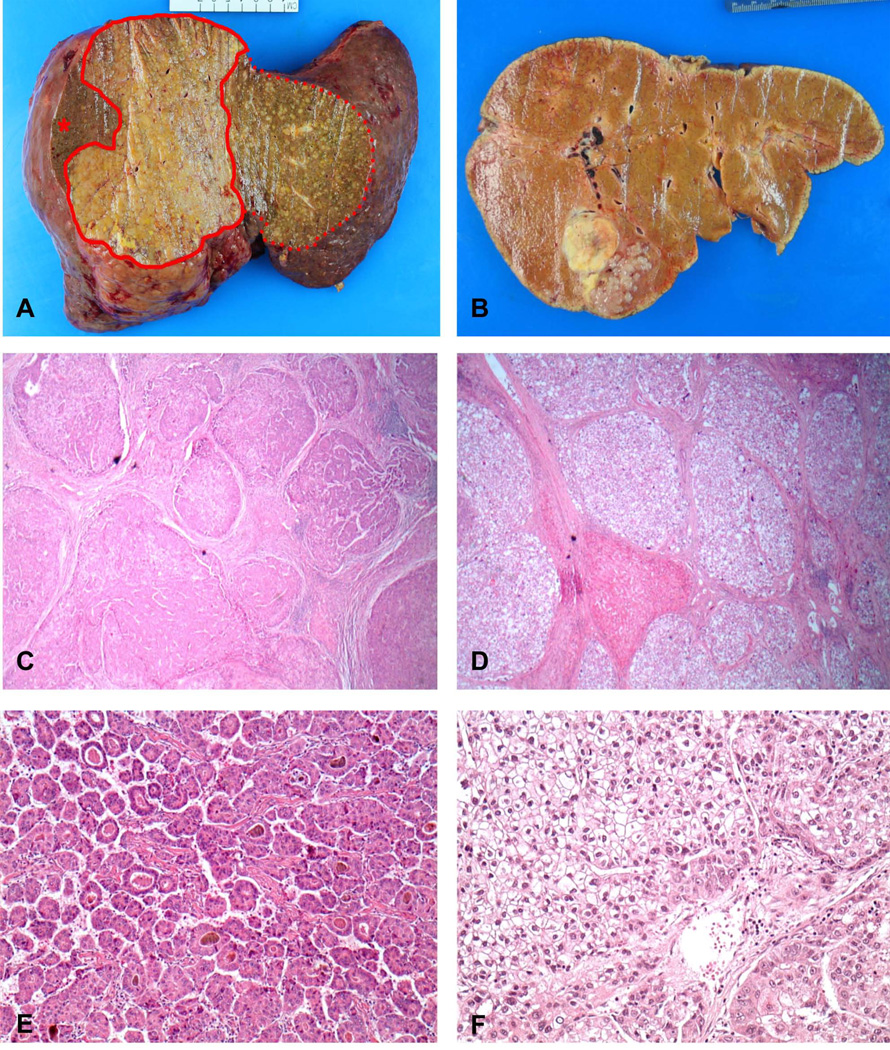
All 26 tumors met the criteria of cirrhotomimetic growth, defined as unanticipated tumor burden distributed amongst non-neoplastic cirrhotic nodules, discovered either during gross examination or histologic assessment of sections. Twenty-three patients (88.5%) showed bilobar involvement by HCC. There was a spectrum of CMM growth, ranging from few small tumor nodules emanating from a dominant nodule (Figure 2B), to numerous small nodules interspersed between regenerative cirrhotic nodules to form a confluent mass (Figure 2A). CMM patterns generally fell into two major categories, “confined” (CMM component involving less than 50% of the entire liver) and “extensive” (CMM component involving >50% of the entire liver). Using these definitions, 14 tumors demonstrated a confined CMM pattern, and 12 demonstrated an extensive pattern. Of note, application of these definitions by a blinded pathologist (HP) yielded near complete concordance in classification.
Figure 2. Macroscopic and microscopic features of cirrhotomimetic HCC that form the basis of tumor classification.
A. Explanted liver with cirrhotomimetic hepatocellular carcinoma, extensive subtype. A confluence of sub-centimeter tumor nodules occupy the right liver (area within solid line), with additional interdigitation among cirrhotic nodules in left lobe (area within dotted line). Non-neoplastic liver in right lobe is denoted by asterisk. B. Explanted liver with cirrhotomimetic HCC, confined type. Cirrhotomimetic spread is limited within a few centimeters of the main nodule. C&D. Microscopic HCC nodules encircled by fibrous bands mimic the architecture of cirrhotic liver. C. Low-power view of trabecular architecture in extensive subtype (2.5x) D. Trabecular architecture and clear cell morphology in confined subtype. A residual nodule of non-neoplastic liver is seen among tumor nodules comprised of clear cells (2.5x) E. Extensive tumor showing trabecular and pseudoglandular architecture (10x) F. Medium-power view of confined tumor with clear cell morphology. A small component of non-clear cells is seen in the lower right corner (20x)
Using the TNM staging system, most patients, on average, fell into the T2 category defined by dominant nodule size (3.02 +/− 1.44 cm), but demonstrated overall tumor burden of 4.91 +/− 2.96 cm, often greater than the stated 5 cm cutoff for T3. When viewing tumors at explant, most of these cases would exceed the exclusionary criteria delineated by Milan and UCSF criteria. Eleven tumors (11/26) exhibited trabecular architecture, and fifteen (15/26) showed a mix of trabecular and pseudoglandular architecture. No tumors showed exclusively pseudoglandular architecture. For histologic grade, the majority of tumors clustered within moderate differentiation, with fewer numbers exhibiting poor and well differentiation, respectively. Fifteen (57.7%) of tumors showed clear cell morphology, defined as optical clearing of cytoplasm identified in >10% of tumor composition. None of the patients showed invasion of major vessels. Twenty-three patients (88.5%) showed microscopic evidence of lymphovascular invasion. None of the patients had positive surgical resection margins (defined as tumor present at porta hepatis or hepatic vein resection margins), positive lymph nodes, or metastases. Independent pathologists’ review showed nearly complete concordance with regards to pathologic features.
Defining a cirrhotomimetic classification system
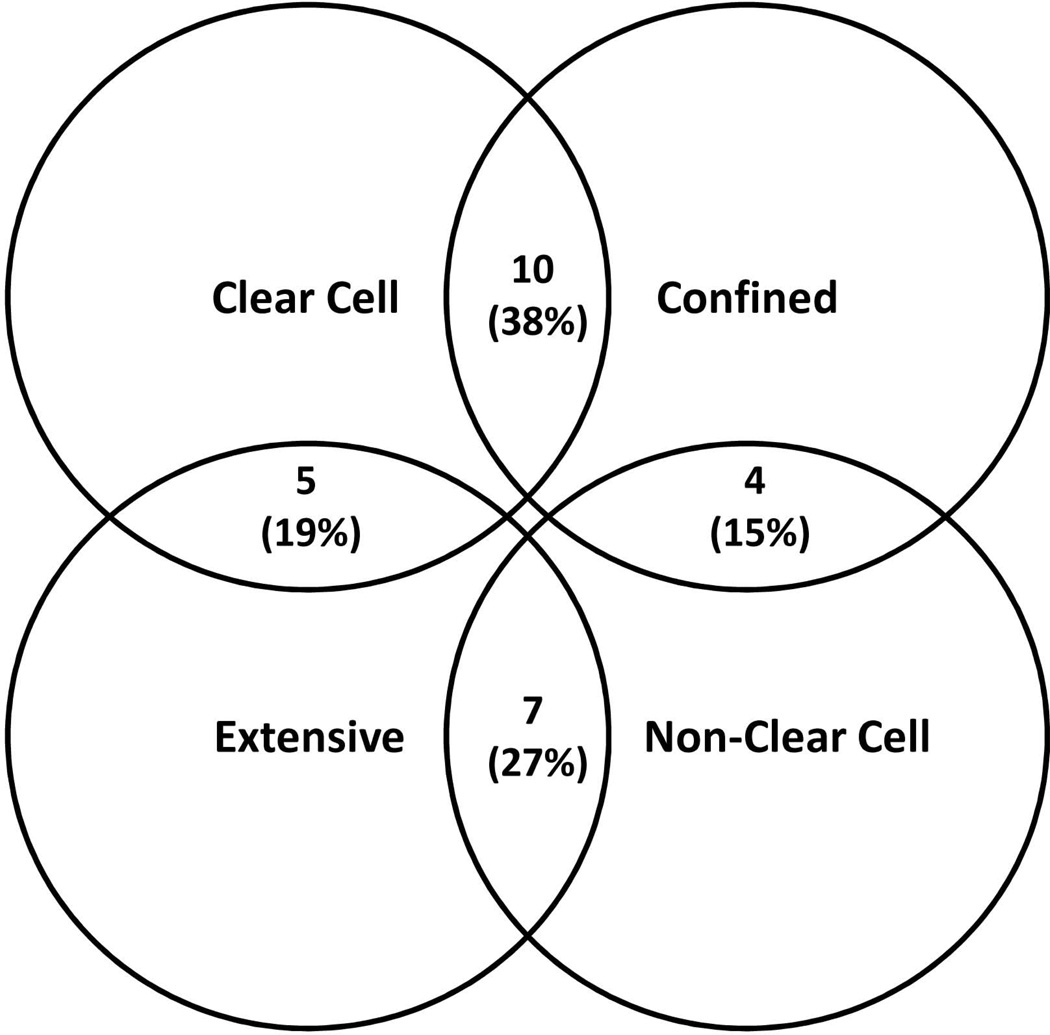
We noted that some patients showed substantially better than expected survival following transplantation. After stratifying the patients on the basis of tumor characteristics, we determined that patients who had confined tumors with clear cell morphology had generally favorable outcome compared to patients whose tumors did not fit these criteria, and we divided the study population into Clear and Confined (n = 10; 38%) and Other (n = 16; 62%) groups for further analysis.
We noted that the majority of clear cell tumors had confined extent and that the majority of non-clear cell tumors had extensive growth within the liver, although there was not complete concordance between these tumor characteristics (Figure 3). Of the tumors with a confined pattern, 10 (71.4%) showed trabecular architecture and 4 (28.6%) showed mixed trabecular and pseudoglandular architecture. Eleven of the 12 extensive tumors (91.7%) exhibited both trabecular and pseudoglandular architecture. No tumors in either subtype showed an exclusively pseudoglandular architecture. Tumors with clear cell morphology included 10 patients (66.7%) with confined CMM growth and 5 patients (33.3%) with extensive CMM growth. There was no significant correlation between tumor grade and presence of clear cells. Examples of tumors with trabecular, pseudoglandular, and clear cell morphologies are shown in Figure 2 (C–F).
Figure 3. Venn diagram illustrating distribution between tumor extent and cellular histology groups.
The distribution and intersection between clear cell versus non-clear cell pathology and confined versus extensive tumor extent on explant within the cirrhotomimetic tumor explant specimens is shown.
Patient Demographics by Classification Groups
Patient demographics by tumor-defined group criteria are described in Table 1. Patients were assigned to groups based upon tumor characteristics with the Clear and Confined group demonstrating both clear cell tumor cellular pathology and confined extent of disease and the Other group demonstrating one or none of these pathological features. There were no significant differences in patient demographics, disease etiology, or liver donor data between these groups with the exception of cold ischemic time (CIT) which was significantly longer in the Other group. This difference is likely not clinically relevant as the median times were short in both groups and early graft function was not compromised in either group. In all other categories, characteristics were similar between groups. The Other group had somewhat more Hepatitis C virus positivity than the Clear and Confined group (93% vs. 64%) but this did not reach significance. As shown in Table 2, top panel, the extent of known tumor burden at the time of transplant and the treatment of tumors preoperatively did not differ between groups.
Tumor Recurrence and Patient Survival
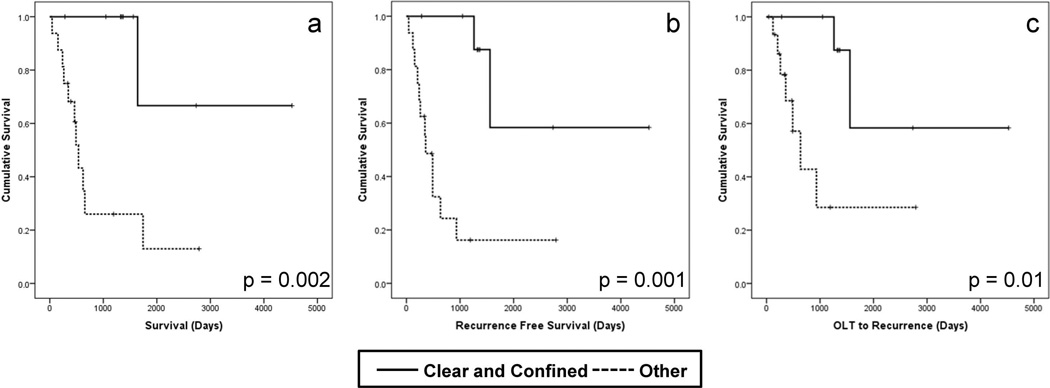
The median post-transplant survival of patients with CMM pathology was 1.75 years (639 days). The overall recurrence rate was 57%. Recurrence free survival was 67% and 50% at 1 and 3 years respectively. Only two recurrences were noted beyond three years from transplant and only two deaths occurred beyond three years after transplant – both in patients with tumor recurrence. There were a total of 4 deaths without documented tumor recurrence. Survival, recurrence free survival, and overall recurrence rates at 1, 3 and 5 years by classified group are shown in Table 3 and Kaplan-Meier survival curves are shown in Figure 4.
Table 3.
| Survival | 1 yr | 3 yr | 5 yr | Median Survival Time (days) |
|---|---|---|---|---|
| Overall | 80.4% | 56.9% | 34.2% | 1641 |
| Clear and Confined | 100.0% | 100.0% | 66.7% | *3252 |
| Other | 68.2% | 26.0% | 13.0% | 537 p =0.002 |
| Recurrence Free Survival | 1 yr | 3 yr | 5 yr | Median RFS Time (days) |
| Overall | 68.4% | 50.1% | 33.4% | 1261 |
| Clear and Confined | 100.0% | 100.0% | 58.3% | *3252 |
| Other | 48.6% | 16.2% | 16.2% | 359 p = 0.001 |
| Patients without Recurrence | 1 yr | 3 yr | 5 yr | Median Time to Recurrence (days) |
| Overall | 82.3% | 65.0% | 43.3% | 1560 |
| Clear and Confined | 100.0% | 100.0% | 58.3% | *3252 |
| Other | 68.5% | 28.6% | 28.6% | 636 p =0.01 |
Estimated median time; more than half pts surviving at last follow-up, median cannot be calculated
Figure 4. Patient and tumor-specific survival after liver transplantation in cirrhotomimetic HCC.
Kaplan-Meier survival curves showing patient survival (a), recurrence free survival (b), and time for liver transplant to recurrence (c) of the Clear and Confined (solid lines) versus Other (hatched lines) after transplant in patients with cirrhotomimetic hepatocellular carcinoma.
Tumor Classification and Correlation with Patient Outcomes
Grouping patients by CMM growth pattern categories yielded substantial differences in tumor-specific outcomes (Figure 4a–c). For those in the Clear and Confined category, all patients survived to the end of the analysis (283 – 4525 days). There was one late tumor recurrence (at 1008 days) in the clear and confined group. This is in sharp contrast to patients in the Other group of CMM growth pattern who either had non-clear cell (4) or non-confined (2) or neither characteristic (10). This group of patients had very poor post-transplant outcomes with a median post-transplant survival of 467 days (42–2789 days), a tumor recurrence rate of 80%, and a median time to tumor recurrence of 320 days. All of these outcome differences were highly significant (Table 3).
By comparison, the recurrence free survival rates for all patients transplanted for HCC at our transplant center in 2002–2011 within the MELD era are 70% at 3 years and 63% at 5 years (Figure S1). Additional subgrouping of all patients transplanted for HCC at our center to better define comparison groups is shown (Figure S2). In this classification, group 2 represents patients most comparable to the majority of CMM HCC patients in this study - namely those determined to be within Milan criteria by imaging pre-transplant and determined to have tumors more extensive than Milan criteria upon pathological examination of the explants. Our Clear and Confined CMM HCC patients had better recurrence free survival than this comparison group of nodular HCC and the Other group of CMM HCC had substantially worse survival than this comparison group.
Discussion
CMM HCC is a rare entity described in very few published studies largely as case reports or small series 9, 10, 12, 13. The outcomes following transplantation for CMM HCC have not been well characterized. While this is primarily due to the rarity of CMM HCCs, it is also due to the difficulty in applying traditional tumor stage-based prognostic schemes to this type of tumor. Our series of 26 patients with CMM HCC who underwent liver transplantation over a 13 year period constitutes the largest described experience thus far.
CMM HCC have been previously described as “diffuse cirrhosis-like” or having “extranodular growth” 10, 12. Our cohort is similar to the previously studied cohorts in the following respects: the tumors arose in patients with a clinical history of hepatitis and background of cirrhosis, with most cases of extensive CMM growth undetected prior to orthotopic liver transplant. The unexpectedly favorable survival in CMM HCC has been recognized in a single patient report after transplantation and in a report of post-resection outcome 9, 12.
It is important to note that the tumor nodule measurements described in Table 2 are distinct variables from the cirrhotomimetic extent. While the tumor sizes summarized in the table refer solely to the discrete nodular components of tumor, the extent (confined versus extensive) refers specifically to the tumor component that infiltrates out into the surrounding liver parenchyma as cirrhosis-like nodules. As cirrhotomimetic spread is often discovered incidentally on histologic sections, it is difficult to ascribe a linear distance for which a numeric measurement of extent can be given. Thus, the extent is best characterized by the proportion of the liver involved (<50% or >50%) by the cirrhotomimetic component of tumor. It is also important to note that the measurable nodular components of the tumors are not significantly different between the “clear and confined” and “other” groups, which underscores the point that it is the cirrhotomimetic extent, rather than the measurable nodule size(s) that correlates with prognostic significance.
While our subtypes of confined and extensive are similar to those previously characterized by Shimada et al as “single nodular with extranodular growth” and “confluent multinodular,” respectively, significant differences exist between these reports 12. First, our study describes whole liver explants after transplantation while Shimada et al describe partial hepatectomy specimens. Only approximately half of the Shimada patients were cirrhotic, although a higher proportion of patients with more aggressive growth patterns arose in a background of cirrhosis. Nonetheless, they report worse tumor-specific outcomes and patient survival in patients with single nodular with extranodular growth tumors (equivalent to our confined CMM) when compared with single nodular (i.e. conventional) HCCs and those with confluent multinodular growth (similar to our extensive CMM) groups. In contrast, our cohort showed confined CMM tumors correlating with improved recurrence free survival (similar to patients transplanted for typical nodular hepatocellular carcinoma) compared to the extensive group. More importantly, Shimada’s characterization of extent is limited, as their data was derived from examination of partial hepatectomy specimens. Our examination is based on whole explanted livers, which is therefore more complete and not directly comparable to previously published data derived from partial resections.
All tumors in our study were entirely submitted, affording an opportunity to thoroughly assess histologic features. Differing opinions exist regarding the prognostic significance of HCC tumor architecture. Lai et al reported the architectural pattern to be prognostically insignificant, while Lauwers et al showed that patients with macrotrabecular and acinar architecture had poorer prognosis than those with a compact growth pattern14, 15. Hepatocellular carcinomas most commonly exhibit trabecular architecture, frequently admixed with a pseudoglandular component 2. Jakate et al reported frequent presence of pseudoglandular architecture in their CMM cohort 10. We also show that the pseudoglandular architecture is prevalent in CMM tumors and more likely to be seen in those of the extensive subtype. Of note, the vast majority (91.7%) of patients with the extensive pattern show pseudoglandular architecture, and have significantly lower rates of disease-free survival.
To our knowledge, this is the first study demonstrating the significance of clear cell morphology within a CMM cohort. Hepatocellular carcinomas often exhibit clear cells--ranging from small foci which are thought to have no clinicopathologic significance and are thus not mentioned in the pathology report, to a clear cell variant of hepatocellular carcinoma, defined as >30% of tumor and occurring at reported frequencies ranging from 0.4 to 37% of all hepatocellular carcinomas 16–20. Ultrastructural study shows that lipid accumulation in the cytoplasm as well as swollen mitochondria account for the clear cell morphology21. However, the correlation with tumor biology remains poorly defined and the prognostic significance of clear cells in hepatocellular carcinoma is controversial. Some report no significant difference in clinical outcome 13, while others report later recurrence in patients with the clear cell variant 22 as well as improved survival, with survival correlating to the proportion of clear cells 13, 20, 22. In our study population, patients whose tumors exhibited clear cell morphology had significantly better disease-free survival than those without clear cells, regardless of CMM tumor extent when taken as an entire group although only 2 of 5 clear cell/extensive classified patients had recurrence free survival beyond 2 years. Confined CMM tumors with clear cells had significantly improved survival as compared to all tumors in the Other group.
Further investigation is needed to determine the etiology of the CMM pattern of HCC. 100% of patients in our cohort had tumor arising in a background of cirrhosis, a feature commonly seen in hepatocellular carcinoma. 82% of patients in our cohort had a history of Hepatitis C, which exceeds the prevalence of Hepatitis C in our non-CMM HCC transplant cohort, suggesting a possible viral association, although insufficient numbers exist to investigate this association further in this data set. Molecular studies to evaluate tumor clonality may elucidate whether CMM HCCs represent intraparenchymal lymphovascular metastasis as opposed to synchronous tumor development. Markers of epithelial-mesenchymal transition may reveal differences in the tumor microenvironments of CMM and non-CMM HCCs.
We have noted some association of this growth pattern with hepatic arterial chemoembolization (HACE). Histologic sections often reveal cirrhotomimetic growth emanating from a dominant nodule that is predominantly necrotic and located adjacent to chemoembolization material lodged in arterioles. It is possible that cirrhotomimetic growth may represent tumor escape through venules in response to arterial perturbation in some cases. Nearly all patients with known HCC who were transplanted in our institution since 2007 have undergone HACE, which might explain an increased frequency of observation of this growth pattern since that time. The vast majority of patients treated with HACE in our center do not exhibit this growth pattern, however, regardless of the extent of tumor necrosis.
Our findings imply that Milan tumor classification may not adequately stratify risk in all patients with CMM HCC. All patients transplanted after the introduction of the MELD exception criteria for HCC in 2002 in this study were deemed by cross sectional imaging to be within Milan criteria if tumor was identified pre-transplant. However, subsequent examination of the explanted liver showed that all patients had tumor burden exceeding Milan criteria, belying the difficulty in identifying infiltrative tumor by imaging. Despite their unanticipated extent of tumor, those in the Clear and Confined group had survival similar to patients transplanted with non-CMM HCC who were within Milan criteria. Thus, excluding these patients from transplant would not have been the correct approach, had the tumor extent been identifiable on cross sectional imaging preoperatively. Conversely, those in the Other group had prohibitive rates of tumor recurrence and mortality, highlighting the risk of making an error in this determination.
Our pathology-based subclassification of CMM HCC has the utility of resolving issues that arise when attempting to stage these tumors by the TNM system. Pathology staging (pTNM) of HCC often forms the basis of prognostic models for outcomes following transplantation, with significant weight given to tumor size and number. Using the traditional TNM staging definitions, most CMM HCCS would fall under T2 (e.g. multiple tumors each smaller than 5 cm, or tumors with vascular invasion) as few of the nodules exceed 1 cm, let alone the 5 cm set by the T stage cutoffs. As illustrated in our study, most CMM HCCs do not exhibit a single nodule greater than 5 cm to qualify as T3, despite the fact that the cumulative tumor burden typically exceeds this amount. Additionally, greater than 80% of patients in both CMM groups demonstrated microscopic lymphovascular invasion, which is an unfavorable finding associated with recurrence and metastasis. With a high frequency of these unfavorable features, it is not unreasonable to predict poor outcomes in all CMM HCC patients. However, we demonstrate that patients in the Clear and Confined group have unexpectedly favorable outcomes that reach statistical significance. It is important to note that BOTH groups of patients described here showed actual tumor burden that generally exceeded Milan criteria. Thus, the favorable outcome in the Clear and Confined group is more unexpected than the poor outcome in the Other group. The more favorable outcomes seen in the Clear and Confined group may be appropriate to consider when formulating a post-operative treatment plan including possible consideration of anti-tumor systemic therapy.
The strengths of this study include a single pathologist reviewing all samples in our center for more than 15 years, yielding a consistency that allowed us to discover this variant tumor subtype. In addition, we use definable criteria whose reproducibility is shown by achieving tight correlation when applied by a blinded pathologist.
While we do not propose to create a new formal classification system for HCC as a whole since this describes a rare variant of HCC, we find that our categorization may provide better prognostic modeling for this variant than the traditional TNM staging.
Our study also has some clear limitations, including the single center nature of the study, the limited group of patients due to this being a rare presentation of HCC, and the somewhat more limited follow-up time of a small number of the subjects included in this study. The limited study size precludes a multivariate analysis of factors between the two pathology-defined groups as our cohort size lacks sufficient power. Likewise, the small study population prevents control for variables beyond the pathology-defined groups in the survival analysis. At this time we are also not able to provide a molecular-based explanation for our findings. Despite these limitations, no other major differing factor was identified between the pathology-defined groups in univariate analysis. Furthermore, the outcomes difference between the groups is so large that small variations in factors such as disease etiology are unlikely to account for this substantial difference.
By definition, study of a rare tumor type leads to limited numbers. . By study design, we have identified a group of patients with a cell type and growth pattern that is associated with improved survival and given the small numbers it is possible that this association is due to chance, despite the fact that the outcome difference is very large. This is best addressed by applying this categorization to another center’s patient cohort, which we plan to perform as a multicenter study in the future. We then could also evaluate for any molecular signatures that correlate with the clinical outcome difference that we have identified.
In summary, our institutional experience with CMM HCCs suggests prognostic significance of CMM extent, pseudoglandular architecture, and clear cell morphology. We introduce a clinically relevant sub-classification of confined and extensive, with the confined subtype correlating with improved survival. We demonstrate that pseudoglandular tumor architecture and clear cell morphology, traditionally thought to be clinicopathologically insignificant features in nodular HCC, are indeed relevant in this tumor variant, as tumors with pseudoglandular architecture correlate with lower survival and clear cell morphology correlates with increased survival rates. Despite the high frequency of unfavorable pathologic features of tumor burden and lymphovascular invasion, the patient survival and tumor recurrence rates of those within the Clear and Confined CMM classification group did not substantially diverge from those of non-CMM HCC patients transplanted at our center. In conclusion, the predictive value of cirrhotomimetic extent and histologic features appears to supersede traditional measures of tumor staging and prognosis, and are important parameters to consider when advising patients post-transplant when CMM HCC is discovered in the explanted liver.
Supplementary Material
Acknowledgement
We would like to credit the contribution of Robin Noel to the preparation of the figures in this manuscript.
Financial Support: Dr. Levine’s effort is in part funded by NIDDK grant number 1K08DK092282-01.
Abbreviations
- (HCC)
Hepatocellular carcinoma
- (CMM)
Cirrhotomimetic
- (OLT)
Orthotopic liver transplantation
Footnotes
Conflict of Interest: The authors have no conflict of interest to disclose regarding the data presented in this manuscript. There is no off-label utilization of medications described in this text.
Bibliography
- 1.Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet. 1999;353(9160):1253–1257. doi: 10.1016/S0140-6736(98)09148-X. [DOI] [PubMed] [Google Scholar]
- 2.Aaltonen L, Hamilton S. Pathology and genetics of tumours of the digestive system. Lyon/Oxford: IARC Press/Oxford University Press; 2000. (distributor) [Google Scholar]
- 3.Ishak K, Goodman Z, JT S. Hepatocellular Carcinoma. In: aSL Rosai J., editor. Tumors of the Liver and Intrahepatic Bile Ducts. Washington DC: Armed Forces Institute of Pathology; 2001. pp. 203–205. [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 5.Yao FY, Bass NM, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: lessons from the first year under the Model of End-Stage Liver Disease (MELD) organ allocation policy. Liver Transpl. 2004;10(5):621–630. doi: 10.1002/lt.20159. [DOI] [PubMed] [Google Scholar]
- 6.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 7.Wiesner RH, Freeman RB, Mulligan DC. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology. 2004;127(5 Suppl 1):S261–S267. doi: 10.1053/j.gastro.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 8.Merion RM, Wolfe RA, Dykstra DM, Leichtman AB, Gillespie B, Held PJ. Longitudinal assessment of mortality risk among candidates for liver transplantation. Liver Transpl. 2003;9(1):12–18. doi: 10.1053/jlts.2003.50009. [DOI] [PubMed] [Google Scholar]
- 9.Han YS, Choi DL, Park JB. Cirrhotomimetic type hepatocellular carcinoma diagnosed after liver transplantation--eighteen months of follow-up: a case report. Transplant Proc. 2008;40(8):2835–2836. doi: 10.1016/j.transproceed.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Jakate S, Yabes A, Giusto D, Naini B, Lassman C, Yeh MM, et al. Diffuse cirrhosis-like hepatocellular carcinoma: a clinically and radiographically undetected variant mimicking cirrhosis. Am J Surg Pathol. 2010;34(7):935–941. doi: 10.1097/PAS.0b013e3181ddf52f. [DOI] [PubMed] [Google Scholar]
- 11.PTN data as of September 1, 2013. http://optn.transplant.hrsa.gov.
- 12.Shimada M, Rikimaru T, Hamatsu T, Yamashita Y, Terashi T, Taguchi K, et al. The role of macroscopic classification in nodular-type hepatocellular carcinoma. Am J Surg. 2001;182(2):177–182. doi: 10.1016/s0002-9610(01)00682-1. [DOI] [PubMed] [Google Scholar]
- 13.Yang SH, Watanabe J, Nakashima O, Kojiro M. Clinicopathologic study on clear cell hepatocellular carcinoma. Pathol Int. 1996;46(7):503–509. doi: 10.1111/j.1440-1827.1996.tb03645.x. [DOI] [PubMed] [Google Scholar]
- 14.Lai Q, Merli M, Ginanni Corradini S, Mennini G, Gentili F, Molinaro A, et al. Predictive factors of recurrence of hepatocellular carcinoma after liver transplantation: a multivariate analysis. Transplant Proc. 2009;41(4):1306–1309. doi: 10.1016/j.transproceed.2009.03.094. [DOI] [PubMed] [Google Scholar]
- 15.Lauwers GY, Terris B, Balis UJ, Batts KP, Regimbeau JM, Chang Y, et al. Prognostic histologic indicators of curatively resected hepatocellular carcinomas: a multi-institutional analysis of 425 patients with definition of a histologic prognostic index. Am J Surg Pathol. 2002;26(1):25–34. doi: 10.1097/00000478-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Adamek HE, Spiethoff A, Kaufmann V, Jakobs R, Riemann JF. Primary clear cell carcinoma of noncirrhotic liver: immunohistochemical discrimination of hepatocellular and cholangiocellular origin. Dig Dis Sci. 1998;43(1):33–38. doi: 10.1023/a:1018859617522. [DOI] [PubMed] [Google Scholar]
- 17.Buchanan TF, Jr., Huvos AG. Clear-cell carcinoma of the liver. A clinicopathologic study of 13 patients. Am J Clin Pathol. 1974;61(4):529–539. doi: 10.1093/ajcp/61.4.529. [DOI] [PubMed] [Google Scholar]
- 18.Emile JF, Lemoine A, Azoulay D, Debuire B, Bismuth H, Reynes M. Histological, genomic and clinical heterogeneity of clear cell hepatocellular carcinoma. Histopathology. 2001;38(3):225–231. doi: 10.1046/j.1365-2559.2001.01096.x. [DOI] [PubMed] [Google Scholar]
- 19.Lai CL, Wu PC, Lam KC, Todd D. Histologic prognostic indicators in hepatocellular carcinoma. Cancer. 1979;44(5):1677–1683. doi: 10.1002/1097-0142(197911)44:5<1677::aid-cncr2820440522>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Ma W, Li H, Li Q. Clinicopathological and prognostic features of primary clear cell carcinoma of the liver. Hepatol Res. 2008;38(3):291–299. doi: 10.1111/j.1872-034X.2007.00264.x. [DOI] [PubMed] [Google Scholar]
- 21.Clayton EF, Furth EE, Ziober A, Xu T, Yao Y, Hwang PG, et al. A case of primary clear cell hepatocellular carcinoma in a non-cirrhotic liver: an immunohistochemical and ultrastructural study. Rare Tumors. 2012;4(2):e29. doi: 10.4081/rt.2012.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li T, Fan J, Qin LX, Zhou J, Sun HC, Qiu SJ, et al. Risk factors, prognosis, and management of early and late intrahepatic recurrence after resection of primary clear cell carcinoma of the liver. Ann Surg Oncol. 2011;18(7):1955–1963. doi: 10.1245/s10434-010-1540-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.