Abstract
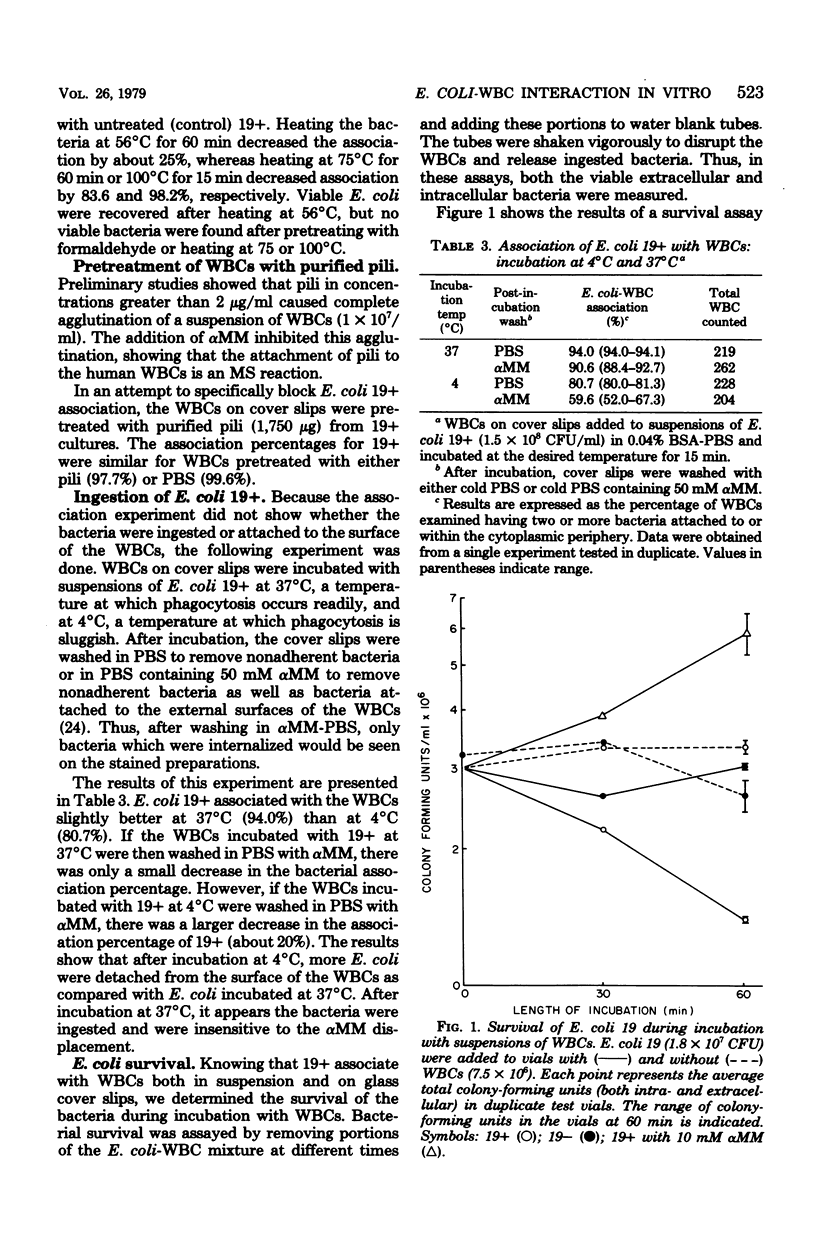
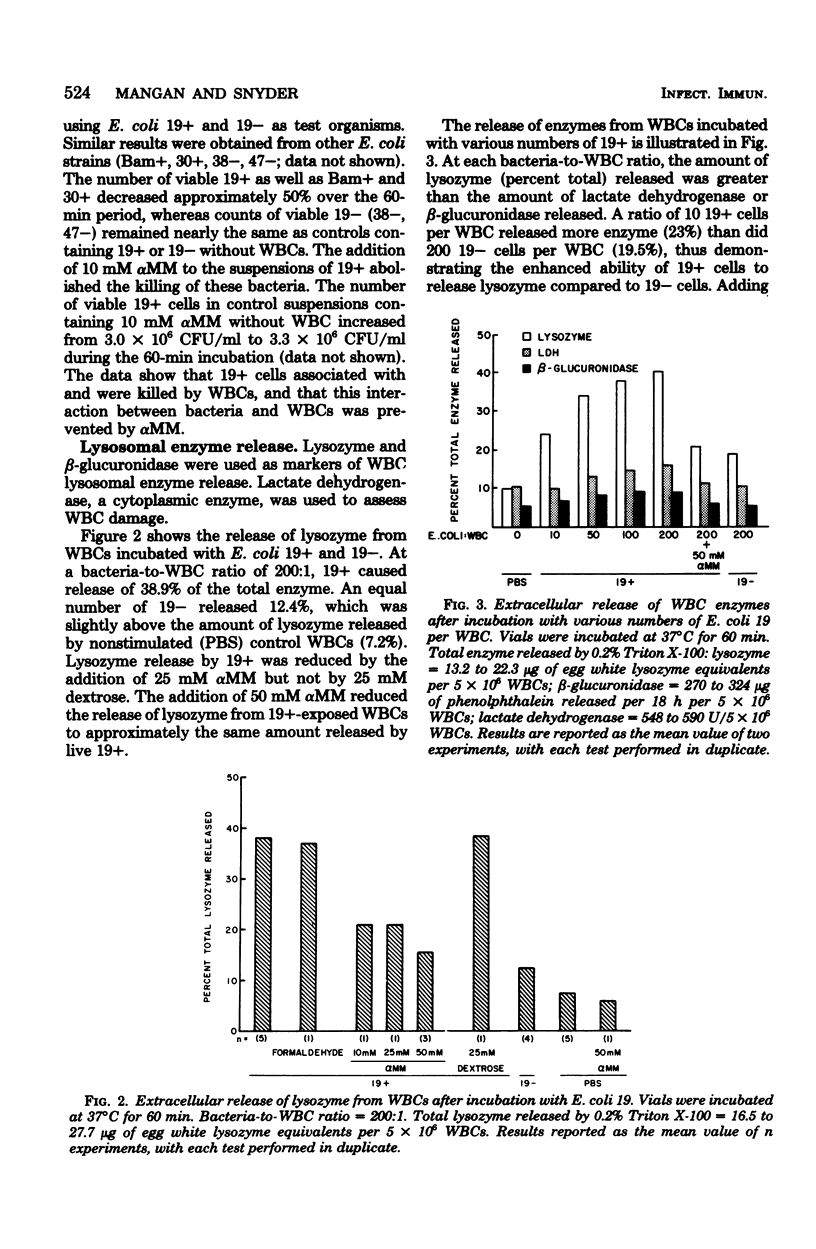
The ability of Escherichia coli which possess or lack mannose-sensitive adherence factors (adhesins) to associate with human peripheral leukocytes in vitro in the absence of serum was studied. E. coli 19+, which have mannose-sensitive adhesins, were derived from E. coli strain 19 by culturing in static Trypticase soy broth at 37 degrees C. E. coli 19-, which lack mannose-sensitive adhesins, were derived from E. coli 19 by culturing in agitated Trypticase soy broth at 30 degrees C. E. coli 19+ attached to leukocytes and stimulated the release of lysozyme but not beta-glucuronidase or lactate dehydrogenase. In contrast, E. coli 19- showed poor attachment to the leukocytes and failed to stimulate lysosomal enzyme release. During a 60-min incubation with the leukocytes, the number of viable 19+ organisms decreased, whereas the number of viable 19- remained constant. Purified type 1 pili from E. coli 19+ agglutinated the leukocytes but did not stimulate lysosomal enzyme release. Pretreatment of leukocytes with type 1 pili failed to prevent the adherence of E. coli 19+. The association of 19+ with leukocytes and subsequent release of lysozyme could be blocked by alpha-methyl-D-mannoside but not by equivalent concentrations of dextrose and sucrose. These results show that mannose-sensitive adhesins on E. coli mediate association of the organisms with leukocytes in the absence of serum components. The identity of the adhesins involved in leukocyte association has yet to be determined.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit Z., Ofek I., Goldman R., Mirelman D., Sharon N. Mannose residues on phagocytes as receptors for the attachment of Escherichia coli and Salmonella typhi. Biochem Biophys Res Commun. 1977 Sep 9;78(1):455–460. doi: 10.1016/0006-291x(77)91276-1. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Brittinger G., Hirschhorn R., Douglas S. D., Weissmann G. Studies on lysosomes. XI. Characterization of a hydrolase-rich fraction from human lymphocytes. J Cell Biol. 1968 May;37(2):394–411. doi: 10.1083/jcb.37.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- Clark R. A., Kimball H. R. Defective granulocyte chemotaxis in the Chediak-Higashi syndrome. J Clin Invest. 1971 Dec;50(12):2645–2652. doi: 10.1172/JCI106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder J. G., Martin R. R., White A. Release of histamine and lysosomal enzymes by human leukocytes during phagocytosis of staphylococci. J Lab Clin Med. 1969 Sep;74(3):436–444. [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Reeke G. N., Jr, Becker J. W., Waxdal M. J., Wang J. L. The covalent and three-dimensional structure of concanavalin A. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2580–2584. doi: 10.1073/pnas.69.9.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshdat Y., Ofek I., Yashouv-Gan Y., Sharon N., Mirelman D. Isolation of a mannose-specific lectin from Escherichia coli and its role in the adherence of the bacteria to epithelial cells. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1551–1559. doi: 10.1016/0006-291x(78)91179-8. [DOI] [PubMed] [Google Scholar]
- Glynn A. A., Howard C. J. The sensitivity to complement of strains of Escherichia coli related to their K antigens. Immunology. 1970 Mar;18(3):331–346. [PMC free article] [PubMed] [Google Scholar]
- Hoffstein S., Goldstein I. M., Weissmann G. Role of microtubule assembly in lysosomal enzyme secretion from human polymorphonuclear leukocytes. A reevaluation. J Cell Biol. 1977 Apr;73(1):242–256. doi: 10.1083/jcb.73.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstein S., Soberman R., Goldstein I., Weissmann G. Concanavalin A induces microtubule assembly and specific granule discharge in human polymorphonuclear leukocytes. J Cell Biol. 1976 Mar;68(3):781–787. doi: 10.1083/jcb.68.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Glynn A. A. Some physical properties of K antigens of Escherichia coli related to their biological activity. Infect Immun. 1971 Jul;4(1):6–11. doi: 10.1128/iai.4.1.6-11.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Glynn A. A. The virulence for mice of strains of Escherichia coli related to the effects of K antigens on their resistance to phagocytosis and killing by complement. Immunology. 1971 May;20(5):767–777. [PMC free article] [PubMed] [Google Scholar]
- Klempner M. S., Dinarello C. A., Gallin J. I. Human leukocytic pyrogen induces release of specific granule contents from human neutrophils. J Clin Invest. 1978 May;61(5):1330–1336. doi: 10.1172/JCI109050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Novotny C., Knight W. S., Brinton C. C., Jr Inhibition of bacterial conjugation by ribonucleic acid and deoxyribonucleic acid male-specific bacteriophages. J Bacteriol. 1968 Feb;95(2):314–326. doi: 10.1128/jb.95.2.314-326.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Old D. C. Inhibition of the interaction between fimbrial haemagglutinins and erythrocytes by D-mannose and other carbohydrates. J Gen Microbiol. 1972 Jun;71(1):149–157. doi: 10.1099/00221287-71-1-149. [DOI] [PubMed] [Google Scholar]
- PARRY R. M., Jr, CHANDAN R. C., SHAHANI K. M. A RAPID AND SENSITIVE ASSAY OF MURAMIDASE. Proc Soc Exp Biol Med. 1965 Jun;119:384–386. doi: 10.3181/00379727-119-30188. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Dalldorf F. G., Leffell M. S., Folds J. D., Welsh I. R., Cooney M. H., Martin L. E. Character of azurophil and specific granules purified from human polymorphonuclear leukocytes. Lab Invest. 1974 Jun;30(6):774–785. [PubMed] [Google Scholar]
- Swanson J., Sparks E., Young D., King G. Studies on Gonococcus infection. X. Pili and leukocyte association factor as mediators of interactions between gonococci and eukaryotic cells in vitro. Infect Immun. 1975 Jun;11(6):1352–1361. doi: 10.1128/iai.11.6.1352-1361.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Zeligs B., Siam M. A., Parrott C. Studies on gonococcus infection. V. Observations on in vitro interactions of gonococci and human neutrophils. Infect Immun. 1974 Sep;10(3):633–644. doi: 10.1128/iai.10.3.633-644.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman N. S., McArthur W. P. Interaction of inflammatory cells and oral bacteria: release of lysosomal hydrolases from rabbit polymorphonuclear leukocytes exposed to gram-positive plaque bacteria. Arch Oral Biol. 1976;21(4):257–263. doi: 10.1016/0003-9969(76)90044-3. [DOI] [PubMed] [Google Scholar]
- Tew J. G., Hess W. M., Donaldson D. M. Lysozyme and beta-lysin release stimulated by antigen-antibody complexes and bacteria. J Immunol. 1969 Mar;102(3):743–750. [PubMed] [Google Scholar]
- ULMER D. D., VALLEE B. L., WACKER W. E. Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med. 1956 Sep 6;255(10):450–456. doi: 10.1056/NEJM195609062551001. [DOI] [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. Contact angles and phagocytosis of non-opsonized bacteria. J Reticuloendothel Soc. 1972 Sep;12(3):283–292. [PubMed] [Google Scholar]
- Weissmann G. Leukocytes as secretory organs of inflammation. Hosp Pract. 1978 Sep;13(9):53–62. doi: 10.1080/21548331.1978.11707397. [DOI] [PubMed] [Google Scholar]
- Wright D. G., Bralove D. A., Gallin J. I. The differential mobilization of human neutrophil granules. Effects of phorbol myristate acetate and ionophore A23187. Am J Pathol. 1977 May;87(2):273–284. [PMC free article] [PubMed] [Google Scholar]
- Wright D. G., Malawista S. E. The mobilization and extracellular release of granular enzymes from human leukocytes during phagocytosis. J Cell Biol. 1972 Jun;53(3):788–797. doi: 10.1083/jcb.53.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oss C. J. Phagocytosis as a surface phenomenon. Annu Rev Microbiol. 1978;32:19–39. doi: 10.1146/annurev.mi.32.100178.000315. [DOI] [PubMed] [Google Scholar]



