Abstract
Objective
Saphenous vein grafts (SVG) used for coronary artery bypass surgery (CABG) often develop a gradual luminal narrowing over the first year due to neointimal hyperplasia (NH). Although the basic science of NH is well studied, our clinical understanding of this issue is limited. The purpose of this cohort study was to investigate clinical risk factors for NH by monitoring luminal narrowing within SVG using multichannel CT angiography (CTA).
Methods
Thirty patients underwent CABG involving SVG (N = 44) and arterial grafts (N = 36). Patient variables were recorded and the baseline quality of each conduit determined intraoperatively by analyzing surplus segments for intima-media thickness ratio (IMT) by histology and matrix metalloproteinase-2 by enzyme-linked immunosorbent assay. Percent luminal narrowing (%LN) was calculated for each patent graft by comparing the CTA appearance on day 5 to a repeat study at 1 y.
Results
Compared with arterial grafts, SVG showed significantly higher IMT at baseline (0.9 ± 0.65 versus 0.22 ± 0.17, P < 0.0001) and more %LN over the first year (6.9 ± 7.5 versus 25.3 ± 13.3% LN, P< 0.0001). Of all of the measured variables, the only significant predictors of %LN included baseline IMT (r = 0.58, P = 0.002) and matrix metalloproteinase-2 levels (r = 0.60, P = 0.002) in SVG.
Conclusions
The degree of NH at baseline, a phenomenon exclusive to SVG and not found in arterial grafts, was significantly related to the development of lumen loss in the conduit over the first year after CABG. The study of SVG using serial CTA may provide unique insights into the natural history of SVG remodeling and to identify factors that influence the long-term function of this conduit.
Keywords: coronary artery bypass, neointimal hyperplasia, matrix metalloproteinase, endothelium, computed tomographic angiography
INTRODUCTION
The success of saphenous vein grafts (SVG) used during coronary artery bypass surgery (CABG) has been limited by the development of neointimal hyperplasia (NH), a process that leads to a progressive narrowing of the graft lumen [1–3]. Basic science studies have established that NH is caused by smooth muscle cell migration and proliferation in response to the introduction of a venous conduit into an arterial environment [4]. NH in lower extremity veins is also seen as a process of aging due to the chronic effects of high venous pressure from valvular incompetence [5]. A prerequisite for smooth muscle cell migration is the degradation of the extracellular matrix, which is achieved by the secretion of matrix-degrading gelatinases including matrix metalloproteinase-2 (MMP-2) [6, 7].
While the mechanisms of NH have been thoroughly studied in animal models [8–10], translation of this knowledge into a better clinical understanding of this problem has been negligible [11]. The inability to establish this link is attributable in large part to the fact that conventional coronary angiography is an insufficient tool for the study of this process for several reasons. First, a single angiographic examination at 1 y is unable to differentiate SVG that failed due to thrombosis versus NH. Second, conventional angiography only provides an assessment of the SVG luminal diameter (i.e., luminography), which is insensitive to mild-moderate degrees of NH. While comparison of SVG lumen at early and late time points using quantitative coronary angiography may improve the sensitivity, this invasive protocol is inconvenient for patients, associated with complications, and costly. The gold standard method for defining NH, intravascular ultrasound (IVUS), is associated with more expense and morbidity, making it even less applicable for clinical studies that are appropriately powered to address pathophysiology. Recent advances in CT coronary angiography (CTA) provide a noninvasive and highly accurate means of documenting the patency and luminal diameter of bypass grafts. The purpose of this cohort study was to use serial CTA follow-up to investigate the risk factors for luminal narrowing within SVG in the first year after CABG.
METHODS
Patient Selection and Enrollment
Informed consent (University of Maryland Institutional Review Board protocols H25350) was obtained from 30 consecutive patients undergoing CABG. Exclusion criteria at the time of enrollment included patients with chronic renal insufficiency (creatinine >2.0 mg/dL), and allergy to radiographic contrast. Patient variables that were recorded included age, BMI, history of diabetes, history of peripheral vascular disease, past history of smoking, current smoking status, and medication regimen.
Surgery
After median sternotomy, the left internal thoracic artery was procured in all patients; the saphenous vein was harvested using an endoscopic (VasoView5; Guidant Systems, Inc., Minneapolis, MN) or open approach, based on anatomical considerations. Conduits were stored in heparinized saline after harvest. Proximal anastomoses were performed using a partial occluding aortic clamp. All distal anastomoses were facilitated by suction-based exposure and stabilizing devices (Octopus 4.3; Medtronic, Inc., Minneapolis, MN) without intracoronary shunts. Shed mediastinal blood was collected intraoperatively using a cell-saving device (Cobe BRAT 2; Cobe Cardiovascular, Arvada, CO), processed, and re-transfused. Graft flow and pulsatility index (maximum-minimum)/mean blood flow) were recorded intraoperatively using transit time ultrasound technology (Medi-Stim ASA, Oslo, Norway). Bypass grafts with flow that remained less than 10 cc/min and pulsatility index greater than 5, despite revision, were excluded from the analysis.
Neointimal Hyperplasia and Graft Failure
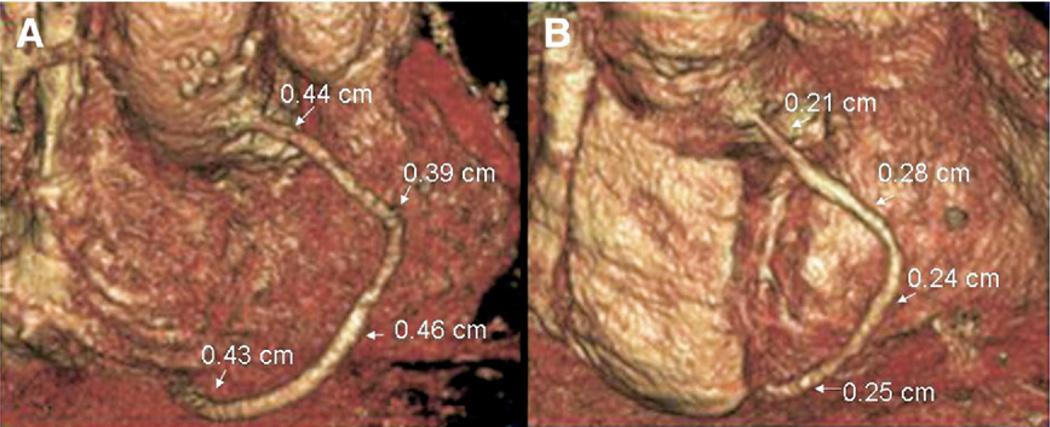
The luminal diameter (LD) of SVG was assessed by 16-channel CTA (420 ms rotation, with 100 to 150 mL contrast agent administered intravenously at 5 cc/s, and retrospective electrocardiographic gating; Philips MX8000, Cleveland, OH) at both 5 d (postoperative) and 1 y (follow-up) after surgery. LD was measured in four areas of each graft (os, proximal, middle, and distal) by two separate, masked expert reviewers (C.W. and J.J.). The percent luminal narrowing (%LN) at each segment of each graft was calculated as %LN = (LDpostop − LDfollow-up)/(LDpostop). The overall %LN for each graft was calculated as the average %LN for each of four measured segments in that graft and the lack of LN was defined as an average LN <15%. (Fig. 1). Patency at both time points was defined as any flow through the entire graft regardless of the presence of stenosis. The graft was classified as nonpatent if a stump was seen or if there was no contrast in an area known by operative report to contain a graft, a recently validated definition of postoperative bypass graft failure [12].
FIG. 1.
Representative 3D reconstructions of a SVG obtained using serial multi-channel CTA at 5 d (A) and 1 y (B). At 5 d the luminal diameter was measured at four points along the graft (os, proximal, middle, and distal) and the % change at 1 y was calculated and averaged as the mean % LN. (Color version of figure is available online.)
Preexisting Intimal Thickening
Surplus segments from each bypass conduit were stored in balanced salt solution at room temperature for 30 to 60 min, embedded in cutting compound (Tissue-Tek O.C.T., Redding, CA) and then frozen in liquid nitrogen. Four separate 5 µ thick sections were cut from each graft. Intimal and media thickness was measured in hematoxylin and eosin stained sections using image analysis software (Bioquant Nova Prime, Nashville, TN) and the intima-media ratio was calculated for each graft [13].
MMP-2 Tissue Levels
Additional portions of each graft were snap-frozen in liquid nitrogen and stored at −80°C until analysis. Two to 3 mm axial ring segments were ground to a fine powder in liquid nitrogen using a mortar and pestle, transferred to Eppendorf vials, weighed, and then incubated with a cell lysis buffer (RIPA lysis buffer; Upstate, Lake Placid, NY) at room temperature for 1 h. After spinning samples at 16,000 g on a tabletop centrifuge for 10 min, MMP-2 levels in the supernatant were determined using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (MMP-2 ELISA Kit QIA63; Calbiochem, San Diego, CA) and normalized with respect to the weight of homogenized tissue [14].
Statistical Methods
The primary end point of this trial was an analysis of intraoperative factors associated with the development of luminal narrowing at 1 y follow-up. Correlations were examined using linear regression for parametric data. Groups were compared using unpaired Student’s t-test for continuous variables and the Fisher’s exact test for categorical variables. Results are described as mean ± standard deviation. Data points categorized as outliers were excluded as described at: http://www.graphpad.com/quickcalcs/Grubbs1.cfm (n = 1). Statistical analysis was performed using the InStat statistical package with the assistance of a biostatistician.
RESULTS
Patients received an average of 2.7 grafts with an average of 1.5 SVG. All patients received a LIMA graft, with 4 right internal mammary arteries and 4 radial arteries also used.
CT Angiographic Follow-Up
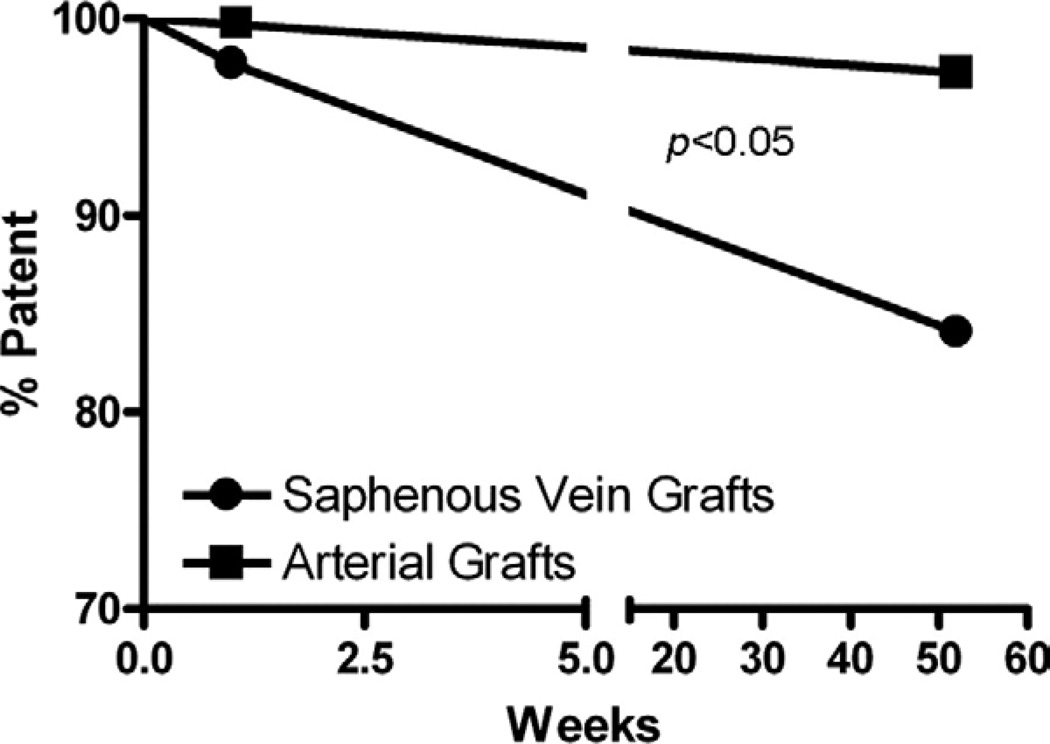
Initial postoperative CTA analysis on postoperative day 5 showed a 98% SVG patency rate (1/46 failed) compared with a 100% arterial patency rate (0/34 failed). One year CTA analysis showed an 86% SVG patency rate (6/46 failed) compared with a 97% arterial graft patency rate (1/34 failed) with one failed radial artery graft. Kaplan-Meier analysis revealed that the patency rate over this timeframe for SVG was significantly poorer compared to arterial grafts (Fig. 2).
FIG. 2.
A Kaplan-Meier survival curve analysis of graft patency rate over 1 y. Patency was determined at two time points, 1 and 52 wk, using CT angiography. SVG performed significantly poorer compared with arterial grafts.
All 30 patients completed follow-up at both early and late CT angiographic time points. This allowed a comparison of the luminal diameter of each patent graft. When compared with arterial grafts, the presence of luminal narrowing above our threshold for detection (9% versus 73%, P < 0.0001) and the severity of luminal narrowing (6.9 ± 7.5 versus 25.3 ± 13.3% LN, P < 0.0001) were significantly more severe in SVG over this timeframe. The degree of luminal narrowing was relatively homogenous throughout a given SVG (i.e., low intra-graft variation) with a 17% coefficient of variation noted for the four points of measurement (os, proximal, middle, and distal graft). However, in those patients receiving more than one SVG, a comparison of the average luminal narrowing for each graft showed a 31% coefficient of variation between grafts (i.e., high inter-graft variation).
Risk Factors for Luminal Narrowing
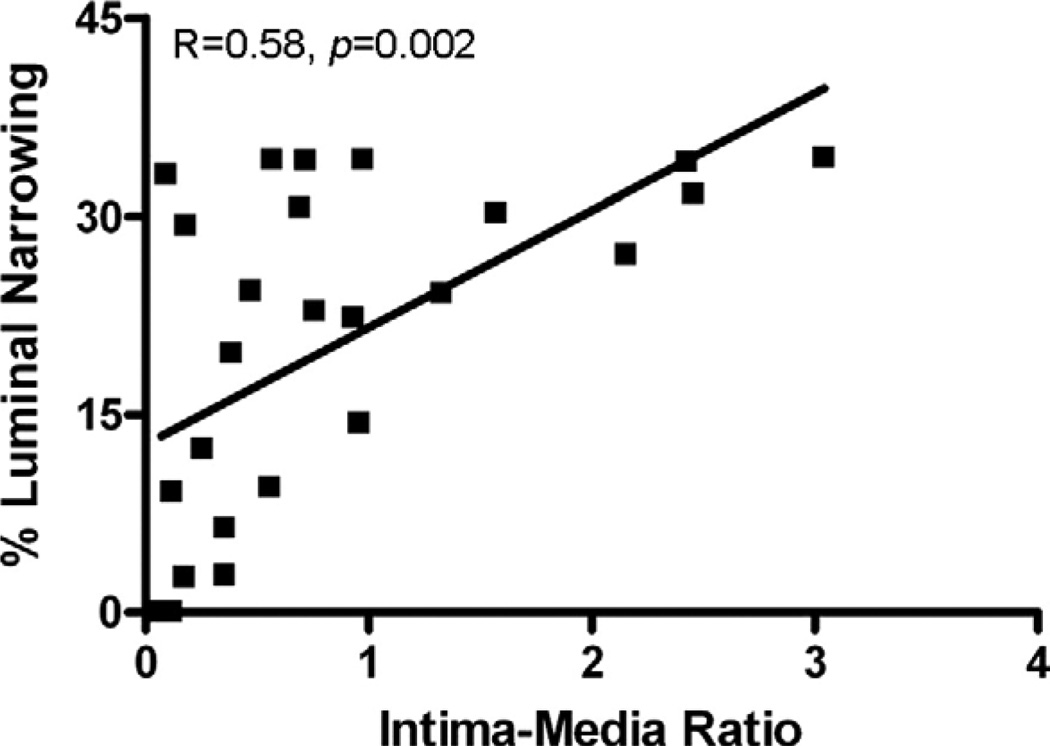
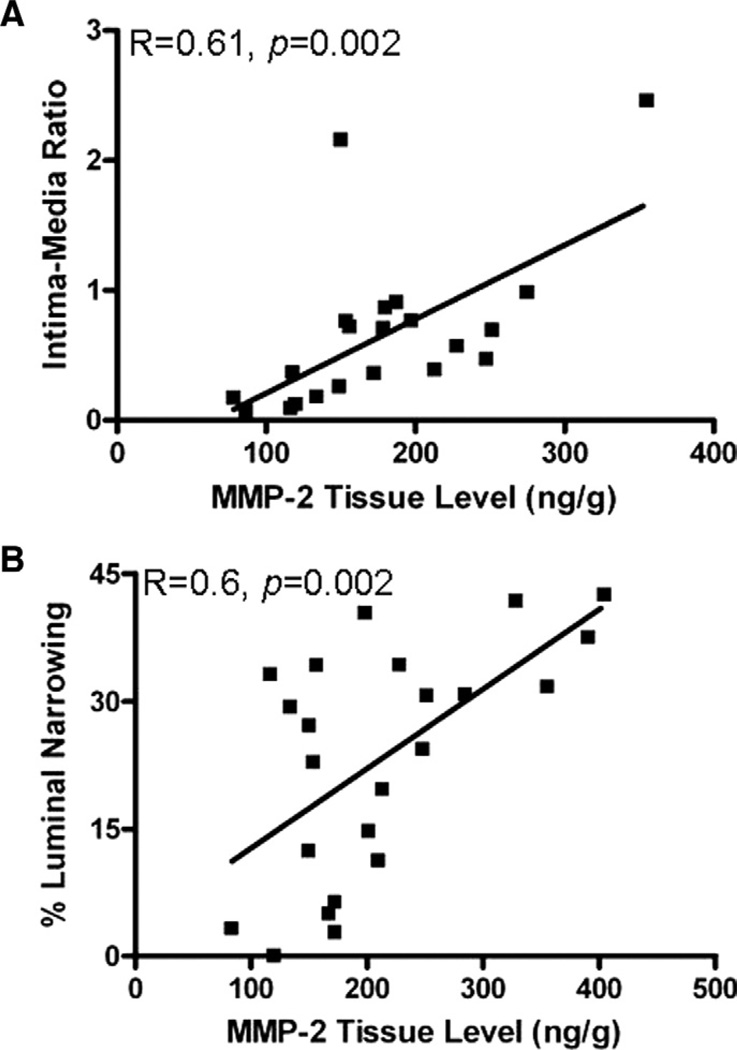
Analysis of surplus graft segments obtained intraoperatively revealed significantly more thickened intima in venous compared with arterial segments (0.9 ± 0.65 versus 0.22 ± 0.17 IMT, P < 0.0001) (Table 1). Intraoperative intima-media ratio was shown to have a strong linear correlation with the degree of graft luminal narrowing at 1 y (r = 0.58, P = 0.002) (Fig. 3). None of the clinical variables that were analyzed were associated with the development of luminal narrowing at 1 y (Table 2). The baseline level of MMP-2 in the SVG was found have a strong linear correlation with both the degree of luminal narrowing at 1 y (r = 0.6, P = >0.002) and intimamedia ratio measured from intraoperative surplus graft segments (r = 0.61, P = 0.002) (Fig. 4).
TABLE 1.
SVG Versus Arterial Graft Analysis
| Saphenous vein grafts |
Arterial grafts |
P-value | |
|---|---|---|---|
| Preoperative | |||
| Intima-media ratio | 0.9 ± 0.65 | 0.22 ± 0.17 | <0.0001 |
| At one year | |||
| Grafts with luminal narrowing | 73% | 9% | <0.0001 |
| Mean % luminal narrowing | 25.3 ± 13.3 | 6.9 ± 7.5 | <0.0001 |
FIG. 3.
Using hematoxylin and eosin staining of surplus graft segments and imaging software, intraoperative graft intimal, and medial thickness was measured and an intima-media ratio was calculated. Intimal thickening showed a strong linear correlation with the degree of LN at 1 y.
TABLE 2.
Risk Factors for Luminal Narrowing
| <15% LN | ≥15% LN | P-value | |
|---|---|---|---|
| Patient characteristics | |||
| Age (y) | 69 ± 11 | 63 ± 13 | NS |
| Gender (M) | 67% | 88% | NS |
| BMI | 30.2 ± 6.3 | 30.3 ± 6.3 | NS |
| Diabetes | 25% | 31% | NS |
| Hypertension (>90 and/or >140) | 92% | 88% | NS |
| History of smoking | 92% | 85% | NS |
| Current smoker | 25% | 15% | NS |
| Peripheral vascular disease | 17% | 19% | NS |
| Medications at surgery | |||
| ACE inhibitor | 58% | 42% | NS |
| Aspirin | 83% | 73% | NS |
| Beta blocker | 92% | 88% | NS |
| Diuretics | 50% | 38% | NS |
| Statins | 67% | 62% | NS |
| Graft analysis | |||
| MMP-2 (ng/g) | 168 ± 50 | 243 ± 98 | 0.046 |
| Intima-media ratio | 0.46 ± 0.34 | 1.18 ± 0.84 | 0.024 |
FIG. 4.
Intraoperative MMP-2 graft tissue levels assessed in homogenized surplus graft segments using ELISA. MMP-2 tissue level was found to show a strong linear correlation with both the intraoperative intima-media ratio (A) and the degree of LN at 1 y (B).
DISCUSSION
Prior studies have proven that CTA is a highly sensitive method for detecting bypass graft occlusions due to thrombosis early after CABG and focal stenoses due to atherosclerotic lesions at late follow-up [15, 16]. The novel approach of this study was the use of serial CTA to compare the luminal diameter of the same bypass graft at 1 wk and 1 y following CABG. Unlike the focal stenoses reported in prior studies, we noted that >70% of SVG developed widespread caliber narrowing during that timeframe. The relationship to risk factors that have been previously linked to NH in other studies (venous versus arterial grafts, preexisting intimal disease, MMP-2) suggests that this luminal narrowing identified by CTA is the direct result of NH. Of particular interest was the association between NH and baseline MMP-2 expression [17]. MMP-2 breaks down the extracellular matrix of the basement membrane, allowing smooth muscle cells to proliferate and migrate into the intimal layer reducing the luminal diameter. This observation illustrates the potential of serial CTA to provide a novel, noninvasive tool that can be exploited to establish the link between our basic science understanding of NH and the long term success of CABG.
General features of the patient such as diabetes, HTN, and medical therapies were not found to be related to NH in the graft. While this study was not appropriately powered to rule out any impact of these factors, our data suggest that these clinical variables are likely to have less of an impact on the end point of NH when compared with the initial quality of the conduit. Furthermore, the effect appeared to be specific to the vein segment used; SVG within the same individuals who were found to be of higher quality (i.e., lower IMT at baseline) tended to remain widely patent whereas those of lesser quality (i.e., higher IMT at baseline) developed a more severe form of NH.
Despite our current limited understanding of clinical risk factors, numerous therapeutic approaches have attempted to manage and treat NH [18–20], with the largest trial being the PREVENT IV trial using the gene therapy agent, E2F decoy. While this gene therapy approach was found to be highly efficacious in several animal models [8, 10], it proved to be no better than placebo in CABG patients [11]. Our data suggest that a possible explanation for this difference in the animal and clinical results may be the more prevalent finding of preexisting NH within SVG even prior to grafting. Saphenous veins used for CABG are obtained from elderly CABG patients. Lower extremity veins procured from young animals may be less likely to experience the chronic effects of high venous pressure from valvular incompetence. The ability to block the progression of NH after grafting that is already established in a conduit before grafting is obviously a more complicated challenge than merely preventing its initiation in vessel from an animal that is normal at baseline.
Limitations
There are several limitations to this study. First, CTA was able to determine luminal narrowing, but not changes in wall thickness, to the SVG. This method is insensitive to vessel remodeling that occurs during the early stages of NH. As CTA technology improves (e.g., 64-slice scanners), we likely will be able to directly measure wall thickening more convincingly and reliably [21]. In addition, our study did not incorporate a gold standard method for defining NH such as IVUS to confirm that NH was indeed responsible for the luminal narrowing detected by CTA. IVUS is invasive, expensive, and potentially morbid when performed on diseased bypass grafts. We consider SVG luminal narrowing to be a clinically relevant finding because it is highly likely to influence graft function irregardless of how it is measured or what underlying process it actually represents. As such, establishing a clear definition of the risk factors for SVG lumen loss is likely to provide an important clinical benefit. We enrolled only a small number of patients in this study. As a result, this limited our ability to perform a multivariate analysis to detect unidentified factors that might have also influenced our study end point. Although mechanistically consistent, this pilot analysis of the use of serial CTA to define predictors of NH should be considered only as hypothesis generating pending prospective confirmation with a larger cohort that utilizes these more powerful statistical techniques.
CONCLUSIONS
Serial CTA is a rapid, inexpensive, and noninvasive means of monitoring SVG function over the critical first postoperative year that is widely acceptable to patients, as illustrated by the 100% follow-up at 1 y in this cohort. Luminal narrowing detected by serial CTA was related to risk factors that have been previously linked to NH in other studies (venous versus arterial grafts, preexisting intimal disease, MMP-2), suggesting that this CTA finding is the direct result of NH within the SVG. This preliminary analysis supports our ongoing use of serial CTA as a means of further investigating and treating NH, a major factor that limits the success of CABG.
ACKNOWLEDGMENTS
R.S.P. is supported by a Scientist Development Grant from the American Heart Association, an Intramural Grant from the University of Maryland, a faculty pilot grant from the Tobacco Restitution Fund at the University of Maryland, and by the NIH (RO1 HL084080-01A1).
REFERENCES
- 1.Fitzgibbon GM, Kafka HP, Leach AJ, et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5065 grafts related to survival and reoperation in 1388 patients during 25 years. J Am Coll Cardiol. 1996;28:616. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 2.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: Pathogenesis, predisposition, and prevention. Circulation. 1998;97:916. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 3.Reardon MJ, Conklin LD, Reardon PR, et al. Coronary artery bypass conduits: Review of current status. J Cardiovasc Sur. 1997;97:916. [PubMed] [Google Scholar]
- 4.Zwolak RM, Adams MC, Clowes AW. Kinetics of vein graft hyperplasia: Association with tangential stress. J Vasc Surg. 1987;5:126. [PubMed] [Google Scholar]
- 5.Thiene G, Miazzi P, Valsecchi M, et al. Histological survey of the saphenous vein before its use as autologous aortocoronary bypass graft. Thorax. 1980;35:519. doi: 10.1136/thx.35.7.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, the ugly. Circ Res. 2002;90:251. [PubMed] [Google Scholar]
- 7.Uzui H, Lee JD, Shimizu H, et al. The role of proteintyrosine phosphorylation and gelatinase production in the migration and proliferation of smooth muscle cells. Atherosclerosis. 2000;149:51. doi: 10.1016/s0021-9150(99)00295-6. [DOI] [PubMed] [Google Scholar]
- 8.Morishita R, Gibbons GH, Horiuchi M, et al. A gene therapy strategy using a transcription factor decoy of the E2F binding site inhibits smooth muscle proliferation in vivo. Proc Natl Acad Sci. 1995;92:5855. doi: 10.1073/pnas.92.13.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mann MJ, Gibbons GH, Kernoff RS, et al. Genetic engineering of vein grafts resistant to atherosclerosis. Proc Natl Acad Sci. 1995;92:4502. doi: 10.1073/pnas.92.10.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehsan A, Mann MJ, Dell’Acqua G, et al. Long-term stabilization of vein graft wall architecture and prolonged resistance to experimental atherosclerosis after E2F decoy oligonucleotide gene therapy. J Thorac Cardiovasc Sur. 2001;121:714. doi: 10.1067/mtc.2001.111204. [DOI] [PubMed] [Google Scholar]
- 11.PREVENT IV Investigators. Efficacy and safety of Edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: A Randomized Controlled Trial. JAMA. 2005;294:2446. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 12.Martuscelli E, Romagnoli A, D’Eliseo A, et al. Evaluation of venous and arterial conduit patency by 16-slice spiral computed tomography. Circulation. 2004;110:3234. doi: 10.1161/01.CIR.0000147277.52036.07. [DOI] [PubMed] [Google Scholar]
- 13.Burris N, Schwartz K, Tang C-M, et al. Catheter-based infrared light scanner as a tool to assess conduit quality in coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2007;133:419. doi: 10.1016/j.jtcvs.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 14.Patterson MA, Leville CD, Hower CD, et al. Shear force regulates matrix metalloproteinase activity in human saphenous vein organ culture. J Surg Res. 2001;95:67. doi: 10.1006/jsre.2000.6045. [DOI] [PubMed] [Google Scholar]
- 15.Lesser JR, Flygenring B, Knickelbine T, et al. Clinical utility of coronary CT angiography: Coronary stenosis detection and prognosis in ambulatory patients. Catheter Cardiovasc Interv. 2006;69:64. doi: 10.1002/ccd.20904. [DOI] [PubMed] [Google Scholar]
- 16.Marano R, Storto ML, Maddestra N, et al. Noninvasive assessment of coronary artery bypass graft with retrospectively ECG-gated four-row multi-detector spiral computed tomography. Eur Radiol. 2004;14:1353. doi: 10.1007/s00330-004-2323-3. [DOI] [PubMed] [Google Scholar]
- 17.von Offenberg SN, Cummins PM, Birney YA, et al. Cyclic strain-induced endothelial MMP-2: Role in vascular smooth muscle cell migration. Biochem Biophys Res Commun. 2004;320:325. doi: 10.1016/j.bbrc.2004.05.174. [DOI] [PubMed] [Google Scholar]
- 18.Blankenhorn DH, Nessim SA, Johnson RL, et al. Beneficial effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. JAMA. 1987;257:3233. [PubMed] [Google Scholar]
- 19.Cashin-Hemphill L, Mack WJ, Pogoda JM, et al. Beneficial effects of colestipol-niacin on coronary atherosclerosis: A 4-year follow-up. JAMA. 1990;264:3013. [PubMed] [Google Scholar]
- 20.The post-coronary artery bypass graft trial investigators. The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. N Engl J Med. 1997;336:153. doi: 10.1056/NEJM199701163360301. [DOI] [PubMed] [Google Scholar]
- 21.Lau GT, Ridley LJ, Bannon PG, et al. Lumen loss in the first year in saphenous vein grafts is predominantly a result of negative remodeling of the whole vessel rather than a result of changes in wall thickness. Circulation. 2006;114:435. doi: 10.1161/CIRCULATIONAHA.105.001008. [DOI] [PubMed] [Google Scholar]