Abstract
The potential influence of diversity on ecosystem structure and function remains a topic of significant debate, especially for tropical forests where diversity can range widely. We used Center for Tropical Forest Science (CTFS) methodology to establish forest dynamics plots in montane wet forest and lowland dry forest on Hawai‘i Island. We compared the species diversity, tree density, basal area, biomass, and size class distributions between the two forest types. We then examined these variables across tropical forests within the CTFS network. Consistent with other island forests, the Hawai‘i forests were characterized by low species richness and very high relative dominance. The two Hawai‘i forests were floristically distinct, yet similar in species richness (15 vs. 21 species) and stem density (3078 vs. 3486/ha). While these forests were selected for their low invasive species cover relative to surrounding forests, both forests averaged 5–>50% invasive species cover; ongoing removal will be necessary to reduce or prevent competitive impacts, especially from woody species. The montane wet forest had much larger trees, resulting in eightfold higher basal area and above-ground biomass. Across the CTFS network, the Hawaiian montane wet forest was similar to other tropical forests with respect to diameter distributions, density, and aboveground biomass, while the Hawai‘i lowland dry forest was similar in density to tropical forests with much higher diversity. These findings suggest that forest structural variables can be similar across tropical forests independently of species richness. The inclusion of low-diversity Pacific Island forests in the CTFS network provides an ∼80-fold range in species richness (15–1182 species), six-fold variation in mean annual rainfall (835–5272 mm yr−1) and 1.8-fold variation in mean annual temperature (16.0–28.4°C). Thus, the Hawaiian forest plots expand the global forest plot network to enable testing of ecological theory for links among species diversity, environmental variation and ecosystem function.
Introduction
High species richness is a hallmark of many tropical forests [1], [2]. Indeed, the latitudinal gradient and equatorial peak in plant diversity has attracted attention for centuries e.g., [1], [3], [4], [5]. Numerous studies have focused on the causes of high diversity in tropical forests [1], [6], [7], [8], [9], [10], and theories have been formulated to explain how species or functional diversity in turn affects ecosystem function [11], [12]. However, these linkages have rarely been tested, and not all tropical forests are diverse. For example, legume-dominated swamp forests, peat forests, pine savannas, and oceanic islands that are geographically isolated can have low to very low diversity [13], [14], [15], [16]. Such low-diversity forests are understudied, and there is no clear answer to the simple question of whether the structure of a low-diversity tropical forest would be expected to be similar to or different from that of a high-diversity tropical forest with comparable climate.
Indeed, the question of how forest structure—i.e., physiognomy, basal area, density, diameter size class distributions, biomass, and evenness—varies with species diversity is itself understudied, likely an effect of the paucity of studies of the structure of low-diversity tropical forests. Some have hypothesized that forest structure and species-richness might be related, if structure acts as a habitat scaffold or template that precedes and enables species assembly and diversity by providing an increased variety of habitat niches (e.g., nurse logs for seedlings, perches for birds that disperse seeds, climbing structures for vines [17], [18]. Alternatively, higher diversity may enhance forest structure, if more species correspond to a wider variety of size classes, strata, and crown architectures [17]. Both processes are not mutually exclusive and may operate simultaneously, creating a positive feedback cycle that would enhance diversity and influence various forest structural attributes. Recent efforts have examined some structural variables, such as latitudinal trends in height across forests e.g., [19], [20] and the effects of diversity and spatial scale on standing forest biomass [12], but very low-diversity tropical forests were not considered in these analyses. The tropical forests in the Hawaiian Islands represent a low-diversity extreme, as a result of its young geological origins [21] and extreme isolation from continental land masses: at approximately 4000 km from the nearest continent, Hawai‘i is the world's most isolated archipelago. The resulting native flora in Hawai‘i is disharmonic (i.e., missing many functional groups) and is about 90% endemic [22]. While long-term plot-based ecological measurements across the tropics have focused on high-diversity forests, there have been surprisingly few data from low-diversity tropical forests [23], [24], [25], [26]. Such low-diversity forests present many interesting contrasts to other tropical forests, and within Hawai‘i they also fall across striking environmental gradients (Table 1).
Table 1. Distinctive structural and demographic features of Hawaiian forests.
| Environmental Conditions |
| Large variation in elevation, rainfall, temperature and soils among forests that are geographically close 1–2 |
| High light levels in intact wet, mesic, and dry forest (1.9–40% diffuse light transmission) 3–9 |
| Species Composition and Diversity Patterns |
| A global biodiversity hotspot due to high endemism and number of endangered species 10–11 |
| Same species distributed in many habitats differing in environmental conditions, demonstrating exceptional phenotypic plasticity 10–14 |
| Tree ferns common and often the understory dominant in wet forests at all elevations, whereas outside of Hawai‘i they tend to be more restricted 10 |
| Monodominance by a few canopy species 15 |
| Autecology of Plant Species |
| Metrosideros polymorpha dominant in wet forests throughout succession (as pioneer and late successional species) 15–17 |
| Extremely slow growth of primary pioneer species, M. polymorpha (1–2 mm/year diameter) 18–20 |
| Nurse logs serve as a substrate for seedling regeneration 21 |
| Dieback and regeneration of canopy dominant M. polymorpha in cohorts contribute strongly to gap dynamics 16, 22 |
| Trophic Interactions |
| Evolution without land mammals 23, 24 |
| Documented extinctions of plants, pollinators and dispersers may influence present day evenness and rarity measures 23 |
| Animal dispersal of seeds conducted entirely by birds before human contact 24, 25 |
| Apparently low rates of insect herbivory 26 and seed predation 27 |
| Presence of invasive weeds, ungulates, and birds may alter present-day plant-animal interactions 25–26, 28 |
Superscripts refer to references listed in Table S5 in File S2.
The aim of this study was to: 1) characterize and compare two extremely low-diversity Hawaiian forests, montane wet and lowland dry forest, and 2) compare the structural attributes of these two forests to more diverse tropical forests within the Center for Tropical Forest Science (CTFS) permanent plot network. Including the Hawaiian plots as part of a cross-plot analysis allows, for the first time, examination of forest structure along a diversity gradient that varies almost 80-fold across large-scale plots with consistent measurement protocols.
We used the initial census of large-scale permanent plots in Hawai‘i to examine structural and floristic characteristics of two forests that are geographically close but located in widely contrasting environments. The two Hawaiian forest types examined in this first census were montane wet forest (MWF) and lowland dry forest (LDF). Many studies have shown that forests established in areas with higher rainfall or temperature have higher diversity [1], [27], [28], and also greater basal area, tree height, and above-ground biomass [28], [29], [30], [31]. Further, forests in higher rainfall areas tend to have a greater representation of larger trees, but lower tree densities [32]. We therefore ask: 1) How do the two Hawaiian forests compare in terms floristic and life form composition, stand structure, species diversity, and non-native species cover? Our study was not designed to specifically examine the effects of climate on forest structure and composition, but we used this study design, to test a prediction based on the previous literature that Hawaiian dry forest would have greater stem density, lower diversity, and smaller diameter trees than wet forest [32]. To place our findings in a broader context, we also asked: 2) Can the extremely low forests of Hawai‘i have similar structural attributes to more diverse tropical forests? To examine this question, we compared Hawaiian forests with others in the CTFS network enabling the comparison of forest structural variables across a range of environments and diversity levels [1], [28], [31], [33], [34], [35]. If Hawaiian forests converge with other tropical forests, the importance of climate in determining forest structure is highlighted.
Materials and Methods
Study Sites
In 2008 and 2009, we established two forest dynamics plots (FDPs) on Hawai‘i Island – one within montane wet forest (MWF) and one within lowland dry forest (LDF), to initiate the Hawai‘i Permanent Plot Network (HIPPNET; Fig. 1). We focused our study on Hawai‘i Island, because it has the greatest area of intact forests, a complete map of lava flow ages, and excellent infrastructure for ecological studies. As the youngest island in the archipelago (<700,000 years), it has had the least time for plant colonization and subsequent speciation, and thus has lower species richness relative to its size than the older islands [36]. We selected areas in excellent ecological condition that are representative of a given forest type, with high native species cover, and a commitment by ownership to long-term conservation objectives. Notably, all forests in Hawai‘i are affected to some degree by altered trophic interactions due to invasion of non-native species or extinction of the native species [37], but this is not unique to Hawai‘i [38]. Non-native stems that were encountered were measured for percent cover, and then controlled mechanically or chemically (see “Plot Establishment and Vegetation Measurements” below) and were not considered in the census of stems.
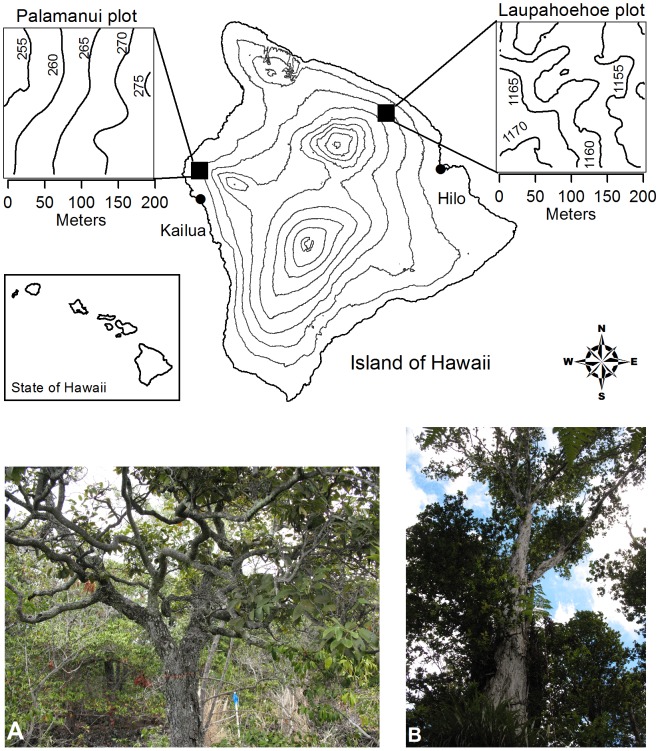
Figure 1. Contour map of the two 4-ha forest plots on Hawai‘i Island.
Pālamanui site in west Hawai‘i is lowland dry forest (LDF; left panel showing the dominant canopy tree Diospyros sandwicensis and the open canopy and understory structure of small trees and shrubs); Laupāhoehoe plot in east Hawai‘i is montane wet forest (MWF; right panel showing Metrosideros polymorpha tree and Cibotium spp. tree fern understory).
Montane wet forest (MWF)
The 4-ha Laupāhoehoe FDP (19°55' N, 155°17' W) is located within the state-owned Laupāhoehoe Natural Area Reserve section of the Hawai‘i Experimental Tropical Forest (HETF) on the northeast slope of Mauna Kea volcano. Permits were obtained for work in the HETF through the Institute of Pacific Islands Forestry and the Hawai‘i Division of Forestry and Wildlife/Department of Land and Natural Resources. The mean elevation of the plot is 1120 m.a.s.l. with slopes of 0–20%, and the overall direction of downslope is northwards towards the Pacific Ocean. The substrate within the plot is 4000-14,000 years old [39]. Soils were formed from weathered volcanic material, and are deep, rocky, and moderately well-drained silty clay loam in the Akaka series, and classified as hydrous, ferrihydritic, isothermic Acrudoxic Hydrudands (websoilsurvey.nrcs.usda.gov). Rainfall at the MWF is dominated by tradewind-driven precipitation [40]. Interpolated mean annual precipitation, based on analysis of climate station data over 30 years, is 3440 mm with no distinct dry season [41] and mean annual air temperature is 16°C [42]. The forest consists of evergreen broad-leaved trees, and the ∼25–28 m canopy is dominated by Metrosideros polymorpha (Myrtaceae; Fig. 1) and to a lesser extent, Acacia koa (Fabaceae). Vegetation at the MWF is highly representative of this forest type in Hawai‘i [43] (see references in Table 1).
The dominant pre-human contact disturbance regime in this forest type was single-to multiple-tree falls, with the maximum gap size averaging 21.5 m2 [44]. Larger openings coincide with dieback due to cohort senescence of older M. polymorpha stands [45]. Following contact, large A. koa trees were occasionally harvested for traditional canoe building. In modern times, limited A. koa logging occurred in the HETF but was restricted to <100 m of an unimproved road that traverses areas. There is no evidence of logging within the MWF [46], which >500 m from the road. Non-native wild pigs disturb soils while rooting, as well as tree ferns [47], with damage over a large area.
Lowland dry forest (LDF)
The 4-ha Pālamanui FDP is an example of one of the world's most endangered forest types, and is located on a privately-owned tract of dry forest on the northwest slope of Hualālai Volcano in the district of North Kona (240 m elevation, 19°44' N, 155°59' W). A memorandum of understanding was established with the land owners and managers, the Palāmani Group, for permission to conduct research in the lowland dry forest site. The mean elevation of the plot is 240 m.a.s.l. Geological substrate in the Pālamanui area consists of ‘a‘ā lava with scattered pāhoehoe flows dating to 1,500–3,000 years old [48]. Soils developing at this site are shallow, rocky, highly organic, and classified as euic, isothermic, shallow Lithic Ustifolist (websoilsurvey.nrcs.usda.gov). Interpolated mean annual precipitation at the LDF site is 835 mm [41], [49], with large within- and between-year variability [50]. For the LDF, major rainfall events typically occur in the winter as low pressure storms (“Kona lows”) while summers tend to be dry and characterized by small convective storms. Mean daily air temperature is approximately 20°C (wrcc.dri.edu). Native vegetation consists of evergreen broad-leaved trees and shrubs that form an open-canopy forest that reaches heights of ∼7–8 m dominated by Diospyros sandwicensis (Ebenaceae) and Psydrax odorata (Rubiaceae; Fig. 1). One species (Erythrina sandwicensis) is drought deciduous and is only represented by a few individuals.
Pre-contact disturbance regimes likely included tree falls. Following contact, selective harvesting of valuable woods (e.g., sandalwood) occurred throughout the area but we do not know of any logging that occurred within the plot. In the last 200 years, much of the lowland dry forest in Hawai‘i has been subjected to grazing and browsing by exotic ungulates, with remnants impacted by wildfire carried by non-native grasses [51]. These factors have reduced the native forest to a fraction of its original extent [52]. While the area containing the FDP has not been burned or significantly browsed by ungulates, the surrounding area is a matrix of degraded LDF and open grassland, and in 2009, a fence and firebreak were installed around the area to protect it from ungulates and fire.
Plot Establishment and Vegetation Measurements
We applied field methodology developed by the Center for Tropical Forest Science global FDP network [53]. Both of our 4-ha FDPs (200×200 m) were oriented north-south and located at the center of a 16 ha buffer area, with all edges at least 100 m from any road or major trail where possible. From 2008 to 2009, we tagged all live, native woody plants ≥1 cm diameter at breast height (DBH, at 130 cm), and mapped tagged plants relative to 5 m×5 m grids installed throughout the plots. Each tagged plant was identified to species and measured for DBH. More detailed methods are in Methods S1 in File S1.
Finally, we estimated and mapped cover of abundant non-native herbaceous, shrub and tree species, which will be important for understanding long-term vegetation change. At each site, we chose six abundant focal species or life forms that were considered “invasive pests” according to their Hawai‘i Weed Risk Assessment scores (Daehler 2004; www.botany.hawaii.edu/faculty/daehler/wra/full_table.asp). Percent cover within each 5×5 m subquadrat was estimated in the following categories: 0: absent, 1: <5%, 2: 5–25%, 3: 25–50%, 4: 50–100%. Non-native trees with stems ≥1 cm at 130 cm were individually mapped. The DBH of the largest stem of non-native trees <5 cm was estimated to the nearest centimeter and measured to the nearest centimeter if >5 cm. For trees with multiple stems, we counted the total number of stems ≥1 cm at 130 cm. After the non-native trees were mapped, they were girdled and sprayed with herbicide. We did not spray herbicide on the grasses in the LDF, nor the vine Passiflora tarminiana in the MWF.
Data Analyses
Stand structure
We determined stand structural characteristics based on DBH measurements. We considered multiple-stemmed plants as single individuals for the calculation of stem density, and summed the basal area of all stems for the calculation of basal area (m2/ha). For each species, we calculated relative abundance (RA, %) as the number of individuals of that species/total number of individuals, relative dominance (RD, %) as the basal area of that species/total basal area, and relative frequency (RF, %) as the number of quadrats with that species/total number of quadrats.
Above-ground biomass
To estimate above-ground biomass (AGB) for the two plots, we used site-specific and species-specific information whenever possible for wood specific gravity, tree height, and DBH (equations derived from 52,54,55,56; see Table S1 in File S2). When these were not available, we compiled data from global databases, utilizing equations based on other sites, and in some cases for other species from within the same genera [56], [57]. Previous studies have reported that genus means are reasonable proxies for species values for specific gravity (r 2>0.70; [58], [59]).
To determine tree height, we applied species-specific equations of [54] giving the relationship of tree height vs. DBH, to each individual tree for 12 of the MWF species and 4 of the LDF species (Table S1 in File S2). For the other species, we used the general wet and dry forest equations [55] to determine tree height. We used these tree height estimates to calculate AGB for each tree using published equations that also included DBH and wood specific gravity. Hawai‘i-specific equations for AGB were available for 5 MWF species and 4 LDF species. For another two species of the LDF, D. sandwicensis and P. odorata, equations were available that were developed specifically from our study site [52] (Table S1 in File S2).
Species richness and diversity
Species area curves were generated by plotting cumulative number of species against area for the 20 m×20 m quadrats. Rarefaction analyses were based on 999 permutations (PRIMER-E v. 6, PRIMER-E Ltd, Plymouth, UK), which randomized the sampling order and resulted in a robust average curve. We present several indices: Sobs (the observed number of species), Chao 1 based on rare species (non-parametric), and Michaelis-Menten (parametric), given uncertainty in the ideal estimator [60], [61], [62]. We used the program EstimateS 9.1.0 to calculate species diversity indices and an estimate of error. We report Fisher's alpha, Shannon diversity index, and Simpson's index (inverse form) following standard formulas [63]. Overlap in species composition between the two sites was determined using the Sørenson similarity index (SI):
Forest type comparisons
We compiled data for 19 additional mainland and island CTFS tropical plots for which climate and structure data were available (Table 2). Differences between the Hawai‘i plots and other CTFS plots were assessed using one sample t-tests. Differences in the characteristics of island and mainland plots were assessed using Wilcoxon signed-rank tests [64]. These statistics were analyzed with JMP v. 6 [29], [65].
Table 2. Diversity and forest structure characteristics of plots in the Center of Tropical Forest Science global plot network, including the Hawaiian plots, arranged in order of descending species richness.
| CTFS plot location | Plot code | Latitude | Mean annual rain (mm) | Dry season months | Mean elevation (m) | Mean annual temp (°C) | Land type | Plot size (ha) | No. of species | No. of families | Mean species/family | Fisher's α/ha | H'/ha | Trees/ha | Basal area (m2/ha) | Dom. of most common family | Above-ground biomass (Mg/ha)7 |
| Lambir, Malaysia | LAM | 4.19 | 2664 | 0 | 170 | 26.6 | I | 52 | 1182 | 83 | 14.2 | 165 | 2.40 | 6915 | 43.5 | 41.0 | 497.2 |
| Yasuní, Ecuador | YAS | −0.69 | 3081 | 0 | 230 | 28.4 | M | 50 | 1114 | 81 | 13.8 | 187 | 2.44 | 3026 | 33.0 | 14.9 | 282.4 |
| Pasoh, Malaysia | PAS | 2.98 | 1788 | 1 | 80 | 28.0 | M | 50 | 814 | 82 | 9.93 | 124 | 2.31 | 6708 | 31.0 | 28.2 | 339.8 |
| Khao Chong, Thailand | KHA | 7.54 | 19851 | 32 | 140 | 27.3 | M | 24 | 593 | na | na | na | Na | 5063 | na | na | na |
| Korup, Cameroon | KOR | 5.07 | 5272 | 3 | 200 | 26.7 | M | 50 | 494 | 62 | 7.97 | 48.0 | 1.75 | 6580 | 32.0 | 16.1 | na |
| Ituri, Dem. Rep. of Congo3 | ITU | 1.44 | 1700 | 3–4 | 780 | 23.1 | M | 404 | 445 | na | na | na | Na | 7200 | na | na | na |
| Palanan, Philippines | PAL | 17.04 | 3379 | 4–5 | 110 | 23.5 | I | 16 | 335 | 60 | 5.58 | 43.4 | 1.92 | 4125 | 39.8 | 52.8 | 290.1 |
| Bukit Timan, Singapore | BUK | 1.25 | 2473 | 0 | 150 | 26.9 | I | 2 | 329 | 62 | 5.31 | 60.0 | 1.90 | 5950 | 34.5 | 38.4 | na |
| BCI, Panama | BCI | 9.15 | 2551 | 3 | 140 | 26.9 | M | 50 | 299 | 58 | 5.16 | 34.6 | 1.62 | 4168 | 32.1 | 11.4 | 306.5 |
| Mo Singto, Thailand | MOS | 14.43 | 2200 | 5 | 770 | 23.0 | M | 30.5 | 262 | na | na | na | Na | Na | na | na | na |
| Huai Kha Khaeng, Thailand | HKK | 15.63 | 1474 | 6 | 590 | 24.1 | M | 50 | 251 | 58 | 4.33 | 23.3 | 1.50 | 1450 | 31.2 | 21.2 | 211.2 |
| La Planada, Colombia | LPL | 1.16 | 4084 | 0 | 1840 | 19.0 | M | 25 | 240 | 54 | 4.44 | 30.6 | 1.72 | 4216 | 29.8 | 14.9 | 177.6 |
| Dinghushan, China | DIN | 23.16 | 1985 | 0 | 350 | 20.9 | M | 20 | 210 | na | na | na | Na | 3581 | na | na | na |
| Sinharaja, Sri Lanka | SIN | 6.4 | 5016 | 0 | 500 | 22.6 | I | 25 | 204 | 46 | 4.43 | 24.4 | 1.53 | 7736 | 45.6 | 26.7 | 357.9 |
| Doi Inthanon, Thailand | DOI | 18.52 | 1908 | 6 | 1700 | 21.4 | M | 15 | 192 | na | na | na | Na | 4913 | na | na | na |
| Luquillo, Puerto Rico | LUQ | 18.33 | 3548 | 0 | 380 | 22.8 | I | 16 | 138 | 47 | 2.94 | 13.5 | 1.45 | 4194 | 38.3 | 17.3 | 276.1 |
| Nanjenshan, Taiwan | NAN | 22.06 | 3582 | 0 | 320 | 23.5 | I | 3 | 125 | 41 | 3.05 | 15.6 | 1.64 | 12133 | 36.3 | 32.3 | na |
| Ilha do Cardoso, Brazil5 | ILH | −25.1 | 2100 | 0 | 5 | 22.4 | M | 10 | 106 | na | na | na | Na | Na | na | na | na |
| Mudumalai, India | MUD | 11.6 | 1250 | 6 | 1050 | 22.8 | M | 50 | 72 | 29 | 2.48 | 6.20 | 0.944 | 510 | 25.5 | 28.2 | 174.2 |
| Laupāhoehoe, USA | LAU | 19.93 | 3440 | 1 | 1150 | 16.0 | I | 4 | 21 | 15 | 1.40 | 2.58 | 1.82 | 3078 | 67.36 | 37.4 | 247.9 |
| Pālamanui, USA | PLN | 19.74 | 835 | 12 | 240 | 20.0 | I | 4 | 15 | 15 | 1.00 | 1.18 | 1.00 | 3487 | 8.6 | 74.2 | 29.4 |
Southern hemisphere latitudes are negative; land types are island (I) and mainland (M); dry season months are as those with <100 mm precipitation (Richards 1996). Data are from [104] and ctfs.si.edu unless indicated by footnotes.
Mean annual rainfall data for the nearby city of Songkhla, Thailand (www.world-climates.com)
Kira T (1998) NPP Tropical Forest: Khao Chong, Thailand, 1962–1965. Data set. Available on-line [http://www.daac.ornl.gov] from Oak Ridge National Laboratory Distributed Active Archive Center, Oak Ridge, Tennessee, U.S.A.
Average of 4 plots (2 monodominant forest, 2 mixed forest)
Divided into four 10-ha plots
Data from Ferreira de Lima RA, Oliveira AAD, Martini AMZ, Sampaio D, Souza VC, Rodrigues RR (2011) Structure, diversity, and spatial patterns in a permanent plot of a high restinga forest in Southeastern Brazil. Acta Botanica Brasilica 25: 633–645.
Basal area including tree ferns; 36.1m2/ha without tree ferns
Chave J and 37 others (2008) Assessing evidence for a pervasive alteration in tropical tree communities. PLoS Biology 6: 455–462.
Results
Comparison of Floristics and Life Forms in Hawaiian Forests
The two Hawai‘i forests were distinct in floristic composition (Table S2 in File S2). The plots had a very low Sørenson similarity index of 0.06 (a value of 1 would indicate complete overlap). Only M. polymorpha occurred in both forests; it was the second most common species in MWF but was represented by only 5 individuals in the 4-ha LDF plot. Species richness was 21 in the MWF and 15 in the LDF. Fifteen families were represented at each site, and the canopy trees at the two sites were from different families, though four families were represented in the understory or the midstory at both sites (Euphorbiaceae, Fabaceae, Myrtaceae, and Rubiaceae).
The plots differed in their distribution of plant life forms. In the MWF, 68% of stems were trees, 4.5% were shrubs, and 28% were tree ferns, accounting for 45%, 8.3% and 46% of the basal area respectively. In the LDF, 82% of stems were trees and 18% were shrubs, accounting for 95% and 5% of basal area respectively (Fig. 2). In the MWF, a large proportion of stems (31%) were growing on non-soil substrates, primarily tree ferns, logs or rocks, with 17% of all individuals growing on dead tree ferns (Table S3 in File S2). In contrast, in the LDF, all trees were growing on soil or broken lava, and tree ferns were absent.
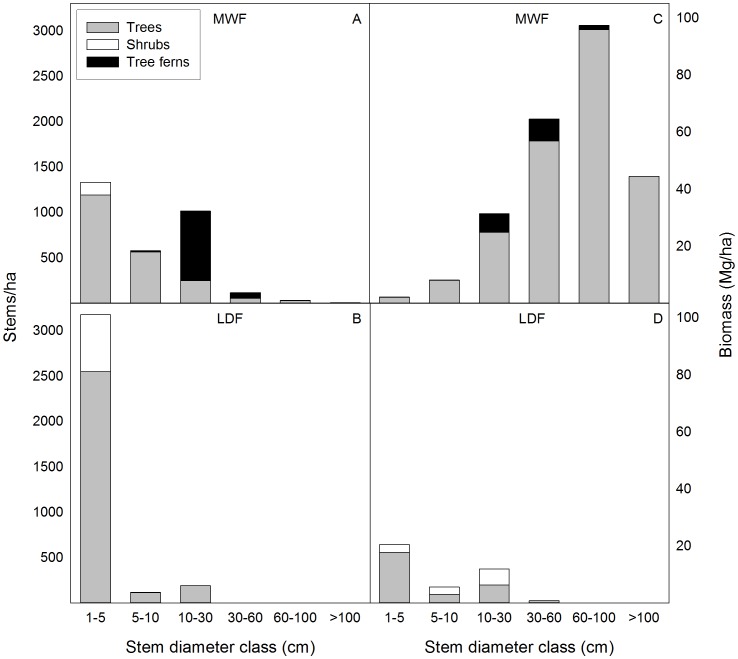
Figure 2. Life form distribution of stems and biomass by diameter size intervals.
In (A) Hawaiian montane wet forest (MWF) and (B) lowland dry forest (LDF), stems represent the number of main stems (i.e., one per individual, not including other multiple stems). In (C) MWF and (D) (LDF), biomass calculations were made for all stems (including multiple stemmed individuals). Diameter classes are 1–4.99 cm, 5 - <9.99 cm, 10–29.99 cm, 30–59.99 cm, 60–99.99 cm, and ≥100 cm.
Comparison of Stand Structure in Hawaiian Forests
The MWF had larger trees and lower stem density than the LDF (3078±1.21 and 3487±1.40 stems/ha respectively; Table 3). The tree size class distributions differed between the two forests as expected based on their contrasting climates: the LDF had mainly small stems and the MWF had a much more even spread of size classes (Fig. 2). Because the stems in the LDF were small, total basal area and biomass values were low. Thus, the MWF had a nearly eight-fold higher basal area than the LDF (67.3 vs. 8.6 m2/ha respectively; Tables 3–4), and tree ferns accounted for 31.2 m2/ha basal area. Above-ground biomass in the MWF was also more than eight times higher than the LDF (248 Mg/ha vs. 29.4 Mg/ha respectively; with 15.6 Mg/ha in the MWF accounted for by tree ferns; Table 5). The above-ground biomass value for the MWF was consistent with that previously estimated for surrounding forest in the same reserve [66]. In both forest types, the two most common canopy species represented 87–88% of biomass (Table 5). In the MWF, the very large trees (≥60 cm) made up the greatest proportion of the biomass, but in the LDF the majority of the biomass was in the 1–5 cm size class (Fig. 2). More multi-stemmed individuals make up the LDF, a mean of 3.2 stems/individual, compared to 1.4 stems/individual in the MWF plot) (Table S4 in File S2).
Table 3. Statistics on abundance, basal area, and frequency of the species in the Laupāhoehoe (montane wet forest) plot, with data displayed on an absolute and a relative basis.
| Laupāhoehoe montane wet forest | |||||||
| Species | No. individuals | Basal area (m2/ha) | Presence (no. of quadrats) | Relative abundance (%) | Relative dominance (%) | Relative frequency (%) | IV (%) |
| METPOL | 2631 | 25.2 | 100 | 21.4 | 37.5 | 10.2 | 69.1 |
| CIBGLA | 2274 | 17.7 | 100 | 18.5 | 26.4 | 10.2 | 55.1 |
| CHETRI | 3320 | 4.17 | 100 | 27.0 | 6.20 | 10.2 | 43.4 |
| CIBMEN | 1076 | 13.2 | 100 | 8.74 | 19.6 | 10.2 | 38.6 |
| COPRHY | 972 | 0.585 | 99 | 7.90 | 0.870 | 10.1 | 18.9 |
| ILEANO | 965 | 0.466 | 99 | 7.84 | 0.692 | 10.1 | 18.7 |
| ACAKOA | 141 | 5.49 | 57 | 1.15 | 8.16 | 5.84 | 15.1 |
| BROARG | 271 | 0.0454 | 74 | 2.20 | 0.067 | 7.58 | 9.85 |
| MYRLES | 237 | 0.0571 | 70 | 1.93 | 0.085 | 7.17 | 9.18 |
| VACCAL | 255 | 0.0328 | 51 | 2.07 | 0.0488 | 5.23 | 7.35 |
| HEDHIL | 43 | 0.0200 | 29 | 0.349 | 0.0297 | 2.97 | 3.35 |
| PERSAN | 35 | 0.0084 | 28 | 0.284 | 0.0125 | 2.87 | 3.17 |
| CIBCHA | 34 | 0.232 | 17 | 0.276 | 0.345 | 1.74 | 2.36 |
| CLEPAR | 19 | 0.00230 | 17 | 0.154 | 0.00342 | 1.74 | 1.90 |
| MELCLU | 13 | 0.00235 | 11 | 0.106 | 0.00349 | 1.13 | 1.24 |
| PSYHAW | 10 | 0.00219 | 9 | 0.0812 | 0.00326 | 0.922 | 1.01 |
| MYRSAN | 6 | 0.00166 | 6 | 0.0487 | 0.00246 | 0.615 | 0.666 |
| PIPALB | 4 | 0.00226 | 4 | 0.0325 | 0.00335 | 0.410 | 0.446 |
| TREGRA | 2 | 0.000107 | 2 | 0.0162 | 0.000159 | 0.205 | 0.221 |
| LEPTAM | 2 | 0.0000682 | 2 | 0.0162 | 0.000101 | 0.205 | 0.221 |
| ANTPLA | 1 | 0.000154 | 1 | 0.0081 | 0.000229 | 0.102 | 0.111 |
| Total | 12311 | 67.3 | |||||
Table 4. Statistics on abundance, basal area, and frequency of the species in the Pālamanui (lowland dry forest) plot, with data displayed on an absolute and a relative basis.
| Pālamanui lowland dry forest | |||||||
| Species | No. individuals | Basal area (m2/ha) | Presence (no. of quadrats) | Relative abundance (%) | Relative dominance (%) | Relative frequency (%) | IV (%) |
| DIOSAN | 2208 | 6.41 | 99 | 15.8 | 74.2 | 18.3 | 108.3 |
| PSYODO | 8640 | 1.27 | 100 | 62.0 | 14.7 | 18.5 | 95.2 |
| DODVIS | 2301 | 0.359 | 94 | 16.5 | 4.15 | 17.4 | 38.1 |
| SOPCHR | 5 | 0.21 | 4 | 0.0359 | 2.38 | 0.741 | 3.20 |
| SANPAN | 275 | 0.156 | 32 | 1.97 | 1.81 | 5.93 | 9.70 |
| OSTANT | 147 | 0.0900 | 40 | 1.05 | 1.04 | 7.41 | 9.50 |
| WIKSAN | 88 | 0.0890 | 44 | 0.631 | 1.03 | 8.15 | 9.81 |
| EUPMUL | 134 | 0.023800 | 54 | 0.961 | 0.275 | 10.0 | 11.2 |
| SENGAU | 70 | 0.013300 | 27 | 0.502 | 0.154 | 5.00 | 5.66 |
| METPOL | 12 | 0.013 | 9 | 0.0860 | 0.152 | 1.67 | 1.90 |
| MYOSAN | 54 | 0.007480 | 26 | 0.387 | 0.087 | 4.81 | 5.29 |
| SIDFAL | 1 | 0.0008 | 1 | 0.0072 | 0.00947 | 0.185 | 0.202 |
| PLEHAW | 1 | 0.0007 | 1 | 0.0072 | 0.00843 | 0.185 | 0.201 |
| ERYSAN | 2 | 0.000513 | 2 | 0.0143 | 0.00594 | 0.370 | 0.391 |
| PITTER | 8 | 0.00048 | 7 | 0.0574 | 0.00551 | 1.30 | 1.36 |
| Total | 13946 | 8.64 | |||||
Presence based on 100 20×20 m quadrats per plot; species sorted by importance value (IV), which is the sum of the three relative measures (max 300%); species abbreviations as in Table S2 in File S2.
Table 5. Aboveground biomass listed by species for the two Hawai‘i forest plots; species abbreviations as in Table S2 in File S2.
| Laupāhoehoe montane wet forest | Pālamanui lowland dry forest | ||||
| Species | Biomass (Mg/ha) | Relative biomass (%) | Species | Biomass (Mg/ha) | Relative biomass (%) |
| METPOL | 186 | 74.9 | PSYODO | 15.3 | 51.9 |
| ACAKOA | 31.1 | 12.5 | DIOSAN | 10.5 | 35.8 |
| CHETRI | 12.4 | 4.99 | METPOL | 1.40 | 4.78 |
| CIBMEN | 10.9 | 4.39 | DODVIS | 0.921 | 3.14 |
| CIBGLA | 4.55 | 1.83 | OSTANT | 0.525 | 1.79 |
| COPRHY | 1.59 | 0.64 | SANPAN | 0.359 | 1.22 |
| ILEANO | 1.27 | 0.51 | WIKSAN | 0.181 | 0.615 |
| CIBCHA | 0.184 | 0.07 | MYOSAN | 0.109 | 0.372 |
| MYRLES | 0.109 | 0.04 | SOPCHR | 0.0446 | 0.152 |
| VACCAL | 0.0947 | 0.04 | SENGAU | 0.0398 | 0.135 |
| HEDHIL | 0.0456 | 0.02 | EUPMUL | 0.0199 | 0.068 |
| BROARG | 0.0394 | 0.02 | PITTER | 0.00435 | 0.0148 |
| PERSAN | 0.0124 | 0.00499 | PLEHAW | 0.00217 | 0.00740 |
| PSYHAW | 0.00384 | 0.00155 | SIDFAL | 0.00187 | 0.00637 |
| MELCLU | 0.00343 | 0.00138 | ERYSAN | 0.000871 | 0.00297 |
| CLEPAR | 0.00327 | 0.00132 | |||
| MYRSAN | 0.00319 | 0.00128 | |||
| PIPALB | 0.00274 | 0.00110 | Total | 29.4 | |
| ANTPLA | 0.000287 | 0.000116 | |||
| LEPTAM | 0.00017 | 0.0000685 | |||
| TREGRA | 0.0000703 | 0.0000283 | |||
| Total | 247.9 | ||||
Comparison of Community Structure in Hawaiian Forests
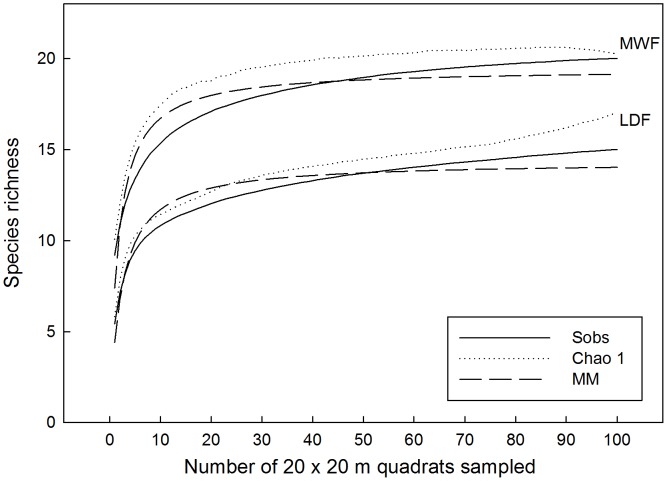
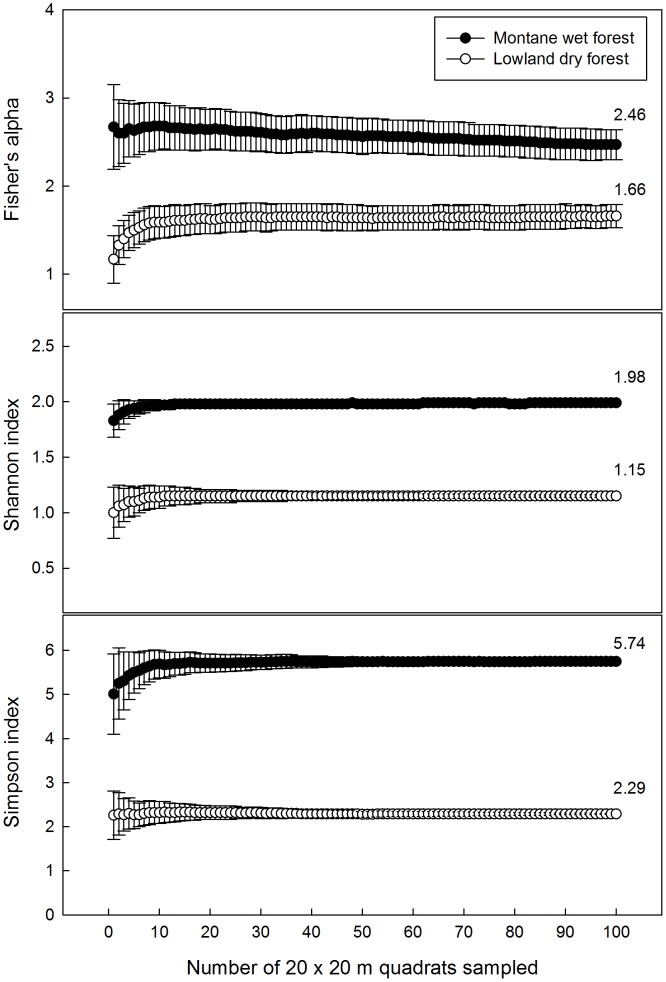
For both the Hawaiian MWF and LDF, rarefaction curves indicated that a 1 ha sample was sufficient to capture 90% of the species present in the larger 4-ha area (Fig. 3). In the MWF, diversity values for the plot were 2.46 (Fisher's alpha), 1.98 (Shannon), and 5.74 (Simpson); in the LDF values were 1.66 (Fisher's alpha), 1.15 (Shannon), and 2.29 (Simpson). When viewed graphically, there was no overlap in any index value between the two forests (Fig. 4): the Hawaiian MWF was more diverse than the LDF. In the MWF, species evenness was higher than in the LDF, primarily because P. odorata in the LDF had a relative abundance of over 60%. In contrast the forests were similar in the abundance of uncommon species, and ∼20% of species were rare, i.e., having ≤1 stem/ha (Tables 3–4).
Figure 3. Species accumulation curves.
Species number is shown cumulatively, as additional 20 m×20 m quadrats are sampled, until the entire 4-ha plot is represented (100 quadrats), for Hawaiian montane wet forest (MWF) and lowland dry forest (LDF). Three rarefaction techniques are used: Sobs (observed species number), Chao 1, and MM (Michaelis-Menten).
Figure 4. Species diversity indices.
Fisher's alpha, Shannon index, and Simpson index for the Hawaiian montane wet forest (MWF) and lowland dry forest (LDF). Each 20×20 m subplot is shown, with the values being cumulative and number above each line representing the entire plot area (4-ha). Values are the diversity index and standard deviation, as estimated by the program EstimateS.
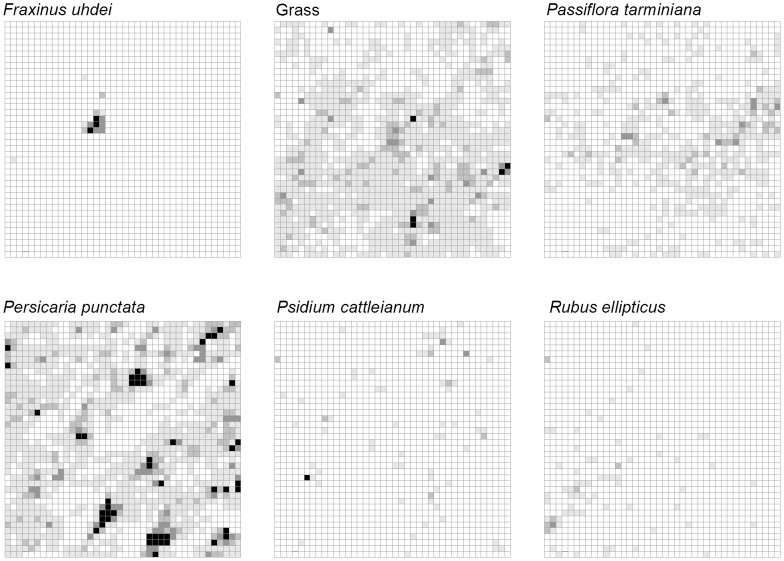
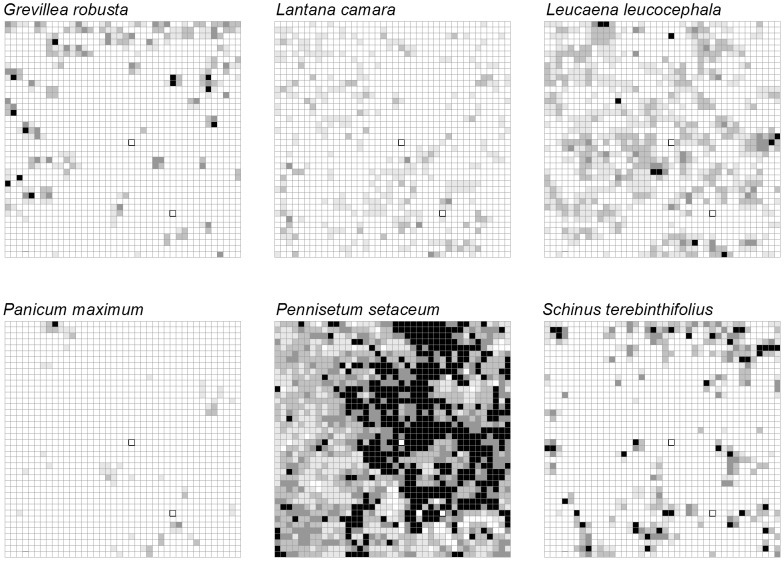
Non-native Species in the Plots
Invasive species made up a larger presence in LDF than MWF (Figs.5–6). The grass Pennisetum setaceum was most widespread in the LDF and the herbaceous weed Persicaria punctata was most common in the MWF, where it tended to dominate low-lying boggy areas. In the LDF there was a greater overall weed cover, particularly of woody weeds (Fig. 7; see also Methods S1 in File S1). In the MWF there were only a few stems that qualified for DBH measurements (>5 cm). F. uhdei averaged 32.3 cm (n = 1) and P. cattleianum averaged 6.2 cm (n = 1). In the LDF, average DBH for Grevillia robusta was 20.0±8.2 cm SE (n = 27), for Leucaena leucocephalum it was 5.9±0.8 cm SE (n = 7), and for Schinus terebinthius it was 11.0±1.5 cm SE (n = 20).
Figure 5. Invasive species cover distribution.
Map showing percent cover and locations of invasive species in the MWF. Each grid square represents one 5×5-m subquadrat white: absent, light grey: present to <5%, medium grey: 5–25%, dark grey: 25–50%, black: >50% cover).
Figure 6. Map showing percent cover and locations of invasive species in the LDF.
Each grid square represents one 5×5-m subquadrat (white: absent, light grey: present to <5%, medium grey: 5–25%, dark grey: 25–50%, black: >50% cover).
Figure 7. Combined invasive species cover.
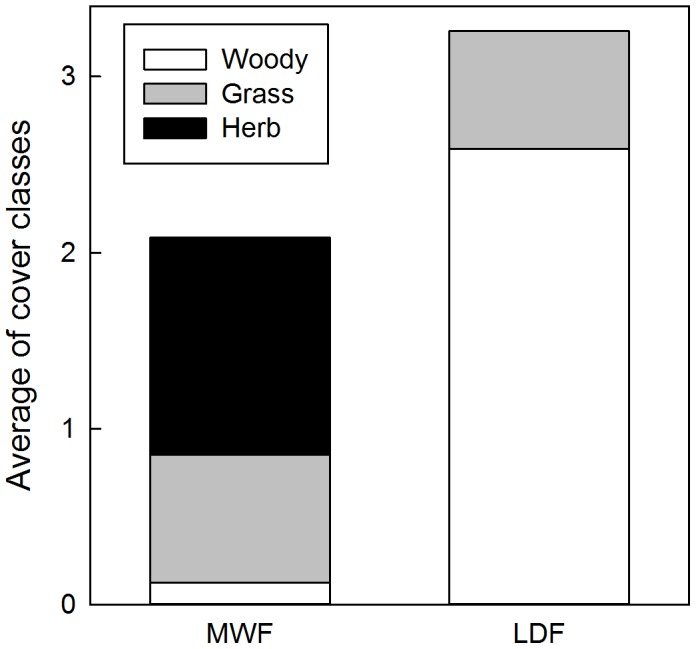
In each 5×5 m subquadrat a cover score from 0–4 was given based on cover classes (see Methods). The y axis represents the average cover class across the 400 subquadrats, separated by life form: grasses, herbaceous, or woody (shrubs and trees). The combined cover represents the species shown in Figure 5.
Comparison of Hawai‘i to Other CTFS Plots
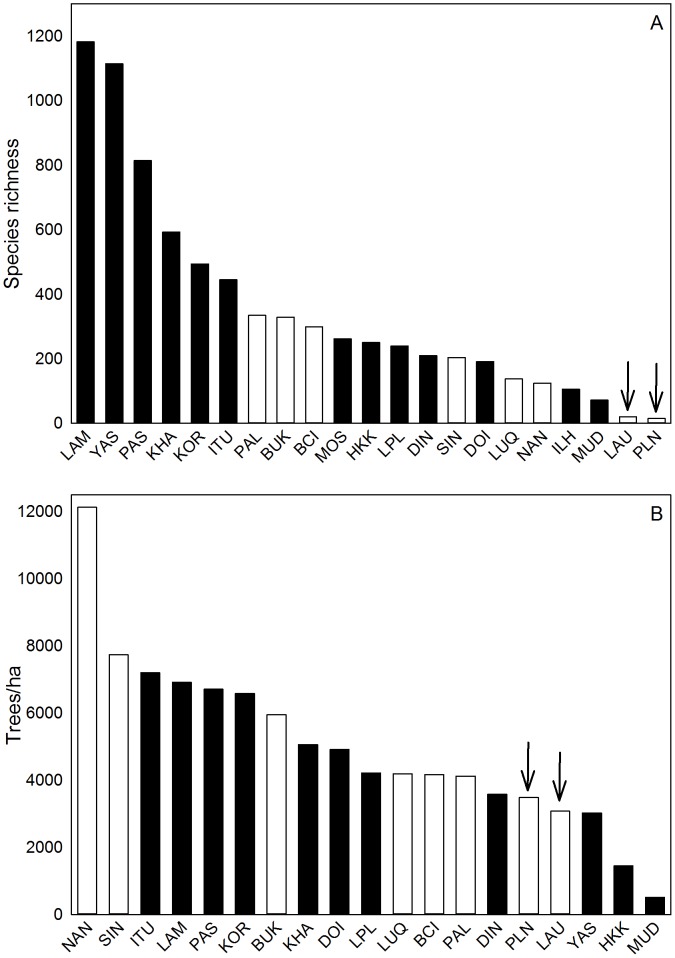
Diversity of both Hawai‘i forests was very low relative to other CTFS forests, including those on islands and with dry climates (Fig. 8). Across the CTFS network, the mean Fisher's alpha per ha ± SE was 59.7±16.6 (n = 13), and the two Hawaiian forests were statistical outliers, with diversity values more than 2 SD lower (t = 3.45 and 3.53, P<0.005). The MWF had approximately 15% as many species as the most comparable island site with tropical wet forest (Luquillo, Puerto Rico). Compared with the next two driest CTFS sites, the Hawai‘i LDF had 21% of the number of species found in the Mudumalai, India plot and just 6% of the number of species found at the Huai Kha Khaeng, Thailand plot (Table 2).
Figure 8. Comparisons of species richness and stem density across a series of CTFS plots.
Black bars represent continents and open bars represent islands. Abbreviations as in Table 2. Data from Losos and Leigh, Jr. (2004) and www.ctfs.si.edu.
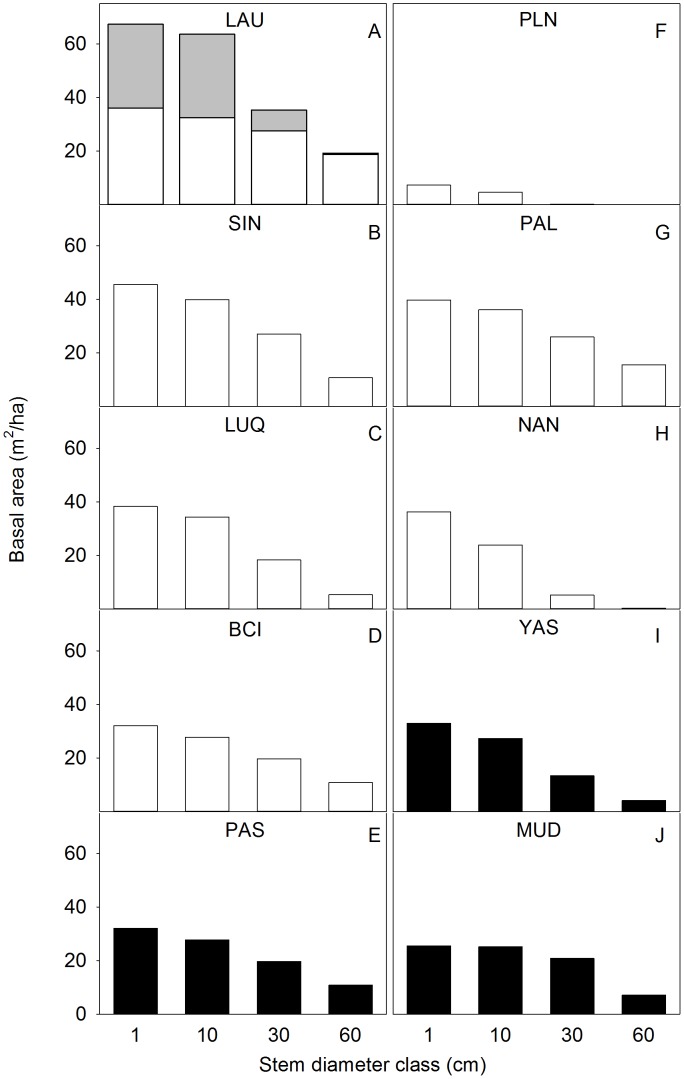
In contrast to biodiversity, the structural comparisons across the CTFS network revealed complicated patterns. The MWF was similar to other CTFS sites with respect to tree size class distribution (Fig. 9), and was not significantly different from other CTFS plots with respect to standing above-ground biomass/ha. However, the MWF had 35% lower stem density than the all-forest mean of 4733±722 SE (t = 2.29, P = 0.039, n = 14). Further, the MWF had a 92% higher basal area than the mean of other tropical FDPs due to its high tree fern abundance (t = −21.6, P<0.0001). When tree ferns were excluded, the basal area of the Hawaiian MWF was within the range of that for other FDPs. For the LDF, stem density was not significantly different than the all-forest mean (t = 1.79, P = 0.097, n = 16, Table 2), but the LDF was an outlier with its very low basal area (t = 10.40, P<0.001, n = 19) and above-ground biomass (t = 7.12, P<0.001, n = 12). The LDF was especially distinctive in having virtually all small stems (Fig. 9).
Figure 9. A reverse-cumulative distribution of basal area by size class.
Size classes are: ≥1 cm, ≥10 cm, ≥30 cm, and ≥60 cm. Data shown for the Hawaiian montane wet forest (LAU) and lowland dry forest (PLN) (top row) and for selected other CTFS plots. Tree ferns (found only at LAU) are symbolized by the gray bars. Island sites are open bars and continental sites are filled bars. Abbreviations as Table 2. Data from Losos and Leigh, Jr. (2004) and www.ctfs.si.edu.
Discussion
Comparing Hawaiian Wet and Dry Forests
Examination of how the two Hawaiian forests compared in terms of composition and structure matched and extended the paradigm for differences between mature wet and dry forests described on other tropical islands [32]. As predicted, our results for Hawai‘i are in agreement with comparisons of mature wet and dry forests in Puerto Rico [32]: wet forest had larger diameter trees, greater basal area, and higher biomass than dry forest, and differences between wet and dry forest in tree density, dominance, and species richness were minor (Tables 3–4). Indeed, across many forests, biomass and basal area are typically correlated with climatic variables such as MAT, MAP, and water deficit within and across sites [31], [70], [71], [72], [73], [74], [75]. However, other variables such as substrate age and type [76] are likely to have contributed to structural and floristic differences between LDF and MWF and we cannot ascribe results solely to climate. While both forests occur on young lava, the much higher rainfall of the MWF and variation in substrate type and texture contributed to greater soil development. Lava flow age and substrate type are important determinants of successional stage in Hawai‘i [39]. In addition, differences in disturbance regimes between the two sites may have influenced their forest structure, and their invasive species cover. In the MWF, almost a third of stems were found growing on a substrate other than soil, such as nurse logs and living tree ferns, which likely reflects preferential survivorship on those substrates. Further, canopy dieback of Metrosideros polymorpha [45], wind storms [44], and invasive animals [77] may be important factors influencing forest structure. Canopy gaps are larger in the MWF due to the much larger and taller trees that make up the canopy. There is also evidence of pig rooting that may affect seedling regeneration preferences [47], [78], explaining why many stems grow on substrates other than soil. The LDF site is currently fenced from ungulates but the large proportion of multi-stemmed trees and the higher prevalence of regeneration by sprouting suggest adaptation to disturbance [79], [80], [81].
The MWF site distinguishes itself in its abundance and dominance of tree ferns, which form a distinct mid-canopy layer approximately 5 m above the ground. Notably, tree ferns also make up a large proportion of stand basal area or stem density in some temperate rain forests [82], [83], [84] and tropical cloud forests [85], [86], [87], [88] but not in other CTFS sites. In Hawai‘i, tree ferns are common in wet forests at all elevations, and are particularly abundant in areas with more well-developed organic soils as opposed to young lava flows. While the dynamics of tree ferns have not been well studied in tropical environments [89], in Hawaiian forests tree ferns undoubtedly influence forest function, due to their long lifespans, high frond area, slow growth [90], and slow decomposition rates [91]. They also play a critical role as a substrate for tree seedlings [47], [78].
Unlike other CTFS plots that are not heavily impacted by non-native plant species, all forests in Hawai‘i have been invaded to some degree. We purposefully chose sites with low non-native species abundance, but cataloged cover before removal for future long-term studies. Because we are removing the invasive species after data collection, we are not examining the consequences of invasion, but previous work in Hawai‘i has shown that invaders can significantly alter forest functioning [50], [51], [52], [92], [94]. A debate in invasion biology is whether invaders owe their success to their introducing a new function to the community (e.g., N-fixing species) or are simply better competitors [92] and we argue that it is the latter case at our sites. At our sites, invasive grasses were widespread, but, woody invaders are a greater competitive threat (Figs. 7). In the LDF, Pennisetum setaceum is widespread, but the vegetation is still dominated by woody species with moderate canopy closure, and reduction of grass cover and fire prevention will reduce its competitive effect in the future. While non-native grasses and herbs are more common than non-native trees across the MWF, their abundance is strongly related to boggy areas, canopy openings, and pig disturbance, and these patches are not likely to expand, but rather to be shaded out in the long term. In MWF, woody invaders such as Psidium cattleianum represent much greater threats based on their extreme abundance elsewhere, and traits such as shade tolerance, vegetative reproduction, and animal-dispersed fruits [93], [94]. At present, the MWF has limited cover of woody invaders (Fig. 7), and in that respect is in better condition that the LDF.
Clearly, site-specific properties influence the structure and species composition between the two sites, but our study also highlights that at the island scale (1 million ha), climate likely exerts a strong influence, both directly and indirectly [67]. These differences matched patterns found in continental forests, where diversity measures as well as structural measures correlate negatively with the length or severity of the dry season [68], [69].
Structure and Diversity across Tropical Forests Globally
The Hawaiian forest data allowed for the examination of the question of how forest structure varies across species diversity gradients across a much wider range of tree species diversity than was previously available. One of the most striking conclusions of our study is that, despite the extremely low species richness of the Hawai‘i FDPs, some structural variables, particularly those for the wet forest, were well within the range of values for the world's most diverse tropical forests (Table 2). For example, stem densities for Hawaiian MWF and LDF were similar to those of the hyper-species-rich Yasun??? FDP (Fig. 8), while biomass and basal area of the Hawai‘i MWF (excluding tree ferns) were similar to those of the higher diversity forests in the CTFS network (Fig. 9). The inclusion of tree ferns increased basal area values by 52%, but only increased biomass by 11% (Tables 3 and 4). Notably, the LDF had among the lowest basal area and biomass in the CTFS network, consistent with this site having the lowest precipitation of all FDPs (Table 2). It should also be noted that the LDF is dry year-round, while other dry sites in the CTFS network are seasonally dry (Table 2).
The low floristic richness and population structure of the Hawaiian forest plots represented strong convergence with other island forests. Hawaiian forests had fewer species per family and greater average population densities for each species, as seen in other very isolated sites [95]. High relative dominance values were consistent with island forests having greater dominance by the most common family than mainland tropical forests (Table 2). In the MWF and LDF, 37% and 74% of basal area respectively were accounted for by a single canopy dominant species. On average 20% of species were rare in Hawaiian forests (defined as ≤1 tree/ha), by contrast with 42% on average across other high-diversity forests [96]. It is likely that the patterns of high basal area dominance in Hawaiian forests arose due to the biogeographic consequences of isolation, but we cannot rule out species loss due to human disturbance and invasive species of multiple trophic levels [97], [98], [99], [100], [101].
In conclusion, Hawaiian forests have among the lowest species richness and highest endemism rates globally, but in a number of key structural variables both of these forests were similar to even the highest diversity tropical forests in the CTFS network. Future work could examine the evolutionary consequences of such a limited species pool. Biodiversity theory developed in high-diversity tropical forests emphasizes that competitive interactions among species are unlikely on evolutionary time scales because any given two species are rarely consistent neighbors [9]. However, in low-diversity forest any two given species have far greater potential for competitive interactions than in high-diversity tropical forests [102], [103]. The addition of Hawai‘i to the global plot network enables investigations of the consequences of such differences across a very wide range in species diversity and environmental gradients.
Supporting Information
Methods S1. Detailed methods and description of situations where field site conditions dictated a different or entirely new methodology by adopted than standardized CTFS protocol in [1].
(DOCX)
Supporting tables. Table S1. Values and equations used for estimating aboveground biomass (AGB) in the montane wet forest (MWF) and lowland dry forest sites (LDF). Table S2. Species ≥1 cm diameter at breast height recorded in Laupāhoehoe (montane wet forest) plot with canopy dominants in bold. Table S3. Percentage of individuals in the Laupāhoehoe (montane wet forest) plot growing on each substrate type. Table S4. Size and multiple stem characteristics of the species species in Laupāhoehoe (montane wet forest) and Pālamanui (lowland dry forest) plots; species abbreviations as in Table S4. Table S5. References from Table 1.
(DOCX)
Acknowledgments
The Hawai‘i Permanent Plot Network thanks the USFS Institute of Pacific Islands Forestry (IPIF) and the Hawai‘i Division of Forestry and Wildlife/Department of Land and Natural Resources for permission to conduct research within the Hawai‘i Experimental Tropical Forest; the Palāmanui Group, especially Roger Harris, for access to the lowland dry forest site. We thank N. DiManno, L. Ellsworth, B. Hwang, R. Moseley, M. Murphy, K. Nelson-Kaula, M. Nullet, C. Perry, J. Schulten, M. Snyder, and J. VanDeMark for logistical assistance, among the many others who served as project interns and volunteers (see hippnet.hawaii.edu); G. Asner, S. Davies, T. Giambelluca, J. Mascaro, D. Metcalfe, J. Michaud, and J. Thompson for technical advice and/or comments on the manuscript
Funding Statement
The major funding for this research came from National Science Foundation's EPSCoR Grants No. 0554657 and No. 0903833 to the University of Hawai ‘i. Major in-kind support was provided by the Pacific Southwest Research Station of the United States Forest Service. Logistical or financial support that was supplemental was provided for by the Smithsonian Tropical Research Institute Center for Tropical Forest Science, and the University of California, Los Angeles. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gentry AH (1988) Changes in plant community diversity and floristic composition on environmental and geographical gradients. Annals of the Missouri Botanical Garden 75: 1–34. [Google Scholar]
- 2. Givnish TJ (1999) On the causes of gradients in tropical tree diversity. Journal of Ecology 87: 193–210. [Google Scholar]
- 3. Phillips O, Gentry AH, Hall P, Sawyer S, Vasquez R (1994) Dynamics and species richness of tropical rain forests. Proceedings of the National Academy of Sciences 91: 2805–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mittelbach GG, Schemske DW, Cornell HV, Allen AP, Brown JM, et al. (2007) Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecology Letters 10: 315–331. [DOI] [PubMed] [Google Scholar]
- 5. Hawkins BA, Rodríguez MÁ, Weller SG (2011) Global angiosperm family richness revisited: linking ecology and evolution to climate. Journal of Biogeography 38: 1253–1266. [Google Scholar]
- 6.Condit R (1998) Tropical forest densus plots: Methods and results from Barro Colorado Island, Panama and a comparison with other plots. Berlin, Germany, and Georgetown, Texas, USA: Springer-Verlag and R. G. Landes Company. 211 p. [Google Scholar]
- 7.Rosenzweig ML (1995) Species diversity in space and time. Cambridge, UK: Cambridge University Press.
- 8. Givnish TJ (1999) On the causes of gradients in tropical tree diversity. Journal of Ecology 87: 193–210. [Google Scholar]
- 9.Hubbell SP (2001) The unified neutral theory of biodiversity and biogeography. Princeton, New Jersey, USA: Princeton University Press.
- 10.Zimmerman JK, Thompson J, Brokaw N (2008) Large tropical forest dynamics plots: testing explanations for the maintenance of species diversity. Chichester, UK: Wiley-Blackwell.
- 11. Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, et al. (2005) Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecological Monographs 75: 3–35. [Google Scholar]
- 12. Chisholm RA, Muller-Landau HC, Abdul Rahman K, Bebber DP, Bin Y, et al. (2013) Scale-dependent relationships between tree species richness and ecosystem function in forests. Journal of Ecology 101: 1214–1224. [Google Scholar]
- 13. Torti SD, Coley PD, Kursar TA (2001) Causes and consequences of monodominance in tropical lowland forests. American Naturalist 157: 141–153. [DOI] [PubMed] [Google Scholar]
- 14.Ghazoul J, Sheil D (2010) Tropical rain forest ecology, diversity, and conservation. Oxford: Oxford University Press.
- 15. Whittaker RJ, Triantis KA, Ladle RJ (2008) A general dynamic theory of oceanic island biogeography. Journal of Biogeography 35: 977–994. [Google Scholar]
- 16. Kier G, Kreft H, Lee TM, Jetz W, Ibisch PL, et al. (2009) A global assessment of endemism and species richness across island and mainland regions. Proceedings of the National Academy of Sciences 106: 9322–9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Terborgh J (1985) The vertical component of plant species diversity in temperate and tropical forests. The American Naturalist 126: 760–776. [Google Scholar]
- 18. Spies TA (1998) Forest structure: A key to the ecosystem. Northwest Science 72: 34–39. [Google Scholar]
- 19.Whitmore TC (1998) An introduction to tropical rain forests. Oxford, UK: Oxford Press.
- 20. Moles AT, Bonser SP, Poore AGB, Wallis IR, Foley WJ (2011) Assessing the evidence for latitudinal gradients in plant defence and herbivory. Functional Ecology 25: 380–388. [Google Scholar]
- 21. Price JP, Clague DA (2002) How old is the Hawaiian biota? Geology and phylogeny suggest recent divergence. Proceedings of the Royal Society of London, Series B Biological Sciences 269: 2429–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner WL, Herbst DR, Sohmer S (1999) Manual of the flowering plants of Hawaii. 2nd ed. Honolulu, HI: Bishop Museum.
- 23. Condit R (1995) Research in large, long-term tropical forest plots. Trends in Ecology and Evolution 10: 18–21. [DOI] [PubMed] [Google Scholar]
- 24. Connell JH, Green PT (2000) Seedling dynamics over thirty-two years in a tropical rain forest. Ecology 81: 568–584. [Google Scholar]
- 25. Hobbie JE, Carpenter SR, Grimm NB, Gosz JR, Seastedt TR (2003) The US long term ecological research program. BioScience 53: 21–32. [Google Scholar]
- 26.Hubbell SP (2004) Two decades of research on the BCI Forest Dynamics Plot. In: Losos EC, Leigh EG Jr, editors. Tropical forest diversity and dynamism: Findings from a large-scale plot network. Chicago: University of Chicago Press. pp. 8–30. [Google Scholar]
- 27. Clinebell RR, Phillips OL, Gentry AH, Stark N, Zuuring H (1995) Prediction of neotropical tree and liana species richness from soil and climatic data. Biodiversity and Conservation 4: 56–90. [Google Scholar]
- 28. Malhi Y, Wood D, Baker TR, Wright J, Phillips OL, et al. (2006) The regional variation of aboveground live biomass in old-growth Amazonian forests. Global Change Biology 12: 1107–1138. [Google Scholar]
- 29. Saatchi SS, Houghton RA, Dos Santos Alvalá RC, Soares JV, Yu Y (2007) Distribution of aboveground live biomass in the Amazon basin. Global Change Biology 13: 816–837. [Google Scholar]
- 30. Slik JWF, Aiba S-I, Brearley FQ, Cannon CH, Forshed O, et al. (2010) Environmental correlates of tree biomass, basal area, wood specific gravity and stem density gradients in Borneo's tropical forests. Global Ecology and Biogeography 19: 50–60. [Google Scholar]
- 31. Toledo M, Poorter L, Peña-Claros M, Alarcón A, Balcázar J, et al. (2011) Climate and soil drive forest structure in Bolivian lowland forests. Journal of Tropical Ecology 27: 333–345. [Google Scholar]
- 32.Lugo AE, Scatena FN, Silver WL, Molina Colón S, Murphy PG (2002) Resilience of tropical wet and dry forests in Puerto Rico. In: Gunderson LH, Pritchard L Jr, editors. Resilience and the behavior of large-scale systems. Washington, DC: Island Press. pp. 195–225. [Google Scholar]
- 33. Bellingham PJ, Stewart GH, Allen RB (1999) Tree species richness and turnover throughout New Zealand forests. Journal of Vegetation Science 10: 825–832. [Google Scholar]
- 34. Sagar R, Singh JS (2006) Tree density, basal area and species diversity in a disturbed dry tropical forest of northern India: implications for conservation. Environmental Conservation 33: 256–262. [Google Scholar]
- 35.Stegen JC, Swenson NG, Enquist BJ, White EP, Phillips OL, et al.. (2011) Variation in above-ground forest biomass across broad climatic gradients. Global Ecology and Biogeography: 744–754.
- 36. Price JP, Wagner WL (2004) Speciation in Hawaiian angiosperm lineages: cause, consequence, and mode. Evolution 58: 2185–2200. [DOI] [PubMed] [Google Scholar]
- 37.Ziegler AC (2002) Hawaiian natural history, ecology, and evolution. Honolulu, HI: University of Hawaii Press.
- 38. Harrison RD (2011) Emptying the forest: Hunting and the extirpation of wildlife from tropical nature reserves. BioScience 61: 919–924. [Google Scholar]
- 39. Vitousek PM, Farrington H (1997) Nutrient limitation and soil development: experimental test of a biogeochemical theory. Biogeochemistry 37: 63–75. [Google Scholar]
- 40.Juvik SP, Juvik JO, Paradise TR (1998) Atlas of Hawaii, 3rd edition. Honolulu, HI: University of Hawaii Press.
- 41. Giambelluca TW, Chen Q, Frazier AG, Price JP, Chen Y-L, et al. (2013) Online Rainfall Atlas of Hawai‘i. Bulletin of the American Meteorological Society 94: 313–316. [Google Scholar]
- 42. Crews TE, Kitayama K, Fownes JH, Riley RH, Herbert DA, et al. (1995) Changes in soil phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology 76: 1407–1424. [Google Scholar]
- 43.Tosi JAJ, Watson V, Bolaños R (2002) Life zone maps Hawaii, Guam, American Samoa, Northern Mariana Islands, Palau, and the Federated States of Micronesia. San Jose, Costa Rica and Hilo, Hawaii, USA: Tropical Science Center and the Institute of Pacific Islands Forestry, USDA Forest Service.
- 44. Kellner JR, Asner GP (2009) Convergent structural responses of tropical forests to diverse disturbance regimes. Ecology Letters 12: 1–11. [DOI] [PubMed] [Google Scholar]
- 45. Mueller-Dombois D (2000) Rain forest establishment and succession in the Hawaiian Islands. Landsc Urban Plann 51: 147–157. [Google Scholar]
- 46. Friday JB, Scowcroft PG, Ares A (2008) Responses of native and invasive plant species to selective logging in an Acacia koa-Metrosideros polymorpha forest in Hawai'i. Applied Vegetation Science 11: 471–482. [Google Scholar]
- 47. Murphy M, Inman-Narahari F, Ostertag R, Litton CM (2014) Invasive feral pigs impact native tree ferns and woody seedlings in Hawaiian forest. Biological Invasions 16: 63–71. [Google Scholar]
- 48.Moore RB, Claque DA, Rubin M, Bohrson WA (1987) Hualalai volcano: A preliminary summary of geologic, petrologic, and geophysical data. In: Decker RW, Wright TL, Stauffer PH, editors. Volcanism in Hawaii. Washington, DC: U.S. Geological Service Professional Paper 1350, U’. Government Printing Office. pp. 571–585. [Google Scholar]
- 49.Giambelluca T, Chen Q, Frazier A, Price J, Chen Y-L, et al. (2011) The Rainfall Atlas of Hawai‘i. Available: http://rainfall.geography.hawaii.edu. Accessed 2014 Aug 10.
- 50. Thaxton JM, Cole TC, Cordell S, Cabin RJ, Sandquist DR, et al. (2010) Native species regeneration following ungulate exclusion and nonnative grass removal in a remnant Hawaiian dry forest. Pacific Science 64: 533–544. [Google Scholar]
- 51. Freifelder RR, Vitousek PM, D'Antonio CM (1998) Microclimate change and effect on fire following forest-grass conversion in seasonally dry tropical woodland. Biotropica 30: 286–297. [Google Scholar]
- 52. Litton CM, Sandquist DR, Cordell S (2006) Effects of non-native grass invasion on aboveground carbon pools and tree population structure in a tropical dry forest of Hawaii. Forest Ecology and Management 231: 105–113. [Google Scholar]
- 53.Condit R (1998) Tropical forest census plots. Berlin: Springer-Verlag, Berlin, Germany.
- 54. Asner GP, Hughes RF, Mascaro J, Uowolo AL, Knapp DE, et al. (2011) High-resolution carbon mapping on the million-hectare island of Hawai‘i. Frontiers in Ecology and the Environment 9: 434–439. [Google Scholar]
- 55. Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, et al. (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145: 87–99. [DOI] [PubMed] [Google Scholar]
- 56.Zanne AE, Lopez-Gonzalez G, Coomes DA, Ilic J, Jansen S, et al.. (2009) Towards a worldwide wood economics spectrum. Dryad Digital Repository doi: 10.5061/dryad.5234. [DOI] [PubMed]
- 57. Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, et al. (2009) Towards a worldwide wood economics spectrum. Ecology Letters 12: 351–366. [DOI] [PubMed] [Google Scholar]
- 58. Slik JWF (2006) Estimating species-specific wood density from the genus average in Indonesian trees. Journal of Tropical Ecology 22: 481–482. [Google Scholar]
- 59. Chave J, Alonso D, Etienne RS (2006) Theoretical biology: Comparing models of species abundance. Nature 441: E1. [DOI] [PubMed] [Google Scholar]
- 60. Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4: 379–391. [Google Scholar]
- 61. Chiarucci A, Enright NJ, Perry GLW, Miller BP, Lamont BB (2003) Performance of nonparametric species richness estimators in a high diversity plant community. Diversity and Distributions 9: 283–295. [Google Scholar]
- 62. Colwell RK, Mao CX, Chang J (2004) Interpolating, extrapolating, and comparing incidence-based species accumulation curves Ecology. 85: 2717–2727. [Google Scholar]
- 63.Magurran A (2004) Measuring biological diversity. Oxford, UK: Blackwell Publishing.
- 64.Sokal RR, Rohlf FJ (1994) Biometry: The principles and practice of statistics in biological research. 3rd edition. New York, New York: W.H. Freeman.
- 65.SAS Institute (2005) JMP introductory guide, release 6. Cary, NC: SAS Institute.
- 66. Asner GP, Hughes RF, Varga TA, Knapp DE, Kennedy-Bowdoin T (2009) Environmental and biotic controls over aboveground biomass throughout a tropical rain forest. Ecosystems 12: 261–278. [Google Scholar]
- 67.Brenes-Arguedas T, Roddy A, Coley P, Kursar T (2010) Do differences in understory light contribute to species distributions along a tropical rainfall gradient? Oecologia: 1–14. [DOI] [PMC free article] [PubMed]
- 68.Ashton PS (2004) Floristics and vegetation of the forest dynamics plots. In: Losos E, Leigh EG Jr, editors. Tropical forest diversity and dynamism: Findings from a large-scale plot network. Chicago, Illinois, USA: University of Chicago Press. [Google Scholar]
- 69. Davidar P, Puyravaud JP, Leigh EG Jr (2005) Changes in rain forest tree diversity, dominance and rarity across a seasonality gradient in the Western Ghats, India. Journal of Biogeography 32: 493–501. [Google Scholar]
- 70. Clark JS, McLachlan JS (2003) Stability of forest diversity. Nature 423: 635–638. [DOI] [PubMed] [Google Scholar]
- 71. Clark DA, Piper SC, Keeling CD, Clark DB (2003) Tropical rain forest tree growth and atmospheric carbon dynamics linked to interannual temperature variation during 1984–2000. Proceedings of the National Academy of Sciences 100: 5852–5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Feeley KJ, Wright SJ, Supardi MNN, Kassim AR, Davies SJ (2007) Decelerating growth in tropical forest trees. Ecology Letters 10: 1–9. [DOI] [PubMed] [Google Scholar]
- 73. Clark DB, Clark DA, Oberbauer SF (2010) Annual wood production in a tropical rain forest in NE Costa Rica linked to climatic variation but not to increasing CO2 . Global Change Biology 16: 747–759. [Google Scholar]
- 74. Stegen JC, Swenson NG, Enquist BJ, White EP, Phillips OL, et al. (2011) Variation in above-ground forest biomass across broad climatic gradients. Global Ecology and Biogeography 20: 744–754. [Google Scholar]
- 75.Martinez-Yrizar A (1995) Biomass distribution and primary productivity of tropical dry forests. In: Bullock SH, Mooney HA, Medina E, editors. Seasonally Dry Tropical Forests. Cambridge, UK: Cambridge University Press. pp. 326–345. [Google Scholar]
- 76. Vitousek P, Asner GP, Chadwick OA, Hotchkiss S (2009) Landscape-level variation in forest structure and biogeochemistry across a substrate age gradient in Hawaii. Ecology 90: 3074–3086. [DOI] [PubMed] [Google Scholar]
- 77. Cole RJ, Litton CM, Koontz MJ, Loh RK (2012) Vegetation recovery 16 years after feral pig removal from a wet Hawaiian forest. Biotropica 44: 463–471. [Google Scholar]
- 78. Inman-Narahari F, Ostertag R, Cordell S, Giardina CP, Nelson-Kaula K, et al. (2013) Seedling recruitment factors in low-diversity Hawaiian wet forest: towards global comparisons among tropical forests. Ecosphere 4: 24 http://dx.doi.org/10.1890/ES1812-00164.00161. [Google Scholar]
- 79. Busby PE, Vitousek PM, Dirzo R (2010) Prevalence of tree regeneration by sprouting and seeding along a rainfall gradient in Hawai'i. Biotropica 42: 80–86. [Google Scholar]
- 80. Kammesheidt L (1999) Forest recovery by root suckers and above-ground sprouts after slash-and-burn agriculture, fire and logging in Paraguay and Venezuela. Journal of Tropical Ecology 15: 143–157. [Google Scholar]
- 81. Bellingham PJ, Sparrow AD (2009) Multi-stemmed trees in montane rain forests: their frequency and demography in relation to elevation, soil nutrients, and disturbance. Journal of Ecology 97: 472–483. [Google Scholar]
- 82. Lehmann A, Leathwick JR, Overton JM (2002) Assessing New Zealand fern diversity from spatial predictions of species assemblages. Biodiversity and Conservation 11: 2217–2238. [Google Scholar]
- 83. Coomes DA, Allen RB, Bentley WA, Burrows LE, Canham CD, et al. (2005) The hare, the tortoise and the crocodile: the ecology of angiosperm dominance, conifer persistence and fern filtering. Journal of Ecology 93: 918–935. [Google Scholar]
- 84. Bellingham PJ, Richardson SJ (2006) Tree seedling growth and survival over 6 years across different microsites in a temperate rain forest. Canadian Journal of Forest Research 36: 910–918. [Google Scholar]
- 85. Tanner EVJ (1983) Leaf demography and growth of the tree-fern Cyathea pubescens Mett ex Kuhn in Jamaica Bot J Linn Soc. 87: 213–227. [Google Scholar]
- 86. Bernabe N, Williams-Linera G, Palacios-Rios M (1999) Tree ferns in the interior and at the edge of a Mexican cloud forest remnant: Spore germination and sporophyte survival and establishment. Biotropica 31: 83–88. [Google Scholar]
- 87. Arens NC (2001) Variation in performance of the tree fern Cyathea caracasana (Cyatheaceae) across a successional mosaic in an Andean cloud forest. American Journal of Botany 88: 545–551. [PubMed] [Google Scholar]
- 88. Williams-Linera G, Palacios-Ríos M, Hernández-Gómez R (2005) Fern richness, tree species surrogacy and fragments complementarity in a Mexican tropical montane cloud forest. Biodiversity and Conservation 14: 119–133. [Google Scholar]
- 89. Jones MM, Olivas Rojas P, Tuomisto H, Clark DB (2007) Environmental and neighbourhood effects on tree fern distributions in a neotropical lowland rain forest. Journal of Vegetation Science 18: 13–24. [Google Scholar]
- 90. Durand LZ, Goldstein G (2001) Growth, leaf characteristics, and spore production in native and invasive tree ferns in Hawaii. American Fern Journal 91: 25–35. [Google Scholar]
- 91. Amatangelo KL, Vitousek PM (2009) Contrasting predictors of fern versus angiosperm decomposition in a common garden. Biotropica 41: 154–161. [Google Scholar]
- 92. Mack MC, D'Antonio CM, Ley RE (2001) Alteration of ecosystem nitrogen dynamics by exotic plants: A case study of C4 grasses in Hawaii. Ecological Applications 11: 1323–1335. [Google Scholar]
- 93. Huenneke LF, Vitousek PM (1989) Seedling and clonal recruitment of the invasive tree, Psidium cattleianum: implications for management of native Hawaiian forests. Biological Conservation 53: 199–211. [Google Scholar]
- 94. Hughes RF, Denslow JS (2005) Invasion by an N2-fixing tree, Falcataria moluccana, alters function, composition, and structure of wet lowland forests of Hawai‘i. Ecological Applications 15: 1615–1628. [Google Scholar]
- 95. Gravel D, Canham CD, Beaudet M, Messier C (2006) Reconciling niche and neutrality: The continuum hypothesis. Ecology Letters 9: 399–409. [DOI] [PubMed] [Google Scholar]
- 96.Hubbell SP, Foster RB (1986) Commonness and rarity in a neotropical forest: Implications for tropical tree conservation. In: Soule ME, editor. Conservation biology: The science of scarcity and diversity: Sunderland, Mass, USA Illus Paper Maps. Sunderland, Massachussetts: Sinauer Associates, Inc. pp. 205–231. [Google Scholar]
- 97. Lach L (2003) Invasive ants: Unwanted partners in ant-plant interactions? Annals of the Missouri Botanical Garden 90: 91–108. [Google Scholar]
- 98. Nogueira SSdC, Nogueira-Filho SLG, Bassford M, Silvius K, Fragoso JMV (2007) Feral pigs in Hawai'i: Using behavior and ecology to refine control techniques. Appl Anim Behav Sci 108: 1–11. [Google Scholar]
- 99. Nogueira-Filho S, Nogueira S, Fragoso J (2009) Ecological impacts of feral pigs in the Hawaiian Islands. Biodiversity and Conservation 18: 3677–3683. [Google Scholar]
- 100.Shiels A, Drake D (2010) Are introduced rats (Rattus rattus) both seed predators and dispersers in Hawaii? Biological Invasions: 1–12.
- 101. Shiels AB (2011) Frugivory by introduced black rats (Rattus rattus) promotes dispersal of invasive plant seeds. Biological Invasions 13: 781–792. [Google Scholar]
- 102. Gilbert B, Lechowicz MJ (2004) Neutrality, niches, and dispersal in a temperate forest understory. Proceedings of the National Academy of Sciences of the United States of America 101: 7651–7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hubbell SP (2005) Neutral theory in community ecology and the hypothesis of functional equivalence. Functional Ecology 19: 166–172. [Google Scholar]
- 104.Losos E, Leigh EG Jr, editors (2004) Tropical forest diversity and dynamism: Findings from a large-scale plot network. Chicago, Illinois: University of Chicago Press.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods S1. Detailed methods and description of situations where field site conditions dictated a different or entirely new methodology by adopted than standardized CTFS protocol in [1].
(DOCX)
Supporting tables. Table S1. Values and equations used for estimating aboveground biomass (AGB) in the montane wet forest (MWF) and lowland dry forest sites (LDF). Table S2. Species ≥1 cm diameter at breast height recorded in Laupāhoehoe (montane wet forest) plot with canopy dominants in bold. Table S3. Percentage of individuals in the Laupāhoehoe (montane wet forest) plot growing on each substrate type. Table S4. Size and multiple stem characteristics of the species species in Laupāhoehoe (montane wet forest) and Pālamanui (lowland dry forest) plots; species abbreviations as in Table S4. Table S5. References from Table 1.
(DOCX)